Abstract
The appearance of biologic agents for the treatment of diverse autoimmune diseases in particular rheumatoid arthritis at the end of the 1990s changed the treatment of these patients. With the introduction of new agents in the treatment of rheumatic diseases, we started to notice the presence of new and sometimes unexpected adverse events. It is well recognized that infections are the main concern with these types of treatments; however, the occurrence of autoimmune abnormalities is also seen and its gaining perhaps more attention as the use of these agents is increasing. The first clinical trials of anti-tumor necrosis factor-α (anti-TNFα) inhibitors showed an increase of antinuclear and anti-double-stranded deoxyribonucleic acid (dsDNA) antibodies in patients treated with these agents. In this paper, we review the frequency of these autoantibodies in patients treated with biologic agents, particularly anti-TNF-α inhibitors, and its correlation with autoimmune processes as well as the clinical relevance of such findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the mid 1980s, a revolution of molecular biology and genetics, together with the intimate relationship between immunology and modern rheumatology, offered enormous promise, due to the advances in the knowledge of mechanisms underlying the pathogenesis of rheumatic diseases and also to the development of more specific targeted therapies for these diseases [1].
In the following years, there were reports on the experimental use of a recombinant cytokine, interferon-Ƴ (IFN-Ƴ), in the treatment of rheumatoid arthritis (RA). These and other similar studies marked a new era in the treatment of RA, and there was also a growing array of recombinant human gene products and a new knowledge on their biological properties and possible role in disease pathogenesis [2, 3].
In a few years, there were a good number of well-characterized recombinant human molecules, like cytokines, cytokines receptors and inhibitors, as well as humanized monoclonal antibodies, directed against specific target molecules of potential pathogenic significance; one of these molecules was directed against the tumor necrosis factor-α (TNF-α) and showed an important clinical efficacy in RA, implicating TNF as a critical cytokine in disease pathogenesis [4, 5].
So far, five inhibitors of TNF-α are approved for the treatment of a variety of inflammatory diseases by the US Food and Drug Administration (FDA) [6]. These agents target specific components of the immune response that are dysregulated and are thought to be central of the disease process; these medications are as follows:
- Etanercept:
-
Etanercept consists of two p75 TNF-α receptors coupled to the constant region of human immunoglobulin G1 (IgG1). Thus, etanercept is a fusion protein.
- Infliximab:
-
Infliximab is comprised of the human constant region of IgG1 coupled to the variable regions of mouse anti-TNF-α. Thus, infliximab is a chimeric (mouse/human) anti-TNF-α antibody.
- Adalimumab:
-
Adalimumab is a human monoclonal antibody comprised of the human constant region of IgG1 attached to human variable regions. Thus, adalimumab is a fully human monoclonal anti-TNF-α antibody.
- Certolizumab pegol:
-
Certolizumab pegol is an antigen-binding fragment (Fab’) of a humanized monoclonal antibody coupled to polyethylene glycol.
- Golimumab:
-
Golimumab is a human IgG1 kappa monoclonal antibody specific for human TNF-α which neutralizes TNF-α activity. Golimumab binds to both the soluble and transmembrane bioactive forms of human TNF-α.
With the use of these biologic agents, it has become evident the occurrence of several adverse events; one of these is the well-recognized appearance of certain infections, particularly tuberculosis. Also, there are reported cases of demyelinating diseases and progressive multifocal leukoencephalopathy in a small number of patients, and the appearance of antinuclear antibodies (ANAs) and clinical autoimmune complications.
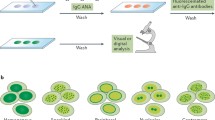
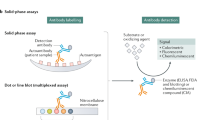
The detection of ANAs has become in the last 35 years one of the most important tests as an aid in the diagnosis of systemic lupus erythematosus (SLE) and other systemic or autoimmune rheumatic diseases. The method of choice for the detection of these antibodies is the indirect immunofluorescence test using HEp-2 cells as a substrate for the detection of the different antinuclear, anti-nucleolar, and anti-cytoplasmic antibodies [7••].
The fluorescein-conjugated secondary antibodies bind to human antibodies, which have reacted with antigens present in the HEp-2 cell substrate. After washing to remove unbound fluoresceinated antibodies, slides are examined using an epi-fluorescent microscope. If fluorescence is detected at one or more screening dilutions (often 1:160 and 1:320), the serum is serially diluted and retested. An endpoint is reached when fewer than half of the cells on the slide show detectable fluorescence. The ANA titer is reported as the dilution prior to this endpoint [8].
In this paper, we will review the occurrence of ANAs in patients treated with anti-TNF-α inhibitors and the importance of ANA testing in this group of individuals as well as one of the most common of these autoimmune complications, that is, “lupus-like syndrome” (LLS).
Pathogenesis
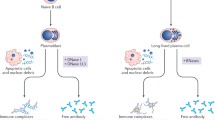
The main concern of the anti-TNF biologic agents is related to infections due to suppression of several immunologic pathways that also play a protective role in the individual. Multiple studies have shown that these agents are safe and in the majority of cases well tolerated; however, since the original studies of infliximab, it was observed that some individuals developed the presence of antinuclear and anti-dsDNA antibodies. Such antibodies have been reported in response to all TNF-α inhibitors but may be more common with infliximab. This might be due to the fact that infliximab may also neutralize the biologic activity of TNF-α by binding the soluble forms and thus preventing its interaction with its p55 and p75 cell receptors. Infliximab also binds the transmembrane form of TNF-α and could induce antibody or complement-dependent cytotoxicity in the cells expressing it [9, 10].
As we know, LLS is an autoimmune disease characterized by the appearance of at least one serological marker and one non-serological marker of systemic lupus erythematosus (SLE) in patients previously healthy. Initially, the syndrome was commonly associated with the use of drugs such as procainamide and hydralazine, although other causes exist, for example, in patients with a paraneoplastic syndrome.
After the introduction of anti-TNF-α therapy in RA, spondyloarthritis, and other autoimmune diseases, the occurrence of this syndrome has been described with the names of LLS and also as “drug-induced lupus erythematosus” (DILE) or as “lupus induced by biologic agents” (LIBAs). However, classification criteria have not been well defined. The mechanism by which this type of therapy induces this syndrome is not yet well understood.
The autoantibody production in these patients is not yet well understood. One hypothesis is related to the fact that the binding of anti-TNF drugs to the cell surface containing TNF induces apoptosis of cells, causing the release of nucleosomal autoantigens and inductions of anti-dsDNA antibodies [11–13]. Another hypothesis is that suppression of T-h1 cells by anti-TNF-α therapy will generate an exuberant T-h2 response that leads to overproduction of autoantibodies [14, 15]. Finally, another hypothesis suggests that in patients using immunosuppressive therapy as anti-TNF-α associated with disease-modifying drugs (DMARDs), they are more susceptible to bacterial infections, leading to polyclonal B cell activation and therefore the production of autoantibodies [16–19].
So, the induction of ANA and anti-dsDNA antibodies during treatment with infliximab is a well-known phenomenon that has already been observed in earlier clinical trials [20–22]. The induction of these autoantibodies is independent of the infliximab dose and is not modified by concomitant treatment with methotrexate (MTX), leflunomide, and corticosteroid. In the other hand, the production of ANAs is not associated with the clinical response to infliximab and even when the development of anti-dsDNA antibodies is observed, the onset of LLS is extremely rare [23–25].
In recent years, there have been reports on the development of ANAs and anti-dsDNA antibodies in patients treated with anti-TNF inhibitors, and they may act as markers of forthcoming treatment failure in some of these patients. Also, it has been demonstrated in patients with RA that the presence of ANAs is a predictive factor of infusion reactions during treatment with infliximab [26••].
In Table 1, we can see the results of nine of the most important studies related to the range of ANAs and anti-dsDNA found in patients treated with anti-TNF-α inhibitors. These studies are from 2003 to 2014, and the majority (eight of them) refers to the use of infliximab, three used etanercept, and three adalimumab. Also, most of the studies were in patients with RA. The development of ANAs ranged from 21 to 82 % and of anti-ds-DNA from 2 to 66 %. The mean time to positivity was 47 weeks. Besides the presence of ANAs and anti-dsDNA, it has been observed that patients treated with infliximab and MTX show a low incidence of induced anticardiolipin antibodies [34].
Autoimmune Diseases
In relation to the autoimmune complications in patients treated with anti-TNF-α agents, more than 140 cases of LIBAs have been reported. Lupus-like mucocutaneous features are frequent and include malar rash, oral ulcers, discoid lupus, or photosensitivity; a recent study described five cases of chilblain lupus induced after anti-TNF-α therapy, a specific cutaneous form of lupus consisting of acral, ischemic-like lesions. Involvement of vital organs (kidneys or central nervous system (CNS)) is infrequent (less than 10 % of reported cases) [35].
Several lines of evidence support the concept that a small minority of patients treated with anti-TNF-α inhibitors develops autoimmune conditions as a result of this treatment [26••]. The majority of reported patients with LLS have rheumatoid arthritis (RA) (72 %). The prevalence for induced SLE is small in patients with inflammatory bowel disease (IBD) (1 %) and 1.75 % in patients with spondyloarthropathies (SpA). Statistical analysis found that SLE was more frequent in older patients and those with baseline-raised anti-dsDNA, but not in those with ANAs. Less than 40 % of reported cases of LLS fulfilled the classification criteria for SLE. Some patients treated with anti-TNF-α inhibitors may develop a systemic drug-induced syndrome (asthenia, malaise, fever, cutaneous rashes, arthralgia, and/or myalgia); this clinical presentation, together with the frequent induction of ANAs/anti-dsDNA by anti-TNF-α agents, may result in a lupus-like presentation of a systemic reaction against the drug rather than a true drug-induced SLE [36].
In Table 2, we can see the main lines of evidence supporting the development of autoimmune conditions in patients treated with anti-TNF-α inhibitors [37].
In a publication analyzing the cases of autoimmune diseases induced by anti-TNF-α agents through December 2006, 113 cases of vasculitis, 92 cases of lupus, and 24 cases of interstitial lung disease were described [37]. In patients with vasculitis, the cutaneous involvement was more frequent (87 %), and the lesions observed included as follows: palpable purpura, erythematous papules, ulcers, and nodules. The visceral involvement and the peripheral nerves and kidneys were affected in a low percent of cases (16 and 13 %, respectively).
In the majority of the cases, vasculitis was confirmed by histological study (73 %), and leukocytoclastic vasculitis was reported in 63 % of cases, necrotizing vasculitis in 17 %, and lymphocytic vasculitis in 6 %. In 92 % of the patients, resolution of the vasculitis occurred after discontinuation of the treatment with the anti-TNF-α.
From the 92 cases of lupus described, the criteria for the diagnosis of SLE were available in 72 patients [38]. Only 37 (51 %) fulfilled four or more criteria, 79 % were ANA positive, 72 % had anti-dsDNA antibodies, 67 % had cutaneous manifestations, and 31 % had arthritis. Serositis was less common than rash and arthritis, and renal and neurological symptoms were rare. In the other hand, the frequency of antihistone antibodies, a hallmark of drug-induced lupus, ranged from 17 to 57 % [39, 40]. Also, in a French registry, the incidence of drug-induced lupus due to anti-TNF-α agents was very rare (approx. 0.2 %) in patients treated with infliximab or etanercept [39].
In the majority of the patients with SLE (71 of the 72), the manifestations of the disease resolved after the discontinuation of the anti-TNF-α. The resolution of SLE has been described in other series; however, some patients received treatment with steroids for the symptoms [40]. It is of interest to note that using methotrexate, which is thought to reduce the frequency of autoantibodies when using anti-TNF-α, did not appear to protect against the development of anti-TNF-induced lupus.
Interstitial lung disease (ILD) has also been reported to occur in association with treatment with anti-TNF-α [37]. In some cases, the lung disease has been aggressive. However, in the majority of the cases, the patients had RA that places them as risk for ILD, so it is difficult to exclude this situation as a factor in these reports.
Finally, uveitis has also been described in association with the use of anti-TNF-α treatment. In one study that included spontaneous reports of uveitis during a period of 8 years (1998–2006), there were 43 cases in patients receiving anti-TNF-α therapy [41]. When the authors excluded patients with diseases associated with a high risk of uveitis, like ankylosing spondylitis, Crohn’s disease, or juvenile idiopathic arthritis, they found 26 cases: 20 taking etanercept, 4 infliximab, and 2 adalimumab. They conclude that if a patient with etanercept treatment develops uveitis, a switch to a different anti-TNF-α agent would be an option to consider.
Conclusions
The treatment of various inflammatory rheumatic diseases with biologic agents has changed the prescription regimen of these disorders especially in their early phases. However, we have also seen the appearance of new adverse events as may occur with the use of novel medications. The occurrence of autoimmune disorders associated with the presence of antinuclear and anti-dsDNA antibodies has been recognized with more frequency, and it has been more common with the use of infliximab, followed by etanercept and adalimumab. Clinically, the most common autoimmune disorders induced by these anti-TNF-α inhibitors have been cases of vasculitis and lupus-like syndromes, and the majority of the cases resolve following discontinuation of the biologic agent. We can conclude that ANA testing in patients receiving this type of treatments is very helpful and recommendable, since it will allow us to increase our knowledge in the association of these autoimmune abnormalities.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Koopman WJ. Dawn of the era of biologics in the treatment of the rheumatic diseases. Arthritis Rheum. 2008;58:S75–8.
Pernice W, Schuchmann L, Dippell J, Suschke J, Vogel P, Truckenbrodt H, et al. Therapy for systemic juvenile rheumatoid arthritis with gamma-interferon: a pilot study of nine patients. Arthritis Rheum. 1989;32:643–6.
Cannon GW, Pincus SH, Emkey RD, Denes A, Cohen SA, Wolfe F, et al. Double-blind trial of recombinant gamma-interferon versus placebo in the treatment of rheumatoid arthritis. Arthritis Rheum. 1989;32:964–73.
Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–12.
Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440.
Tutuncu Z, Kavanaugh A. Anticytokine therapies. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O’Dell JR, editors. Kelley’s textbook of rheumatology. 9th ed. Philadelphia: Elsevier; 2013. p. 957–77.
Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17–23. Important study in which two groups of experts formulated 25 recommendations intended to clarify and standardize clinical and technical aspects pertaining to the determination of ANAs and related antigen-specific antibodies.
Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601–11.
Lügering A, Schmidt N, Lügering M, et al. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology. 2001;121:1145–57.
Bell DA, Morrison B. The spontaneous apoptotic cell death of normal human lymphocytes in vitro: the release of, and immunoproliferative response to, nucleosomes in vitro. Clin Immunol Immunopathol. 1991;60:13–26.
Eriksson C, Engstrand S, Sundqvist KG, et al. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF. Ann Rheum Dis. 2005;64:403–7.
Ferraccioli GF, Assaloni R, Perin A. Drug-induced systemic lupus erythematosus and TNF-alpha blockers. Lancet. 2002;360:645.
Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to antitumor necrosis factor alpha agents. Semin Arthritis Rheum. 2008;37:381–7.
Spillane AP, Xia Y, Sniezek PJ. Drug-induced lupus erythematosus in a patient treated with adalimumab. J Am Acad Dermatol. 2007;56(5 Suppl):S114–6.
Ferraro-Peyret C, Coury F, Tebib JG, Bienvenu J, Fabien N. Infliximab therapy in rheumatoid arthritis and ankylosing spondylitis-induced specific antinuclear and antiphospholipid autoantibodies without autoimmune clinical manifestations: a two-year prospective study. Arthritis Res Ther. 2004;6(6):R535–43.
Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156(2):329–36.
Callen JP. Complications and adverse reactions in the use of newer biologic agents. Semin Cutan Med Surg. 2007;26(1):6–14.
Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatol Treat. 2004;15(5):280–9.
Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58(1):94–105.
Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63.
Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial: ATTRACT Study Group. Lancet. 1999;354:1932–9.
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis: Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–602.
Bacquet-Deschryver H, Jouen F, Quillard M, Ménard JF, Goëb V, Lequerré T, et al. Impact of three anti- TNFalpha biologics on existing and emergent autoimmunity in rheumatoid arthritis and spondyloarthropathies patients. J Clin Immunol. 2008;28:445–55.
Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 2000;43:2383–90.
Eriksson C, Engstrand S, Sundqvist KG, Rantapää-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis. 2005;64:403–7.
Yukawa N, Fujii T, Kondo-Ishikawa S, et al. Correlation of antinuclear antibody and anti-double-stranded DNA antibody with clinical response to infliximab in patients with rheumatoid arthritis: a retrospective clinical study. Arthritis Res Ther. 2011;13:R213. This study suggests that the ANA titer before starting infliximab in RA patients predicts the clinical response to this anti-TNF-α inhibitor. The increased titers of ANA or anti-ds-DNA antibodies after infliximab therapy may be useful markers of no response.
De Rycke L, Kruithof E, Van Damme N, Hoffman IEA, Van de Bossche N, Van den Bosch F, et al. Antinuclear antibodies following infliximab treatment in patients with rheumatoid arthritis or spondyloarthropathy. Arthritis Rheum. 2003;48(4):1015–23.
Bobbio-Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long term infliximab treatment. Arthritis Res Ther. 2004;6:264–72.
Allanore Y, Sellam J, Batteux F, Job Deslandre C, Weill B, Kahan A. Induction of autoantibodies in refractory rheumatoid arthritis treated by infliximab. Clin Exp Rheumatol. 2004;22:756–8.
Atzeni F, Sarzi-Puttini P, Dell’Acqua D, de Portu S, Cecchini G, Cruini C, et al. Adalimumab clinical efficacy is associated with rheumatoid factor and anti-cyclic citrullinated peptide antibody titer reduction: a one year prospective study. Arthritis Res Ther. 2006;8(1):R3.
Gonnet-Gracia C, Barnetche T, Richez C, Blanco P, Dehais J, Schaeverbeke T. Anti-nuclear antibodies, anti-DNA and C4 complement evolution in rheumatoid arthritis and ankylosing spondylitis treated with TNF-α blockers. Clin Exp Rheumatol. 2008;26:401–7.
Benucci M, Saviola G, Baiardi P, Cammelli E, Manfredi M. Anti-nucleosome antibodies as prediction factor of development of autoantibodies during therapy with three different TNFalpha blocking agents in rheumatoid arthritis. Clin Rheumatol. 2008;27:91–5.
Takase K, Horton SC, Ganesha A, Das S, McHugh A, Emery P, et al. What is the utility of routine ANA testing in predicting development of biological DMARD-induced lupus and vasculitis in patients with rheumatoid arthritis? Data from a single-centre cohort. Ann Rheum Dis. 2014;73:1695–9. The study demonstrates no utility of serial ANA/dsDNA testing that could be used to predict onset of seroconversion and therefore the development of lupus/vasculitis. Also it is suggested that there is an association between seroconversion and the development of a secondary non-response to bDMARD therapy.
Atzeni F, Turiel M, Capsoni F, Doria A, Meroni P, Sarzi-Puttini P. Autoimmunity and anti-TNF-α agents. Ann NY Acad Sci. 2005;1051:559–69.
Perez-Alvarez R, Perez-de-Lis M, Ramos-Casals M, on behalf of the BIOGEAS Study Group. Biologics-induced autoimmune diseases. Curr Opin Rheumatol. 2013;25:56–64.
De Bandt M. Lessons for lupus from tumour necrosis factor blockade. Lupus. 2006;15:762–7.
Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242–51.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545–51.
Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology (Oxford). 2009;48:716–20.
Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis Rheum. 2007;56:3248–52.
Acknowledgments
I. García-De La Torre is a member of the SNI (Mexican National Research System) Level II. I. García-Valladares is supported by a Scholarship from CONACyT (Mexican National Council of Science and Technology).
Compliance With Ethics Guidelines
ᅟ
Conflict of Interest
Ignacio García-De La Torre and Ignacio García-Valladares declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Vasculitis
Rights and permissions
About this article
Cite this article
García-De LaTorre, I., García-Valladares, I. Antinuclear Antibody (ANA) Testing in Patients Treated With Biological DMARDs: Is It Useful?. Curr Rheumatol Rep 17, 23 (2015). https://doi.org/10.1007/s11926-015-0500-9
Published:
DOI: https://doi.org/10.1007/s11926-015-0500-9