Abstract
Background
The cornerstone of thrombotic antiphospholipid syndrome (APS) patients’ management is to prevent recurrent thrombosis by long-term anticoagulation.
Purpose of review
The purpose of the review is to summarize available literature on direct oral anticoagulants (DOACs) use in APS patients through a systematic review and to determine factors associated with thrombosis recurrence.
Recent findings
The recent RAPS trial demonstrated that APS patients treated with rivaroxaban had a significant twofold-increased thrombin potential, suggesting a higher thrombotic risk, in comparison with warfarin users. Furthermore, several reports of APS patients treated with DOACs have raised safety issues. Our systematic review identified 122 published APS patients treated with DOACs; among them, 19 experienced a recurrent thrombosis while on DOACs. Of note, triple positivity (positivity of all three laboratory criteria for APS) was associated with a 3.5-fold increased risk for recurrent thrombosis.
Summary
In conclusion, DOACs should be used with caution in APS patients and randomized control trials with clinical primary endpoints assessing clinical efficacy and safety are awaited to establish whether the prescription of DOACs could be a safe alternative to warfarin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antiphospholipid syndrome (APS) is characterized by the association of vascular thrombosis (venous and/or arterial and/or small vessels) and/or pregnancy morbidity, with the presence of persistent antiphospholipid antibodies (aPL) [1].
APS may be primary or associated with autoimmune diseases, especially systemic lupus erythematosus (SLE). Antiphospholipid antibodies tests regroup lupus anticoagulant (LA), IgG or IgM anticardiolipin antibodies (aCL), and IgG or IgM anti-β2-glycoprotein-1 (aβ2GP1), detected in medium to high titers, on a minimum of two consecutive occasions, at least 12 weeks apart in accordance to the international consensus (revised Sapporo criteria) [1]. It has been shown that APS patients carrying all three tests (also known as “triple positivity”) have a higher risk of thrombosis recurrence than other APS patients [2].
To date, the gold standard for the secondary prevention of thrombosis in APS patients is warfarin [3]. However, warfarin use is associated with low quality of life by frequent blood controls and INR instability [4]. Recently, direct oral anticoagulants (DOACs) were developed; however, no randomized controlled clinical trial with clinical primary endpoints has been performed so far in APS [5 6••]. Furthermore, case reports and case series have raised some concerns in terms of thrombosis recurrence while on DOACs. Therefore, the purpose of this systematic review is to assess DOACs efficacy and safety in APS patients and to determine factors associated with thrombosis recurrence based on all available evidence.
Thrombosis Prevention for APS Patients
Current management of thrombosis prevention in APS is based on long-term vitamin K antagonists (VKAs) [1]. With regards to recent guidelines, recommended international normalized ratio (INR) target is 2 to 3 and 3 to 4 in high-risk patients [3]. VKAs monitoring is often challenging and requires frequent controls due the lability of the INR caused by drugs and dietary interactions [7] and variable responsiveness of thromboplastin reagent to LA [8].
Recently, direct oral anticoagulants (DOACs) have been developed and were shown to be non-inferior to VKAs for the secondary prevention of venous thromboembolic event (VTE) and stroke in patients with non-valvular atrial fibrillation (AF). Several DOACs are available as follows: dabigatran etexilate, a direct thrombin inhibitor; rivaroxaban; apixaban; and edoxaban, which are direct factor Xa inhibitors. All four were non-inferior to warfarin for the prevention of thrombosis in non-valvular AF in international, randomized controlled trials [9–12]. Regarding the secondary prevention of recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE), dabigatran, rivaroxaban, apixaban, and recently edoxaban were approved [13–17]. Their use is convenient because of a fixed dose with a predictable effect, fewer drug interactions, and no monitoring. However, in some situations, DOACs are not efficient and safe. For instance, dabigatran etexilate is contraindicated in patients after a heart valve replacement [18]. Likewise, an increased risk of myocardial infarction was demonstrated with dabigatran compared to warfarin users in patients with AF [19]. In APS patients, we do not know whether DOACs are safe and effective in comparison with warfarin and scientific proofs are eagerly awaited. Of course, few APS patients might have been included in phase III trials [20, 21] but this does not validate DOACs use in APS patients. Dedicated randomized controlled trials using clinical endpoints are ongoing, and it will be possible in the future to know whether DOACs use is a safe alternative in APS patients [22••, 23••].
The RAPS Trial: the First Randomized Controlled Trial on DOACs in APS
Recently, Cohen et al. reported the impact of rivaroxaban on thrombin generation in APS patients [6••] (RAPS trial). This controlled, randomized, open-label, non-inferiority trial compared 54 APS patients treated with rivaroxaban versus 56 APS patients treated with warfarin. The primary endpoint was the mean percentage change of endogenous thrombin potential (ETP) determined by thrombin generation before and after the introduction of rivaroxaban. The number of required subject was 58 patients per group to show the non-inferiority of rivaroxaban with less than 20 % increase in mean percentage change ETP compared to warfarin. Patients with only at least one venous thrombosis during no or sub-therapeutic anticoagulant therapy were included, which correspond to low-risk APS patients. Indeed, high-risk APS patients were not included, those with previous arterial manifestations or with recurrent VTE while on therapeutic dose of warfarin. Moreover, a low percentage of patients with triple positivity were included (25 %). Overall, patients treated with rivaroxaban had a significant twofold-increased thrombin potential, suggesting a higher thrombotic risk, in comparison with warfarin users. However, authors stated that no increased thrombotic risk was noticed in the rivaroxaban arm compared to standard-intensity warfarin because no clinical event occurred during the short follow-up (210 days). In conclusion, Cohen et al. demonstrated that rivaroxaban was inferior to warfarin to inhibit thrombin generation in comparison to warfarin in APS patients. Therefore, randomized controlled trials using strong clinical endpoint are mandatory to assess the efficacy and safety or DOACs in APS patients [22••, 23••].
Challenge of LA Testing While on DOACs
It is documented that the presence of direct thrombin inhibitors and factor Xa inhibitors influence diluted prothrombin time, a sensitive screening test for LA, causing false positivity [24]. Regarding rivaroxaban, several studies reported false positivity for LA with dilute Russell’s viper venom time (dRVVT) and LA-partial thromboplastin time (PTT-LA) tests, mainly at peak therapeutic levels [25, 26]. In fact, rivaroxaban and Russell’s viper venom use the same target [27]. These results have been recently confirmed by Sciascia et al. who demonstrated that Taipan/Ecarin time was poorly affected by rivaroxaban; however, false-positive results could be seen with all types of LA reagent; this suggests that a LA test should not be performed in patients receiving rivaroxaban [28]. Furthermore, Merriman et al. reported several false-negative detection of LA with PTT-LA (Triniclot) due to the moderate sensitivity of the reagent to the presence of a LA [27]. Therefore, when possible, LA testing should be done after a wash out period to avoid false-positive LA [29, 30].
One study evaluated a LA detection during treatment by dabigatran etexilate (110 mg BID), and similarly, it has been demonstrated that this treatment affects LA testing both during screening and confirmatory studies suggesting that LA testing should not be performed while on dabigatran etexilate [31].
No data regarding LA detection while on edoxaban or apixaban were published so far.
Safety and Efficacy of DOACs in APS Patients: Systematic Review of the Literature
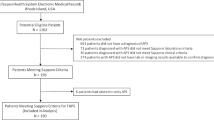
Based on these studies we conducted a systematic literature search of articles published in English or French in PubMed through September 2016, by using the following key words: Antiphospholipid Syndrome, Antibodies, Antiphospholipid, Antibodies Anticardiolipin, Lupus Coagulation Inhibitor, Rivaroxaban, Apixaban, Dabigatran, Edoxaban. Inclusion criteria were APS patients defined according to revised Sapporo criteria, treated by a DOACs with documented thrombosis recurrence or not. Overall, six case reports were published in the literature [32–37] and eight case series [28, 38–44]. An equal number of reports indicated either a good safety and efficacy profile [28, 34, 35, 37–40, 44] or thrombosis recurrences [32, 33, 36, 38, 39, 41–43]. Of note, results from the recent RAPS study were analyzed separately (see section “The RAPS Trial: the First Randomized Controlled Trial on DOACs in APS”). Among 124 cases identified, we excluded two cases with unclear thrombosis recurrence definition. Individual patient data were retrieved from these papers, and authors were contacted if needed (n = 2). Results were summarized using a standardized data form. Variables collected were sample size, design of the study, demographics of patients (mean age, gender), thrombosis history, aPL profile, presence of any underlying autoimmune disease, previous anticoagulation and reason for switching, DOACs use, and duration of follow-up. Then patients were categorized according to thrombosis recurrence to determine associated factors. The association between thrombosis recurrence and variables was tested using non-parametric tests (the Wilcoxon test for quantitative variables and Fisher’s exact test for qualitative variables). Missing data were excluded from analyses. A p ≤ 0.05 was considered significant. SAS 9.1.3 software (SAS Institute Inc., Cary, NC, USA) was used to perform analyses.
Characteristics of APS patients are described in Table 1. Main characteristics of cases reports and cases series are summarized in Table 2. To summarize, among 124 identified patients, 122 were included in the analyses. Two patients were excluded because of unclear definition of thrombosis recurrence. Nineteen had a recurrent thrombosis. Mostly, those with previous arterial or venous manifestation recurred with the same type of thromboses. Indeed, among nine patients with venous thrombosis recurrence, eight had previous venous manifestation of APS (89 %). Likewise, among nine patients with arterial thrombosis recurrence, six had previous arterial thromboses (67 %). Overall aCL positivity tend to be associated with a higher risk of thrombosis recurrence without reaching statistical significance (94 vs. 68 %, p = 0.06) while triple positivity was significantly associated with a 3.5-fold increased risk for thrombosis recurrence (OR = 3.53 (95 % CI 1.14–11.0), p = 0.03) in patients treated with DOACs. Furthermore, a higher number of criteria for definite APS was found in patients with thrombosis recurrence compared to those without (1.63 ± 0.7 vs. 1.23 ± 0.6, p = 0.002). Duration of follow-up was shorter in patients with thrombosis recurrence than those without (5.2 ± 3.2 vs. 14.3 ± 8.5, p < 0.0001). The latter result suggests that thrombosis recurrences occur early after switching for DOACs. Even if previous studies identified history of arterial thromboses as a risk factor for thrombosis recurrence [40, 45], our systematic review did not confirm this finding. Bleeding leading to stop the DOAC was reported in five patients. Considering the large number of patients treated by rivaroxaban (n = 107), we also performed an analysis excluding patients treated with other DOACs. Despite a lower number of patients, triple positivity was still significantly associated with thrombosis recurrence (OR = 3.69 (95 % CI 1.12–12.14), p = 0.05).
According to these results, high-risk APS patients with triple positivity or with several clinical criteria for definite APS developed more frequently a thrombosis recurrence while on DOACs in comparison to warfarin. This result is based on a systematic review of more than 100 patients treated with DOACs. These results are in contrast with the recent RAPS study [6••] in which no clinical event was recorded among 54 patients during a 210-day follow-up. This finding could be explained by baseline characteristics of included patients: No patients with a history of arterial thrombosis were included in the RAPS study while up to 30 % of patients described in the available literature had history of arterial thrombosis. Furthermore, in comparison patients descriptions published so far, a lower proportion of lupus patients as well as triple positivity patients were included in the RAPS study. This leads to a different risk profile among RAPS patients and published case series so far. Furthermore, the short follow-up time (210 days) in the RAPS study may have been insufficient to record any clinical event. Finally, no information was recorded in the RAPS study regarding the mechanism (provoked or unprovoked) of the initial venous thromboembolic event (VTE); we may hypothesize that patients with spontaneous VTE may be at higher risk of recurrence.
Reasons for failure of DOACs in APS are unclear. Calibrated automated thrombin generation measurement is useful to characterize the overall coagulation system. RAPS study demonstrated that thrombin potential was less inhibited with rivaroxaban than warfarin in APS patients. We may hypothesize that DOACs have a focused anticoagulant action on only one factor that may be less efficient for thrombosis prevention in high-risk APS patients than a multi-targeted coagulation factors inhibition as provided by VKA. Future studies assessing mechanisms of thrombosis prevention in APS patients treated with DOACs will be very useful to understand our findings [46•].
Limitations of VKAs and DOACs Maintenance in APS
In APS, a good adherence to anticoagulation treatments is of paramount importance. Long-term VKA can be restrictive for some patients requiring frequent INR control and potentially inducing many drug and dietary interactions. Nevertheless, thanks to its long half-life, the risk of thrombosis recurrence in case of underdosing exists, but is minimized; indeed, INR lability can increase the risk of recurrence. DOACs are less restrictive in everyday life. A bridging therapy with low molecular weight heparin is not necessary due to its rapid onset [43]. Moreover, it requires no diet adjustments and no routine laboratory monitoring because of their predictable pharmacokinetic effects [47]. However, DOACs have a short half-life that could be responsible for a high risk of recurrence in case of missing doses, especially in patients with a high thrombotic risk. Furthermore, in case of poor adherence to VKA, bridging from VKA to DOACs is not a good option because laboratory testing will not be possible in patients with DOACs to ensure that patients are taking their treatments.
Futures Directions
To date, excepting RAPS study, almost all studies assessing DOACs use in APS are case series. Among them, important data are missing, especially aPL profile [28, 42] or underlying autoimmune diseases [39, 41, 42]. Given the publication bias and also the low evidence level of studies published so far, results from this systematic review should be used cautiously. Now it is time to shift to randomized controlled trial to determine the efficacy and safety of DOACs in APS patients using clinical endpoints such as thrombosis or death and a long follow-up. Several trials in thrombotic APS (TRAPS and ASTRO-APS) are ongoing [22••, 23••].
Conclusion
Based on this systematic review of the available evidence, DOACs do not seem to be efficient in preventing thrombosis in high-risk APS patients, especially those with triple positivity and several clinical criteria for definite APS. These findings can be explained by a higher thrombin potential determined by thrombin generation in the recent RAPS study. This must be confirmed by ongoing randomized controlled trials which will focus on clinical endpoints. Consequently, even if DOACs seem to be promising in thrombotic APS patients with INR lability or poor adherence to INR monitoring, data are not in favor bridging VKA to DOACs in high-risk APS patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost JTH. 2006;4(2):295–306.
Pengo V, Ruffatti A, Legnani C, Testa S, Fierro T, Marongiu F, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood. 2011;118(17):4714–8.
Erkan D, Aguiar CL, Andrade D, Cohen H, Cuadrado MJ, Danowski A, et al. 14th international congress on antiphospholipid antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev. 2014;13(6):685–96.
Lancaster TR, Singer DE, Sheehan MA, Oertel LB, Maraventano SW, Hughes RA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston area anticoagulation trial for atrial fibrillation investigators. Arch Intern Med. 1991;151(10):1944–9.
Cohen H, Doré CJ, Clawson S, Hunt BJ, Isenberg D, Khamashta M, et al. Rivaroxaban in antiphospholipid syndrome (RAPS) protocol: a prospective, randomized controlled phase II/III clinical trial of rivaroxaban versus warfarin in patients with thrombotic antiphospholipid syndrome, with or without SLE. Lupus. 2015;24(10):1087–94.
•• Cohen H, Hunt BJ, Efthymiou M, Arachchillage DRJ, Mackie IJ, Clawson S, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3(9):e426–36. This first randomized controlled trial of rivaroxaban vs. warfarin in APS patients showed that thrombin potential determined by thrombin generation was doubled.
Levy RA, Signorelli F. Patient-health care provider relationship: how can it impact on APS (Hughes’ syndrome) adherence to treatment? Lupus. 2014;23(12):1265–8.
Tripodi A, Chantarangkul V, Clerici M, Negri B, Galli M, Mannucci PM. Laboratory control of oral anticoagulant treatment by the INR system in patients with the antiphospholipid syndrome and lupus anticoagulant. Results of a collaborative study involving nine commercial thromboplastins. Br J Haematol. 2001;115(3):672–8.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–52.
Investigators EINSTEIN, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–510.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–15.
EINSTEIN–PE Investigators, Büller HR, Prins MH, Lensin AWA, Decousus H, Jacobson BF, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–97.
Van de Werf F, Brueckmann M, Connolly SJ, Friedman J, Granger CB, Härtter S, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am Heart J. 2012;163(6):931–937.e1.
Hohnloser SH, Oldgren J, Yang S, Wallentin L, Ezekowitz M, Reilly P, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (randomized evaluation of long-term anticoagulation therapy) trial. Circulation. 2012;125(5):669–76.
Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res. 2013;65(11):1869–73.
Clark CA, Laskin CA. Unrealistic estimation of population prevalence of antiphospholipid antibody-related pregnancy loss: comment on the article by Andreoli et al. Arthritis Care Res. 2014;66(6):966.
•• Pengo V, Banzato A, Bison E, Zoppellaro G, Padayattil Jose S, Denas G. Efficacy and safety of rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome: rationale and design of the trial on rivaroxaban in antiphospholipid syndrome (TRAPS) trial. Lupus. 2016;25(3):301–6. This paper describes the protocol of an ongoing clinical trial that will compare the efficacy and safety of rivaroxaban vs. warfarin in high-risk APS patients using clinical endpoints (mainly thrombosis and bleeding).
•• Woller SC, Stevens SM, Kaplan DA, Branch DW, Aston VT, Wilson EL, et al. Apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome: study rationale and design (ASTRO-APS). Clin Appl Thromb Off J Int Acad Clin Appl Thromb. 2016;22(3):239–47. This paper describes the protocol of an ongoing clinical trial that will compare the efficacy and safety of edoxaban vs. warfarin in high-risk APS patients using clinical endpoints (mainly thrombosis and bleeding).
Olah Z, Szarvas M, Bereczky Z, Kerenyi A, Kappelmayer J, Boda Z. Direct thrombin inhibitors and factor xa inhibitors can influence the diluted prothrombin time used as the initial screen for lupus anticoagulant. Arch Pathol Lab Med. 2013;137(7):967–73.
Arachchillage DRJ, Mackie IJ, Efthymiou M, Isenberg DA, Machin SJ, Cohen H. Interactions between rivaroxaban and antiphospholipid antibodies in thrombotic antiphospholipid syndrome. J Thromb Haemost JTH. 2015;13(7):1264–73.
van Os GMA, de Laat B, Kamphuisen PW, Meijers JCM, de Groot PG. Detection of lupus anticoagulant in the presence of rivaroxaban using Taipan snake venom time. J Thromb Haemost JTH. 2011;9(8):1657–9.
Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost. 2011;105(2):385–6.
Sciascia S, Savino S, Breen K, Hunt BJ. Rivaroxaban use in patients with antiphospholipid syndrome and previous venous thromboembolism. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2015;26(4):476–7.
Murer LM, Pirruccello SJ, Koepsell SA. Rivaroxaban therapy, false-positive lupus anticoagulant screening results, and confirmatory assay results. Lab Med. 2016;47(4):275–8.
Góralczyk T, Iwaniec T, Wypasek E, Undas A. False-positive lupus anticoagulant in patients receiving rivaroxaban: 24 h since the last dose are needed to exclude antiphospholipid syndrome. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2015;26(4):473–5.
Martinuzzo ME, Barrera LH, D’adamo MA, Otaso JC, Gimenez MI, Oyhamburu J. Frequent false-positive results of lupus anticoagulant tests in plasmas of patients receiving the new oral anticoagulants and enoxaparin. Int J Lab Hematol. 2014;36(2):144–50.
Joalland F, de Boysson H, Darnige L, Johnson A, Jeanjean C, Cheze S, et al. Seronegative antiphospholipid syndrome, catastrophic syndrome, new anticoagulants: learning from a difficult case report. Rev Médecine Interne Fondée Par Société Natl Francaise Médecine Interne. 2014;35(11):752–6.
Delgado MG, Rodríguez S, García R, Sánchez P, Sáiz A, Calleja S. Antiphospholipid syndrome of late onset: a difficult diagnosis of a recurrent embolic stroke. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2015;24(8):e209–11.
Bachmeyer C, Elalamy I. Rivaroxaban as an effective treatment for recurrent superficial thrombophlebitis related to primary antiphospholipid syndrome. Clin Exp Dermatol. 2014;39(7):840–1.
Sugie M, Iizuka N, Shimizu Y, Ichikawa H. Cerebral venous thromboembolism in antiphospholipid syndrome successfully treated with the combined use of an anti-Xa inhibitor and corticosteroid. Intern Med Tokyo Jpn. 2015;54(23):3051–6.
Rokos J, Heger M, Stöllberger C, Finsterer J, Laufer G, Wiedemann D. Bipolar disorder, ischemic stroke, mitral valve vegetation and recurrent venous thrombosis due to antiphospholipid syndrome despite rivaroxaban. Int J Cardiol. 2016;221:383–4.
Reshetnyak TM, Seredavkina NV, Satybaldyeva MA, Nasonov EL, Reshetnyak VI. Liver transplantation in a patient with primary antiphospholipid syndrome and Budd-Chiari syndrome. World J Hepatol. 2015;7(19):2229–36.
Noel N, Dutasta F, Costedoat-Chalumeau N, Bienvenu B, Mariette X, Geffray L, et al. Safety and efficacy of oral direct inhibitors of thrombin and factor Xa in antiphospholipid syndrome. Autoimmun Rev. 2015;14(8):680–5.
Son M, Wypasek E, Celinska-Lowenhoff M, Undas A. The use of rivaroxaban in patients with antiphospholipid syndrome: a series of 12 cases. Thromb Res. 2015;135(5):1035–6.
Betancur JF, Bonilla-Abadía F, Hormaza AA, Jaramillo FJ, Cañas CA, Tobón GJ. Direct oral anticoagulants in antiphospholipid syndrome: a real life case series. Lupus. 2016;25(6):658–62.
Signorelli F, Nogueira F, Domingues V, Mariz HA, Levy RA. Thrombotic events in patients with antiphospholipid syndrome treated with rivaroxaban: a series of eight cases. Clin Rheumatol. 2016;35(3):801–5.
Win K, Rodgers GM. New oral anticoagulants may not be effective to prevent venous thromboembolism in patients with antiphospholipid syndrome. Am J Hematol. 2014;89(10):1017.
Schaefer JK, McBane RD, Black DF, Williams LN, Moder KG, Wysokinski WE. Failure of dabigatran and rivaroxaban to prevent thromboembolism in antiphospholipid syndrome: a case series of three patients. Thromb Haemost. 2014;112(5):947–50.
Haładyj E, Olesińska M. Rivaroxaban—a safe therapeutic option in patients with antiphospholipid syndrome? Our experience in 23 cases. Reumatologia. 2016;54(3):146–9.
Sciascia S, Lopez-Pedrera C, Cecchi I, Pecoraro C, Roccatello D, Cuadrado MJ. Non-vitamin K antagonist oral anticoagulants and antiphospholipid syndrome. Rheumatology (Oxford). 2016;55(10):1726–35.
• Arachchillage DRJ, Mackie IJ, Efthymiou M, Chitolie A, Hunt BJ, Isenberg DA, et al. Rivaroxaban limits complement activation compared to warfarin in antiphospholipid syndrome patients with venous thromboembolism. J Thromb Haemost JTH. 2016. doi:10.1111/jth.13475. An interesting pilot study showing that rivaroxaban may limit complement activation in APS.
Arachchillage DJ, Cohen H. Use of new oral anticoagulants in antiphospholipid syndrome. Curr Rheumatol Rep. 2013;15(6):331.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Antiphospholipid Syndrome
Rights and permissions
About this article
Cite this article
Dufrost, V., Risse, J., Zuily, S. et al. Direct Oral Anticoagulants Use in Antiphospholipid Syndrome: Are These Drugs an Effective and Safe Alternative to Warfarin? A Systematic Review of the Literature. Curr Rheumatol Rep 18, 74 (2016). https://doi.org/10.1007/s11926-016-0623-7
Published:
DOI: https://doi.org/10.1007/s11926-016-0623-7