Abstract
Purpose
The purpose of this systematic review is to evaluate the effects of adipose derived mesenchymal stem cells (ADSCs) in the treatment of osteoarthritis (OA) in the clinical setting.
Methods
A literature search was performed in the MEDLINE, EMBASE, and The Cochrane Library Database up to January 2017 for inclusion and exclusion criteria. Criteria for inclusion were clinical studies demonstrating the effects of ADSCs on OA, and written in English. The following variables were analyzed: donor site, volume of adipose tissue, preparation of ADSCs, clinical outcomes, and complication rate.
Results
Sixteen studies (knee: 14 studies, multiple joints: 1 study, ankle: 1 study) were included in this systematic review. All of the studies prepared ADSCs in the form of the stromal vascular fraction (SVF). Inconsistencies between studies were found with regards to reported clinical variability, donor sites of SVF, and reported clinical outcomes. Nine studies used either platelet-rich plasma (PRP) (7/16) or fibrin (4/16) or both PRP and Fibrin (1/16), as an adjunct at time of SVF injection. All of the studies reported an improvement in clinical outcomes with the use of SVF. Five studies reported a 90% satisfaction rate, and no study reported any complications with liposuction. Five studies reported on complications, with a 5% incidence of swelling and pain.
Conclusions
This systematic review demonstrated that ADSCs are currently used in the form of SVF. While SVF may produce favorable clinical outcomes with minimal risk of side effects on osteoarthritis, the variability in the data and the use of biological adjuvants have confounded the effectiveness of ADSCs. This study will help surgeons understand the limitations in the literature on ADSCs.
Level of evidence
Level IV, systematic review of level IV studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a highly prevalent, progressive, debilitating joint disease characterized by gradual loss of articular cartilage, damage to the subchondral bone and the surrounding soft tissue [1]. The avascular nature of articular cartilage limits its capacity for self-repair, resulting in progressive cartilage loss ultimately contributing to widespread degeneration of the affected joint [2]. Consequently, OA has become a primary focus for orthopaedic surgeons to provide a biologic milieu that will facilitate some form of endogenous cartilage healing [3].
Various biologic adjuncts have been described that may affect repair including growth factors and stem cells. Much of the recent literature has focused on bone marrow-derived mesenchymal stem cells (MSC) for chondrogenesis [4]. However, the clinical use of bone marrow MSCs has presented problems, including donor site morbidity and pain and low cell number upon harvest [5]. This has led to the investigation of alternative sources for MSCs [6]. Several potential donor sites have been identified for harvesting MSCs, including periosteum, muscle, synovial membrane and adipose tissue [7]. All MSCs share similar characteristics in that they have the capacity to differentiate into chondrocytes, osteoblasts, myoblasts, adipocytes, and fibroblasts depending on their differentiation potential and the conditions in which they are stimulated.
Adipose tissue has become an attractive alternative source of MSCs because of its relatively easy accessibility and abundance [8]. Although adipose may not have the same degree of differentiation as bone marrow MSCs, the relative abundance of MSCs may be more advantageous, but there is evidence suggesting that adipose derived mesenchymal stem cells (ADSCs) have reduced chondogrenic potential relating to its bone morphogenic protein characteristics and the increased age of the patient [5, 8,9,10]. However, recent evidence suggests that ADSCs obtained from lipoaspirates can be induced to express gene and matrix markers associated with chondrocyte pathways under specific conditions [11]. Significant chondrogenic effects from the application of ADSCs have been shown in in-vitro studies, proposing that ADSCs possess the CD73, CD90, CD105 and CD106 markers, which are surface markers required for cell differentiation into cartilage [12]. In addition, recent animal model studies confirmed the chondrogenesis effect of ADSCs in vivo [13]. There is also a paracrine effect of ADSCs on OA chondrocytes as they promote inhibitory macrophages and T regulatory cells, which may decrease inflammatory markers and improve clinical outcomes alongside potential cartilage regeneration [14]. This has prompted several studies to investigate the role of ADSCs in human clinical trials.
However, due to the relative novelty of using ADSCs, there has been no systematic review that demonstrates its efficacy in osteoarthritic joints to date. The purpose of this systematic review is to evaluate the effects of ADSCs in the treatment of OA in the clinical setting. The hypothesis is that ADSCs would produce effective outcome on OA repair in clinical settings supported by clinical evidence.
Materials and methods
Search strategy
The following search terms were used in MEDLINE, EMBASE and the Cochrane Library Database, databases on January 7th 2017: “(cartilage OR cartilage injury OR cartilage damage OR cartilage repair OR cartilage defect OR osteochondral injury OR osteoarthritis) AND (adipose stem OR adipose stem cell OR adipose stem cells OR adipose derived OR adipose derived stem cell OR adipose derived stem cells OR adipose derived mesenchymal stem OR adipose derived mesenchymal stem cell OR adipose derived mesenchymal stem cells OR adipose derived msc OR svf OR stromal vascular fraction of adipose tissue)”. No time limit was given to publication date.
Eligibility criteria
The inclusion criteria were: (1) clinical studies demonstrating the effects of ADSCs on OA, (2) published in a peer reviewed journal, (3) written in English, and (4) full-text of studies available. Exclusion criteria were the following: (1) review studies, (2) case report, (3) in vitro studies, and (4) animal studies.
Study selection
Two independent reviewers performed the literature search and reviewed the search results. The title and abstract were reviewed for all search results, and potentially eligible studies received full text review. In addition, the reference lists of all publications, including systematic reviews found in the search results, were manually screened for additional articles, which met the inclusion criteria that were potentially not identified through our electronic search. If a consensus could not be reached, a senior author was consulted who had the final decision.
Data extraction/analysis
The level of evidence (LOE) was evaluated based on previously published criteria [6]. The methodological index for non-randomized studies (MINORS) score [15] was used to evaluate the methodological quality of the clinical evidence (MQOE), with a score of 0–8 for case series and 0–12 for cohort studies.
The data of each clinical study was then extracted using a standardized data sheet with a list of 30 standardized variables (Table 1) [16]. The following variables were analyzed: donor site locations, volume of adipose tissue, donor site complications, adjunctive biologic therapies, concomitant treatment and clinical outcomes. In addition, the preparation and delivery methods of ADSCs was also recorded and analyzed.
Results
Search and literature selection
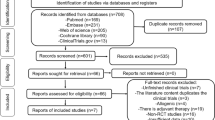
The literature search revealed 2475 total studies and 1728 studies after duplicates were removed. Sixteen clinical studies with 635 joints were included in this review (Fig. 1).
Reported clinical variability in included studies
The clinical variability of the included 16 studies is shown in Table 1. A mean of 61% of the characteristic data was reported. General demographics including age and sex were reported in 94% of studies. While the study design and patient satisfaction were all well reported variables with 88 and 77%, respectively, patient history, clinical variables and imaging data were the least reported criteria with 16, 36, and 38% of the data being reported, respectively.
Donor site locations, volume of adipose tissue, and complications
All of the studies reported the location of the harvesting of adipose tissue. The most common sites included the buttock (nine studies [2, 17,18,19,20,21,22,23, 30]), abdomen (four studies [25,26,27,28]), and infrapatellar fat pad (two studies [24, 29]). One study did not report the harvesting site [31]. The weighted average volume of harvested adipose tissue in the buttock (reported in 6/9 studies [17,18,19,20, 22, 30]) was 140 ml, in the abdomen (reported in 4/4 studies [25,26,27,28]) was 103 ml, and in the infrapatellar fat pad (two studies [24, 29]) was 9.26 g. All of the studies using the buttock or abdomen harvested with liposuction and no study reported complications in the liposuction procedure. The two studies utilizing the infrapateller fat pad harvested via open excision [29, 30].
Preparation method of SVF
All of the studies prepared ADSCs in the form of the stromal vascular fraction (SVF). SVF is a component of the lipoaspirate obtained by liposuction, comprised of ADSCs as well as variety of other cells including pericytes, vascular adventitia cells, fibroblasts, preadipocytes, monocytes, macrophages, and red blood cells [31]. Eleven studies used the Zuk et al. method of ADSC preparation [32]. Eight studies [2, 19,20,21,22,23, 29, 30] reported liposuction the day before surgery, four studies [24,25,26, 28] reported liposuction the day of, and four studies [18, 21, 27, 31] did not report the date of liposuction. After the liposuction, the procedure of the adipose tissue was transported to the laboratory, where adipocytes and connective tissue were separated from the stromal vascular fraction (SVF) by centrifugation, according to the method by Zuk et al. [32]. The majority of the studies analyzed the SVF cells to confirm chondrogenic potential, including 12 studies via flow cytometry, three studies via cell count, 13 studies via culture in a medium. Only two studies did not report analysis of SVF cells [25, 28]. The mean concentration number of ADSCs in the SVF was 6.3 × 106 (range; 1.2 × 106 − 4.2 × 107) in the ten studies that reported this [2, 17,18,19,20, 22, 23, 28,29,30]. The mean percentage of ADSCs in the SVF was 9.2% (range 8.5–9.7%) in the 5 studies [2, 18, 20, 23, 30] that reported this. One study [31] used three different doses with dose escalation: low dose (2 × 106 cells), medium dose (10 × 106), and high dose (50 × 106).
Treatment
Of the 16 studies that treated patients using the SVF, 5 were case series (Table 2) involving OA knees using arthroscopic debridement, with 4 case series using injections in multiple OA joints [17, 18, 22, 24, 25, 26, 28, 29, 31]. All ten studies involved patients with OA joints and reported significant improvement in clinical outcome measures. In six studies, the SVF was used with biologics (PRP or fibrin) [17, 18, 24, 25, 28, 29]. The mean lesion size was reported by three of the ten studies, and ranged between 5.4 and 6.2 cm [2, 17, 22].
Of the 16 studies that treated patients using the SVF, 7 were comparative studies [2, 19, 20, 21, 23, 27, 30] (Table 3), All 7 comparative studies reported significant improvement of functional outcomes including three studies that compared SVF to no SVF. Six studies involved OA knees [2, 20, 21, 23, 27, 30], and one study involved OA ankles [19]. All studies in OA knees used SVF with a biologic (PRP or fibrin), while the study in the ankle compared SVF alone to no biologics. The mean lesion size was reported by three of the seven studies, and ranged between 4.6 and 6.4 cm [2, 20, 30]. The clinical outcomes of the comparative studies are reported in Table 4.
Radiological outcomes
Eight of the included studies reported the radiological outcomes using MRI or X-ray [18, 24, 25, 26, 27, 28, 30, 31]. The reported outcome measures were mixed, but the evidence was promising. MRI findings showed improved cartilage thickness in the majority of the included studies, with only one study finding no difference between the pre and post-treatment scores. Additionally, no study found any evidence of tumor formation. The radiological outcomes reported are shown in Table 5.
Patient satisfaction and complication rate
Five of the included studies reported the satisfaction rate, all of which were above 90% [2, 17, 23, 24, 26]. Seven of the included studies reported the complication rate. Two studies [28, 31] reported a 10 and 37% complication rate of swelling and four studies reported no complications [21, 24, 25, 27]. The other included studies did not mention any complications.
Discussion
The most important finding of this systematic review was that ADSCs in the form of SVF produce favorable clinical outcomes with minimal complications in the treatment of OA. Additionally, several studies showed that patients receiving SVF had better radiographic outcomes compared to the baseline [18, 24, 25, 27, 29]. However, caution should be taken to interpret the outcomes of the present study. This systematic review demonstrates the variability in both the data collected and the use of biological adjuvants between studies, thereby limiting proper cross-study comparisons and potentially confounding the effects of ADSCs. Therefore, while SVF may produce favorable clinical outcomes for patients with OA, the confounding factors limit our understanding of the effectiveness of ADSCs.
There have been many studies evaluating the effects of ADSCs in cartilage repair; however, the terminology describing the use of ADSCs has been inconsistent and confusing with terms including adipose-derived adult stem (ADAS) cells, adipose-derived mesenchymal stem cells (AD-MSCs), adipose MSCs (AMSCs), and adipose stromal/stem cells (ASCs). The results from this systematic review demonstrated inconsistencies on what components of ADSC were used in the various studies reviewed when evaluating the effects of ADSCs. The results demonstrate that analysis has focused on the SVF rather than ADSCs. The SVF contains different proportions of ADSCs, pericytes, vascular adventitia cells, fibroblasts, preadipocytes, monocytes, macrophages, and red blood cells [33]. Therefore, analysis of the SVF limits the ability to evaluate the effectiveness of only the stem cell component of the fraction. There has been limited data evaluating other components of SVF such as pericytes and the vascular fraction and therefore these human trials cannot determine the role each factor has in cartilage regeneration.
The results of this systematic review also demonstrate the use of an adjunctive procedure in the majority of the studies evaluated (12/16 studies). These studies utilized either PRP (7/16) or fibrin (4/16), with one study comparing both biologic adjuncts. These studies demonstrated favorable outcomes when SVF and a biologic were used. Few studies have compared SVF to the adjunctive biologic itself, confounding meaningful analysis. There is evidence that PRP contains growth factors that increases chondrocyte viability and differentiation, as well as the synthetic capacity of MSCs, which may prove beneficial in cartilage repair [34, 35]. There have been several level I and II clinical studies demonstrating significant effects and positive clinical outcomes of PRP applications in the treatment of OA and osteochondral defects [36, 37]. There is also evidence of fibrin as a stem cell carrier [34], however, its effect on cartilage repair is largely unknown. Both PRP and fibrin can act as a scaffold and may enhance ADSC adherence to cartilage lesions and promote their proliferation [38]. The application of either PRP or fibrin confounds the effects of ADSCs in OA; therefore, the advantages of ADSCs beyond the use of these adjuncts cannot be determined from this study.
The results from the current study demonstrate large variability in the data reported thereby limiting the ability to analyze the results through meta-analytical methods. Two different sites including the knee and ankle were evaluated in the literature, which result in variable responses in cartilage repair. In addition, many studies failed to report lesion size, which is an important prognostic factor in the repair process of cartilage [39]. Another reported variable was in the donor sites location, which varied between the buttock and infra-patellar fat pad. Although liposuction has been shown to produce a higher percentage of viable cells in lipoaspirates when compared to surgical resection of adipose tissue [35, 37], the number of nucleated cells can range from 500,000 to 2,000,000 cells per gram of adipose tissue [40]. Recent evidence has shown that different individuals have variable density in different locations of adipose tissue [41] and that there may be differences in the multi-potency of ADSCs depending on the harvest location [42, 43]. In addition to the extraction methods and the differences in adipose tissue, the process and preparation of SVF are an important assessment area in this systematic review.
There was significant variability in the preparation methods reported in the included studies, and in the reported methods of assessing chondrogenic potential. The reported percentage of ADSCs in the SVF was only 9.2% in the studies assessing this, showing that the majority of the injected material was in fact the pericytes, vascular adventitia cells, fibroblasts, preadipocytes, monocytes, macrophages, and red blood cells. Additionally, those studies found a wide-ranging difference in the percentage of included ADSCS in SVF, although no analysis of factors relating to differences where performed. Previously, studies have found that older patients have decreased growth factors and chondrogenic potential in autologous blood products compared to younger patients [44]. While the Zuk method of SVF preparation [32] originally reported using collagenase to separate the ADSC from the surrounding area, the authors in the included studies did not report using any collagenase indicating that the method may have been altered. It is worth noting that collagenase digestion cannot be used in the United States. The concentration and incubation time of collagenase can affect the yield of ADSCs as higher dosages or exposures have been shown to be toxic to ADSCs [38]. Excess amounts of collagenase can decrease the ADSC viability while insufficient amounts may result in inefficient and inadequate amounts of ADSC yield [12, 38]. Although all of the included studies reported improved clinical outcomes, the lack of standardization in the clinical outcome measures, the operative treatment, and concomitant biologic use limits adequate comparison thus confounding any meaningful clinical analysis.
Five of the studies (42%) reported on patient’s reported outcomes with all above 90% satisfaction. However, of the five studies reporting on patient satisfaction only 2 reported radiological findings, with mixed results. Koh et al. found high patient satisfaction alongside improved radiological outcome measures, while Fodor et al. showed high patient satisfaction with no change in radiological measures at 3 months [23, 26]. These findings suggest that the patient satisfaction may be at least partially due to the paracrine effect of the SVF rather than true cartilage repair alone. Six (50%) of the included studies reported an improvement in VAS scores. The complication rate of the liposuction procedure was not reported in any of the included studies. Previous evidence has shown this to be a safe procedure, with complication rates of only 0.1% in a national survey of 112,756 reported patient procedures [45]. The outcomes from this study, therefore, suggest that injection of ADSCs in the form of SVF may be a safe procedure with a low complication rate.
This systematic review has several inherent limitations and potential biases. The search criterion was limited to MEDLINE, EMBASE and The Cochrane Library Database articles published exclusively in English. Eleven of the surgical studies came from one center: The Center for Stem Cell & Arthritis Research, Department of Orthopaedic Surgery, Yonsei Sarang Hospital, Seoul, Korea. This may suggest that the results could be partially attributed to the surgeons’ skill sets, as there is a lack of variance in the operating surgeons for the procedures compared to normal systematic reviews. There were few studies that included a true control, as the majority compared the results to patients treated with PRP alone, which limits the comparison. There is also evidence that ADSCs have a paracrine effect on OA chondrocytes by producing cytokines, anti-inflammatory mediators, immunoregulatory molecules, and there is a potential placebo effect from the injections, both which may explain at least partially explain the improvement in clinical symptoms [14]. The overall quality of evidence of published studies was variable in this review. The LOE in the included studies was low with 14 (82%) of the studies being LOE III or IV, with no reported randomized clinical trials.
Conclusions
This systematic review demonstrates that the majority of studies in the current literature utilize ADSCs in the form of SVF when evaluating the outcomes in the treatment of OA. While SVF may produce favorable clinical outcomes with minimal risk of side effects on osteoarthritis, the variability in the data and the use of biological adjuvants have confounded the effectiveness of ADSCs.
References
Creamer P, Hochberg MC (1997) Osteoarthritis. Lancet 350:503–509
Kim YS, Choi YJ, Suh DS, Heo DB, Kim YI, Ryu JS, Koh YG (2015) Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med 43:176–185
Erickson GR, Alexopoulos LG, Guilak F (2001) Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech 34:1527–1535
Gupta PK, Das AK, Chullikana A, Majumdar AS (2012) Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther 3:25
Chen YC, Chen CH, Chen PL, Huang IY, Shen YS, Chen CM (2006) Donor site morbidity after harvesting of proximal tibia bone. Head Neck 28:496–500
Wright JG, Swiontkowski MF, Heckman JD (2003) Introducing levels of evidence to the journal. J Bone Joint Surg Am 85:1–3
Hass R, Kasper C, Bohm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12
Nathan S, Das De S, Thambyah A, Fen C, Goh J, Lee EH (2003) Cell-based therapy in the repair of osteochondral defects: a novel use for adipose tissue. Tissue Eng 9:733–744
Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W (2007) Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol 211:682–691
Maredziaki M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM (2016) The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int 2016:2152435
Van Pham P, Bui K, Ngo DQ, Vu NB, Truong NH, Phan NL, Le DM, Duong TD, Nguyen TD, Le VT, Phan NK (2013) Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther 4:91
Soriano RA, Lamblet H, Mohammedi SA, Torfi H (2013) Optimization of Roche Liberase TM research grade in the enzymatic digestion of human adipose tissue for the isolation of stem and regenerative cells. Cell Isolation Application Note No. 3. 2013
Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R (2007) Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther 8:272–284
Mei L, Shen B, Ling P, Liu S, Xue J, Liu F, Shao H, Chen J, Ma A, Liu X (2017) Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS One 18:12
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 73(9):712–716
Hannon CP, Murawski CD, Fansa AM, Smyth NA, Do H, Kennedy JG (2013) Microfracture for osteochondral lesions of the talus: a systematic review of reporting of outcome data. Am J Sports Med 41:689–695
Kim YS, Choi YJ, Koh YG (2015) Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes. Am J Sports Med 43:2293–2301
Kim YS, Choi YJ, Lee SW, Kwon OR, Suh DS, Heo DB, Koh YG (2016) Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthr Cartil 24:237–245
Kim YS, Koh YG (2016) Injection of mesenchymal stem cells as a supplementary strategy of marrow stimulation improves cartilage regeneration after lateral sliding calcaneal osteotomy for varus ankle osteoarthritis: clinical and second-look arthroscopic results. Arthroscopy 32:878–889
Kim YS, Kwon OR, Choi YJ, Suh DS, Heo DB, Koh YG (2015) Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am J Sports Med 43:2738–2746
Koh YG, Choi YJ (2012) Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 19:902–907
Koh YG, Choi YJ, Kwon OR, Kim YS (2014) Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med 42:1628–1637
Koh YG, Kwon OR, Kim YS, Choi YJ (2014) Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy 30:1453–1460
Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, Choi Y (2013) Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 29:748–755
Bui K, Duong TD, Nguyen NT, Nguyen TD, Le VT, Mai VT, Phan LC, Le DM, Ngoc NK, Van Pham P (2014) Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomed Res Ther 1(1):2–8
Fodor PB, Paulseth SG (2016) Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J 36(2):229–236
Nguyen PD, Tran TD, Nguyen HT, Vu HT, Le PT, Phan NL, Vu NB, Phan NK, Van Pham P (2017) Comparative clinical observation of arthroscopic microfracture in the presence and absence of a stromal vascular fraction injection for osteoarthritis. Stem Cells Transl Med 6(1):187–195
Pak J, Chang JJ, Lee JH, Lee SH (2013) Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet Disord 1 14:337
Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE (2015) Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 23:1308–1316
Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH (2016) Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 32:97–109
Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noël D, Canovas F, Cyteval C, Lisignoli G, Schrauth J, Haddad D, Domergue S, Noeth U, Jorgensen C (2016) Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med 5(7):847–856
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Rodbell M, Jones AB (1966) Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem 241:140–142
Krüger JP, Freymannx U, Vetterlein S, Neumann K, Endres M, Kaps C (2013) Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus Med Hemother 40:432–440
Schreml S, Babilas P, Fruth S, Orso E, Schmitz G, Mueller MB, Nerlich M, Pranti L (2009) Harvesting human adipose tissue-derived adult stem cells: resection versus liposuction. Cytotherapy 11:947–957
Khoshbin A, Leroux T, Wasserstein D, Marks P, Theodoropoulos J, Ogilivie-Harris D, Gandhi R, Takhar K, Lum G, Chahal J (2013) The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy 29:2037–2048
Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG (2013) Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy 29:1399–1409
Pak J, Lee JH, Kartolo WA, Lee SH (2016) Cartilage regeneration in human with adipose tissue-derived stem cells: current status in clinical implications. BioMed Res Int 4702674
de Windt TS, Bekkers JE, Creemers LB, Dhert WJ, Saris DB (2009) Patient profiling in cartilage regeneration: prognostic factors determining success of treatment for cartilage defects. Am J Sports Med 37(Suppl 1):58S–62S
Baer PC, Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012:812693
Martin AD, Daniel MZ, Drinkwater DT, Clarys JP (1994) Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord 18:79–83
Iwen KA, Priewe AC, Winnefeld M, Rose C, Siemers F, Rohwedel J, Cakiroglu F, Lehnert H, Schepky A, Klein J, Kramer J (2014) Gluteal and abdominal subcutaneous adipose tissue depots as stroma cell source: gluteal cells display increased adipogenic and osteogenic differentiation potentials. Exp Dermatol 23(6):395–400
Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ, van Milligen FJ (2008) Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res 332(3):415–426
Dragoo JL, Korotkova T, Wasterlain AS, Pouliot MA, Kim HJ, Golish SR (2012) Agre-related change of chondrogenic growth factors in platelet-rich plasma. Op Tech Orthop 22:49–55
Teimourian B, Rogers WB (1989) A national survey of complications associated with suction lipectomy: a comparative study. Plast Reconstr Surg 84(4):628–631
Funding
No funding has been received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This manuscript is a systematic review and does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Hurley, E.T., Yasui, Y., Gianakos, A.L. et al. Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surg Sports Traumatol Arthrosc 26, 3499–3507 (2018). https://doi.org/10.1007/s00167-018-4955-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-4955-x