Abstract
Warfarin is recognized as the standard treatment for thrombotic antiphospholipid syndrome (APS); however, direct oral anticoagulants (DOACs) represent appealing therapeutic alternatives given their lack of monitoring and limited drug interactions. A few randomized controlled trials comparing rivaroxaban with warfarin showed an increased risk of recurrent thromboembolism, specifically arterial thrombosis, in patients with high risk forms of APS such as those that are triple antibody positive. We conducted a single-center, retrospective cohort study of all patients within our health system from 2015 to 2020 with a diagnosis of APS (single or double antibody positive) and history of venous thromboembolism. We sought to compare the proportion of patients with a recurrent thrombosis when prescribed a DOAC versus warfarin. Among 96 patients included, 57 were prescribed warfarin and 39 were prescribed a DOAC (90% rivaroxaban). The proportion of patients with a recurrent thromboembolism was almost three times higher in the DOAC group (15.4%) compared to the warfarin group (5.3%), although this was not statistically significant (p = 0.15). Major bleeding was not different between groups. Our findings suggest that rivaroxaban may pose an increased risk for recurrent thromboembolism in low risk APS patients that are single or double-antibody positive compared to warfarin. Results of our study should be cautiously applied to DOACs besides rivaroxaban given their small representation in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Guidelines recommend warfarin as standard treatment for thrombotic APS whereas DOACs may be used for certain low risk APS populations such as those without a history of arterial thrombosis and those without a triple antibody positive profile.
-
This retrospective study compared the proportion of recurrent thromboembolic events among patients with single or double antibody positive APS and a history of only venous thromboembolism that were prescribed a DOAC versus warfarin.
-
Patients in the DOAC group had a non-significant near tripling of risk for a recurrent thrombosis compared to warfarin while bleeding risk was similar between groups.
-
Opportunities for future studies include prospective assessments focusing on lower risk APS populations and studies evaluating the use of DOACs besides rivaroxaban such as dabigatran and apixaban.
Introduction
Antiphospholipid syndrome (APS) is an acquired autoimmune disease characterized by vascular thrombosis and/or pregnancy loss/morbidity with persistently positive antiphospholipid antibodies (aPL; lupus anticoagulant [LA], IgG/IgM anticardiolipin [aCL]) and anti-beta-2-glycoprotein I [aβ2GPI]). Triple aPL-positive represents the presence of all three antibodies (LA, aCL and aβ2GPI); whereas single or double antibody positive APS describes those patients that are positive for any one or two out of the three antibodies [1]. APS is the most common acquired thrombophilia and is associated with both venous and arterial thromboses [2].
Treatment with warfarin is recognized as the standard treatment for thrombotic APS [3,4,5,6]. The 2019 EULAR guidelines recommend warfarin (INR goal 2–3) as preferred treatment for patients with a history of vascular thrombosis and recommend against direct oral anticoagulants (DOACs) for those with arterial thrombosis [6]. The 2020 ISTH guidelines have similar guidance and recommend warfarin for high risk APS patients such as those with triple positive aPL, arterial thrombosis, small vessel thrombosis, or heart valve disease [7]. These guidelines suggest that DOACs can be used in patients with lower risk forms of APS which offers an appealing therapeutic alternative given that DOACs do not require therapeutic monitoring and have fewer dietary and drug interactions.
Three randomized controlled trials (RCTs) have been conducted to date evaluating the safety and efficacy of rivaroxaban compared to warfarin in patients with APS. In the Rivaroxaban in APS (RAPS) trial, rivaroxaban 20 mg daily was compared to standard intensity warfarin (INR range 2.0–3.0) and failed to meet prespecified criteria for noninferiority; however, there were no thrombotic events during the 7-month follow-up [8]. The Trial on Rivaroxaban in APS (TRAPS) compared rivaroxaban 20 mg daily with standard intensity warfarin in triple positive APS patients, but was terminated early due to an excess of thromboembolic events (primarily arterial thromboses) in the rivaroxaban arm [9]. Lastly, a randomized trial comparing rivaroxaban 20 mg daily with standard intensity warfarin in patients with a history of arterial or venous thromboses and at least 1 positive aPL found that rivaroxaban failed to meet the prespecified noninferiority criteria. Patients in the rivaroxaban arm had a non-significant near doubling of risk for recurrent thrombosis [10].
In the context of this prior evidence, our retrospective cohort study aimed to compare patients treated with a DOAC versus standard intensity warfarin, focusing on a lower risk APS population of patients that are single or double antibody positive with a history of only venous thromboembolism.
Methods
Study design and patients
We conducted a single-center retrospective cohort analysis of adult patients at the University of Colorado Health System with a definitive diagnosis of APS and treated with a DOAC (apixaban, dabigatran, or rivaroxaban) or warfarin between January 2015 and January 2020. Epic’s Slicer Dicer® application was used to identify patients with the following: a documented diagnosis of APS using ICD-10 codes, current use of anticoagulation, and laboratory criteria for single or double antibody positive APS as defined by the Revised Sapporo Criteria [11]. Medical records were then manually reviewed to determine thrombosis history, obtain additional pertinent past medical history (PMH), and to ensure that patients met the laboratory criteria for APS diagnosis (at least one or two aPLs persistently positive 12 weeks apart). Patients were excluded if any of the following criteria were present: active cancer requiring chemotherapy any time after the index date (defined as the date of the first positive aPL), triple positive APS, warfarin INR goal range other than 2.0–3.0, or history of arterial thrombosis prior to the index date. Evidence of testing for all three APS antibodies was required to ensure that higher risk, triple positive patients were excluded.
Patients prescribed warfarin were compared to those prescribed a DOAC after the initial thromboembolic event that prompted a work-up for APS. Patients who were treated with warfarin (INR goal range of 2.0–3.0) were allocated to the warfarin group. Patients that were treated with a DOAC at therapeutic doses were allocated to the DOAC group. The Colorado Multiple Institutional Review Board approved this study as exempt.
Outcomes
The primary outcome was the proportion of patients with a documented venous or arterial thromboembolic event while on anticoagulation after the index date. If patients in the warfarin group had an event that met the primary endpoint but had a documented subtherapeutic INR (< 1.8) at the time of the event, it was not included in the primary outcome. The secondary outcome was the proportion of patients with a documented major bleeding event (as defined by ISTH criteria [12]) while on anticoagulation after the index date.
Statistical analysis
For parametric continuous data, mean and standard deviation was reported and for non-parametric data, median and interquartile range (IQR) was reported. Categorical data was reported as a number and percentage of participants. Continuous variables were compared using the t-test for parametric data and the Mann–Whitney U test for non-parametric data. Categorical variables were compared using the chi square test or the Fisher’s exact test if the frequency of the outcome was < 5. For all analyses, a p-value of < 0.05 was considered significant for two-tailed tests. GraphPad Prism 9 software (version 9.1.0) was used to run statistical analyses.
Results
Patient characteristics
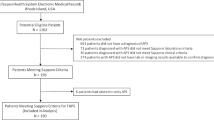
A total of 185 patients with APS and one or two persistently positive aPLs were initially identified. Of these, 96 patients met study criteria and were included in the final analysis (Fig. 1). History of arterial thrombosis prior to the index date was the most common exclusion criteria identified. A total of 57 patients were assigned to the warfarin group and 39 patients were assigned to the DOAC group. Table 1 summarizes the baseline characteristics of the patients included in our study. There were significantly more males in the DOAC group and significantly more patients with a PMH significant for diabetes mellitus in the warfarin group.
Outcomes
Patients in the DOAC group had a numerically higher rate of recurrent thromboembolic events compared with the warfarin group, although this was not statistically significant (15.4% vs 5.3%, p = 0.15). Of the 3 patients in the warfarin group that had a thromboembolism, one of these events was arterial whereas 3 out of the 6 patients in the DOAC group had an arterial event (Fig. 2).
Of the 4 patients with an arterial thromboembolism, only 1 patient in the DOAC group was prescribed an antiplatelet agent prior to their event. Notably, only one other patient meeting the primary endpoint, also in the DOAC group, was prescribed aspirin and experienced a VTE. Most patients (35/39) in the DOAC group were prescribed rivaroxaban (3 dabigatran, 1 apixaban). All patients in the DOAC group meeting the primary endpoint were prescribed rivaroxaban. Approximately 8% of the patients in our study had a BMI ≥ 40 kg/m2. Of those only one patient in the warfarin group with a BMI ≥ 40 kg/m2 had a recurrent thrombosis. None of the patients with a BMI ≥ 40 kg/m2 in the DOAC group had a recurrent thrombosis. Table 2 provides a summary of the characteristics for the patients experiencing a recurrent thromboembolism while on anticoagulation.
Major bleeding events were determined using ISTH criteria. The rates of major bleeding were 7% in the warfarin group and 7.7% in the DOAC group (p = 0.99) (see Fig. 3). No patients died because of bleeding due to anticoagulation. Two patients in each group were prescribed concomitant antiplatelet therapy at the time of the bleeding event.
Several patients in our study switched their anticoagulant to an alternate agent. However, all patients meeting the primary outcome were found to have a thrombosis while on their initial treatment. One patient in the warfarin group that had a recurrent thromboembolism was switched to a DOAC (apixaban) 3 years after their recurrent event due to undergoing a liver transplant. Of the 6 patients in the DOAC group that experienced a recurrent thrombosis, 1 patient continued the DOAC, 2 switched to warfarin with a standard INR goal of 2.0–3.0, and 3 patients switched to warfarin with a high INR goal of 2.5–3.5.
Discussion
Our retrospective study is the first to evaluate DOAC use in lower risk APS patients that are single or double antibody positive without a history of arterial thromboembolism. The risk of having a recurrent thromboembolic event while on a DOAC was almost 3 times higher compared to those patients treated with warfarin. Although this difference was not statistically significant, this suggests that patients treated with DOACs (specifically rivaroxaban) are at a higher risk for recurrent thromboembolic events, regardless of their aPL profile, compared to those treated with warfarin.
All recurrent thromboembolic events within the first 90 days occurred in patients treated with a DOAC and accounted for 50% of events in the DOAC group, the time frame where thromboembolic recurrence is at its highest. The TRAPS trial was terminated early due to an excess of thromboembolic events in the rivaroxaban arm. Of the 7 thromboembolic events in the rivaroxaban arm that occurred in their “as treated” population, 2 of them occurred within the first 90 days from randomization [9]. Ordi-Ros, et al. published another randomized controlled trial in 2019 that compared rivaroxaban to warfarin in thrombotic APS patients with at least 1 positive aPL and found that rivaroxaban failed to meet noninferiority criteria. More than half (60.5%) of the trial’s population included patients with triple-positive APS. While warfarin was not statistically superior to rivaroxaban, the study did find that those treated with rivaroxaban were almost twice as likely to experience a recurrent thrombotic event compared to those treated with warfarin (11.6% vs 6.3%; p = 0.21) [10]. Time to first recurrent thrombotic event was not reported. Our study is unique in that we excluded patients with triple-positive APS, but adds to the evidence that rivaroxaban, especially when used in the first 3 months after a thrombotic event, may be associated with higher rates of recurrent thrombosis regardless of aPL status.
In previous phase 3 trials that evaluated DOACs for VTE treatment, the risk of major bleeding was found to be significantly lower when both rivaroxaban and apixaban were compared with warfarin, providing yet another benefit to prescribing these medications [13,14,15]. In contrast, our study found the risk of major bleeding to be similar between DOAC and warfarin groups. This is comparable to findings in the TRAPS trial that showed 7% in the rivaroxaban group experienced a major bleeding event compared with 3% in the warfarin group (p = 0.3) [9]. In the more recent randomized trial published by Ordi-Ros and colleagues, they also found a similar rate in major bleeding events between groups (rivaroxaban 6.3% vs warfarin 7.4%; p = 0.77) [10].
Most patients in our DOAC group were treated with rivaroxaban. We postulate that rivaroxaban was more commonly prescribed because it is dosed once daily (compared with apixaban twice daily) and it is the preferred DOAC on several insurance formularies [16, 17]. No published randomized controlled trials are available comparing other DOACs with warfarin. Results from the ASTRO-APS trial comparing apixaban to warfarin were not yet available at the time our study was completed [18]. Surrate et al. published a case report describing the successful use of dabigatran for catastrophic antiphospholipid syndrome in a 19 year old patient nonadherent with warfarin. After implementing dabigatran therapy, the patient remained thrombosis free for several years [19]. Noel et al. published a multicenter observational study that followed 26 APS patients of which 11 were treated with dabigatran and 13 treated with rivaroxaban. Only one instance of recurrent thromboembolism occurred with rivaroxaban and none with dabigatran [20]. Dabigatran offers an alternative mechanism of action as it is a direct thrombin inhibitor compared to the other DOACs that inhibit factor Xa which may or may not provide differing results in APS patients [16, 17, 21]. Additional randomized controlled trials are needed to further assess whether DOACs other than rivaroxaban can be used in this patient population.
There are several limitations to our study. There is inherent bias due to its retrospective nature which introduces confounding variables. Any primary or secondary outcomes that occurred outside of our health system could not be evaluated or accounted for in the analysis. Reasonable efforts were made through manual chart review to ensure that patients with a history of arterial thrombosis were excluded. However, there may be some patients included with a history of undocumented or unknown subclinical ischemic strokes, although there is no reason to believe that these patients would not be balanced between treatment groups. Because therapeutic monitoring is not typically done with DOACs and fill history was not obtained, adherence to therapy cannot be determined. Additionally, time in therapeutic range for warfarin was not evaluated. Over the counter medication use with NSAIDs or aspirin could have impacted either the primary or secondary outcomes for our study, but if these medications were not on the patient’s medication list, then they were not accounted for in the analysis.
Our findings suggest that rivaroxaban may pose an increased risk for recurrent thromboembolism in low risk APS patients that are single or double-antibody positive compared to warfarin. Results of our study should be cautiously applied to DOACs besides rivaroxaban given their small representation in this study. Prospective and randomized trials focusing on low risk APS patients are needed to further elucidate the role of DOACs in this population.
Data Availability
Available on request of the editor.
Code availability
Not applicable.
References
Miyakis S, Lockshin MD, Atsumi T et al (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4(2):295–306. https://doi.org/10.1111/j.1538-7836.2006.01753.x
Chaturvedi S, McCrae KR (2017) Diagnosis and management of the antiphospholipid syndrome. Blood Rev 31(6):406–417. https://doi.org/10.1016/j.blre.2017.07.006
Erkan D, Aguiar CL, Andrade D et al (2014) 14th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends. Autoimmun Rev 13(6):685–696. https://doi.org/10.1016/J.AUTREV.2014.01.053
Andrade D, Cervera R, Cohen H et al (2017) 15th International Congress on antiphospholipid antibodies task force on antiphospholipid syndrome treatment trends report. In: Erkan D, Lockshin MD (eds) Antiphospholipid Syndrome. Springer International Publishing, Cham, pp 317–338
Keeling D, Baglin T, Tait C et al (2011) Guidelines on oral anticoagulation with warfarin - fourth edition. Br J Haematol 154(3):311–324. https://doi.org/10.1111/j.1365-2141.2011.08753.x
Tektonidou MG, Andreoli L, Limper M et al (2019) EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 78(10):1296–1304. https://doi.org/10.1136/annrheumdis-2019-215213
Zuily S, Cohen H, Isenberg D et al (2020) Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: Guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 18(9):2126–2137. https://doi.org/10.1111/jth.14935
Cohen H, Hunt BJ, Efthymiou M et al (2016) Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 3(9):e426–e436. https://doi.org/10.1016/S2352-3026(16)30079-5
Pengo V, Denas G, Zoppellaro G et al (2018) Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 132(13):1365–1371. https://doi.org/10.1182/blood-2018-04-848333
Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M et al (2019) Rivaroxaban versus Vitamin K antagonist in antiphospholipid syndrome a randomized noninferiority trial. Ann Intern Med 171(10):685–694. https://doi.org/10.7326/M19-0291
Devreese KMJ, Ortel TL, Pengo V, Laat B (2018) Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J Thromb Haemost 16(4):809–813. https://doi.org/10.1111/jth.13976
Schulman S, Kearon C (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4):692–694. https://doi.org/10.1111/J.1538-7836.2005.01204.X
Agnelli G, Buller HR, Cohen A et al (2013) Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 369(9):799–808. https://doi.org/10.1056/NEJMoa1302507
Bauersachs R, Berkowitz SD, Brenner B, Buller HR (2010) Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 363(26):2499–2510. https://doi.org/10.1056/NEJMoa1007903
Buller HR, Prins MH, Lensing AWA, Decousus H (2012) Oral Rivaroxaban for the Treatment of Symptomatic Pulmonary Embolism. N Engl J Med 366(14):1287–1297. https://doi.org/10.1056/NEJMoa1113572
Xarelto (rivaroxaban) [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals Inc; March 2020.
Eliquis (apixaban) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; April 2021.
Woller SC, Stevens SM, Kaplan DA et al (2016) Apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome: study rationale and design (ASTRO-APS). Clin Appl Thromb 22(3):239–247. https://doi.org/10.1177/1076029615615960
Sarrate E, Olive A, Morales-Indiano C et al (2019) Dabigatran for catastrophic antiphospholipid syndrome. Blood Coagul Fibrinolysis 30(7):364–365. https://doi.org/10.1097/MBC.0000000000000848
Noel N, Dutasta F, Costedoat-Chalumeau N, Bienvenu B (2015) Safety and efficacy of oral direct inhibitors of thrombin and factor Xa in antiphospholipid syndrome. Autoimmun Rev 14(8):680–685. https://doi.org/10.1016/j.autrev.2015.03.007
Pradaxa (dabigatran) [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; July 2020.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of the study proposal. BW collected all data and wrote the initial manuscript draft. All authors revised the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Williams, B., Saseen, J.J., Trujillo, T. et al. Direct oral anticoagulants versus warfarin in patients with single or double antibody-positive antiphospholipid syndrome. J Thromb Thrombolysis 54, 67–73 (2022). https://doi.org/10.1007/s11239-021-02587-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02587-0