Abstract
Purpose of Review
This review article summaries the epidemiology, etiology, clinical presentations, and latest treatment modalities of meralgia paresthetica, including the latest data about peripheral and spinal cord stimulation therapy. Meralgia paresthetica (MP) causes burning, stinging, or numbness in the anterolateral part of the thigh, usually due to compression of the lateral femoral cutaneous nerve (LFCN).
Recent Findings
There are emerging data regarding the benefit of interventional pain procedures, including steroid injection and radiofrequency ablation, and other interventions including spinal cord and peripheral nerve stimulation reserved for refractory cases.
Summary
The strength of evidence for treatment choices in meralgia paraesthetica is weak. Some observational studies are comparing local injection of corticosteroid versus surgical interventions. However, more extensive studies are needed regarding the long-term benefit of peripheral and spinal cord stimulation therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meralgia paresthetica (MP) signifies pain in the anterolateral thigh which is derived from the Greek words meros (thigh) and algos (pain) [1]. It is characterized by symptoms of pain with or without numbness in the area of distribution of the lateral femoral cutaneous nerve (LFCN). It is found to present more commonly in men than in women and is shown to have a strong association with obesity, diabetes mellitus, and pregnancy. In this narrative review, we aim to summarize the prevalence, relevant anatomy, and etiological factors along with aspects of clinical presentation and diagnosis to help in the management of any patient presenting with meralgia paresthetica. We then go on to discuss each of the treatment modalities in light of the available evidence.
Epidemiology
In a hospital-based study in Taiwan that enrolled patients between Jan 2003 and Dec 2013 with clinical and electrodiagnostic confirmation of MP, Weng et al. [2] found the average age at diagnosis was 49.8 ± 12.8 years and about 58% of these patients had risk factors. They also reported the peak age of occurrence in male as 41–50 years and in female as 50–60 years. Several studies have reported that MP showed a slight male preponderance [3]. Parisi et al. [4] reported that the age-adjusted and sex-adjusted incidence of MP is 32.7 per 100,000 patient-years in the general population versus 247 per 100,000 patient-years in patients with diabetes mellitus based on a population-based study performed in Olmsted County, MN, over 10-year period between January 01, 1990, and December 31, 1999. On the contrary, Weng et al. only reported one case of diabetes mellitus in their study population, 50 patients with MP. Both studies showed an increased predilection of the disease in patients who were overweight or obese to develop MP as they have increased susceptibility for direct compression or entrapment of nerve along the course of the nerve.
Anatomy
Anatomically, the LFCN is a purely sensory nerve arising from dorsal divisions of the lumbar nerve roots, most commonly L2 and L3 (58.75%), to supply the anterolateral area of the thigh up to the knee [5]. At other times, the nerve is found arising from L1 and L2 (11%) or only L2 (11.25%) and rarely as a branch from the femoral nerve (7.5%) [5]. Understanding the varied origin of the nerve root is important to aid in diagnosing more central causes of MP, i.e., upper lumbar radiculopathy.
The nerve then runs in the psoas major muscle as part of the lumbar plexus, where the nerve can be affected along with other nerves of the plexus resulting in overlapping clinical signs along with MP. Variability in the course of LFCN in the intra-pelvic course puts it especially at risk of injury during lumbar surgeries especially by the trans-psoas approach [5]. The LFCN exits the pelvis under the inguinal ligament (IL), just medial to the anterior superior iliac spine (ASIS) to enter the thigh. In the thigh, it divides into the anterior and posterior branches that pierce the fascia lata, ultimately supplying the skin on the anterolateral and lateral aspect of the thigh, respectively. Occasionally, it may run lateral to the ASIS or pierce the inguinal ligament. These and other variations in the course of this nerve are not uncommon and have been described in the literature [6,7,8,9,10]. The course of LFCN with respect to the ASIS and the inguinal ligament particularly increases its chance of entrapment [11]. The LFCN may pass through the inguinal ligament and the Sartorius muscle as a single trunk, which are specific sites where it can get entrapped. [12] And when it descends into the thigh lateral to the ASIS, it may get entrapped in the fascia lata of the thigh [13].
It is prudent to keep in mind the variations in the anatomy of the LFCN to avoid prematurely dismissing LFCN entrapment when clinically encountering atypical distributions of sensory loss especially when MP is resultant from more central causes like upper lumbar radiculopathy or lumbar plexopathy.
Etiology
Meralgia paresthetica can result from the following:
-
LFCN nerve entrapment due to its anatomical course and/or mechanical causes
-
Metabolic/inflammatory/infective causes
-
Iatrogenic causes
-
Idiopathic causes
The most common cause of meralgia paresthetica remains entrapment directly related to the nature of its anatomic course with certain variations in the course increasing the risk [11, 12, 14]. Other anatomical factors that increase the risk of meralgia paresthetica can be related to limb length discrepancies [15] resulting from radiological degenerative pubic symphysis [16] or compression from tumors arising in or involving the pelvis [17, 18]. Mechanical pressure on the nerve from tight belts, trousers, or corsets along with attached gadgets has also been described to cause chronic microtrauma to the nerve resulting in symptoms over time [19,20,21]. Etiological associations have been established with obesity, pregnancy, and other conditions associated with abdominal distension including retroperitoneal or abdominopelvic masses and fluid accumulation [22,23,24].
When mechanical causes are absent, meralgia paresthetica can result from involvement of the LFCN in metabolic or infective/inflammatory diseases. Lead poisoning, alcoholism, diabetes mellitus, hypothyroidism, and leprosy have all been implicated in presenting with MP [25, 26].
Iatrogenic causes could be related to pelvic or spine surgeries that may disrupt the course of the nerve either by direct injury or secondary to scarring post-surgery. MP has also been described in association with other procedures including certain cardiac procedures [27,28,29,30,31], gynecological and obstetric procedures [32,33,34], and abdominal and laparoscopic surgeries [35,36,37,38,39,40,41]. Positioning during surgery especially related to elective spine surgery as well as hip arthroplasty has been reported [42,43,44]. More recently MP has been reported in patients recovering after prone ventilation in the ICU [45,46,47].
Despite several varied etiological factors implicated in the development of MP, idiopathic nature of the disease is the second most prevalently reported [2, 48].
Clinical Presentation
Patients typically present with burning type of pain that may be associated with paresthesia in the anterior and lateral aspects of the thigh. If associated with entrapment, positional variations in pain with specific worsening with standing and alleviation on sitting may be noted [25, 26]. Seror and Seror documented the distribution of symptoms in 120 patients with MP using neurophysiological studies and reported sole involvement of the lateral thigh in 73% of the patients, while 26% had symptoms in the anterolateral thigh [49]. Symptoms may be unilateral or bilateral. Bilateral presentation is less common with an incidence reported approximately between 10 and 20% in adult patients [48, 50,51,52]. It is unclear if any specific etiology is more associated with bilateral presentation.
Physical exam confirms the sensory area of pain or altered sensation in the distribution of the LFCN. The absence of muscle wasting with a preserved knee jerk and motor strength distinguishes it from lumbar radiculopathy [26, 53, 54]. Despite high index of clinical suspicion towards the diagnosis of MP, it is important to rule out other pathologies in the vicinity that may lead to similar pain distribution including lumbosacral plexopathy, greater trochanteric pain syndrome [55,56,57,58], hip arthropathy [59, 60], chronic appendicitis, and femoral neuropathy [61] or any mass causing compression along the course of the lateral femoral cutaneous nerve [62].
Diagnosis
Although the diagnosis of MP is mostly clinical, electrodiagnostic studies and imaging studies can help when the presentation is atypical [63]. It is also important to have imaging of the abdomen and pelvis to rule out organic causes for LFCN compression [25, 26].
Electrodiagnostic testing mainly involves either sensory evoked potential (SEP) testing or sensory nerve conduction (SNC) studies. Only very serious nerve damage regularly induces abnormal somatosensory evoked potentials, and SNC studies have been shown to be more reliable than SEP testing in the diagnosis of MP [64]. SNC studies may be limited in obese individuals due to inherent difficulties in obtaining sensory nerve action potential (SNAP) data [49]. SEPs may be tested segmentally or dermatomally. Dermatomal SEPs are more sensitive in diagnosing MP [65], and SEP recording following stimulation in the thigh in diagnosing MP is recommended only in obese patients when SNAP data cannot be obtained [66]. Due to much variability in obtaining results, electrodiagnostic testing is not recommended for routine diagnostic testing of MP [61].
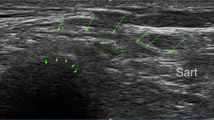
Ultrasound has recently gained increasing popularity in this regard. It not only aids diagnosis but is also able to guide injections and predict treatment success [12], along with being able to identify anatomic variations, thereby improving surgical success [11]. Becciolini et al. [12] describe the use of ultrasound in diagnosing chronic inflammation of the LFCN by the cross-sectional area (CSA) of the nerve at the level of enlargement as compared to the contralateral side. They further suggest that US-guided injection of the nerve proximal to the site of enlargement with 1 cc of local anesthetic with > 50% improvement on the NRS may confirm the diagnosis of MP. A CSA > 5 mm has been proposed for the diagnosis of MP [12, 67].
Three-Tesla MR neurography has made pathology of nerves more conspicuous, making the diagnosis of neuropathies involving smaller nerves like LFCN possible [68•]. Chhabra et al. [69] reported moderate inter-observer agreement with a diagnostic accuracy > 90% and specificity and sensitivity of > 71% and 94% respectively after comparing two readers studying the signal-alteration pattern of LFCN on 3-T MR neurography. They conclude that 3-T MR neurography is a reliable and accurate method for the diagnosis of small nerve neuropathies [69]; especially in the case of diagnostic dilemma, this may prove helpful.
Treatments
The majority of the patients improve without requiring surgical intervention. Cochrane review published in 2012 documents spontaneous recovery in 69% based on a single-center study [70]. Initial conservative management involves avoiding tight-fitting clothes around the waist, weight reduction for obese patients, and symptom alleviation with medications. Medication management usually is related to primarily analgesics, non-steroidal anti-inflammatory medication, neuropathic medications, and various narcotics in some cases [48, 71, 72]. Topical capsaicin [73] and TENS [74] may be beneficial in some cases.
When conservative management is sub-optimal, US-guided injection of the LFCN is sought for both diagnostic and therapeutic indications [12, 75]. Combining corticosteroid injection with conservative measures, the success can be as high as 85–90% in treating MP [75]. Skills in nerve ultrasound and awareness in normal anatomy and variations may assist in diagnosing the correct underlying pathologic process, thereby aiding treatment choice [12]. US may also guide cryo-ablation and radiofrequency ablation.
When peri-surgical scarring is thought to be the reason for the compression, 10–20 cc of 5% glucose is used around the scar [76]. Surgery may be proposed when conservative treatment methods fail. Spinal cord stimulation has also been described in treating LFCN when non-amenable by surgical and other conservative treatment methods, as elaborated in detail below.
Radiofrequency Ablation
Radiofrequency rhizotomy is a minimally invasive procedure that utilizes radio waves to cause thermal damage to the target nerve, which modulates the conduction of pain signals [77]. Cochrane reviews published in 2008 and 2012 reveal a lack of RCTs and quasi RCTs on the effectiveness of interventions in MP [70, 78]. Philip et al. in 2009 reported the first case of successful pulsed RF of LFCN to relieve intractable meralgia. The needle was inserted 1 cm medial and cephalad to ASIS, and RF was performed at t 42 °C for 120 s; bupivacaine and dexamethasone were then injected at the ablation site. The patient reported complete pain relief at 6 months [79]. Dalmau-Carolà in 2009 reported 75% pain relief after pulsed RF of LFCN at 3 weeks after the first procedure and 100% pain relief lasting for 2 years after the second procedure in the same patient. The author reported 100% pain relief after PRF at 3-month follow-up in another patient. The author discusses needle insertion more caudad to the ASIS under the inguinal ligament to locate the nerve, although this technique does not negate the risk of colonic perforation. Dalmau-Carolàhas discussed repeating the procedure for complete and sustained analgesia [80]. Lee JJ in 2016 performed a retrospective review of clinical outcomes after RF in 11 patients with meralgia which was refractory to conservative medical treatment [81]. The authors used the same RF parameters as Philip et al.’s case, that is, pulsed RF at 42 °C for 120 s. They reported dramatic results which include > 5-point reduction in the mean VAS score at 1-, 3-, and 6-month follow-ups, as well as 100% pain relief in 63.6% patients and > 50% pain reduction in 27.3% patients.
Peripheral Nerve Stimulation
Neuromodulation often has a defined role as an invasive therapeutic alternative for chronic pain that is refractory to the initial conservative strategies and injections. PNS is a great option especially in situations when SCS is less preferred due to patient concerns. Although there is a paucity of data supporting the use of peripheral nerve stimulation for meralgia, this is an emerging treatment option [81]. Thompson reported trialing of PNS using SCS leads in postsurgical CRPS-like neuropathy of LFCN [82]. Perryman et al. reported the novel use of a wireless PNS device which was implanted near the nerve [83]. The implanted device included a stimulator electrode with four or eight contacts, a microprocessor, and an antenna which was used to communicate remotely with the external pulse generator by wireless technology. The authors discuss the advantages of the nano stimulation wireless system over traditional SCS systems that are routinely used for PNS including more efficient neurostimulation, minimal surgical intervention, less tissue trauma, reduced operating time, low cost, better cosmesis, and patient comfort. Barna et al. preferred to perform a spinal cord stimulation implant since it is less invasive and less destructive than the PNS implant [84]. In an extensive review of PNS implants for patients with the diagnosis of CRPS in a single center over 30 years, Chmiela et al. reported one case of PNS insertion targeting LFCN [85].
Spinal Cord Stimulation
Since spinal cord stimulation for the treatment of chronic pain was first introduced over 50 years ago, there has been extensive research to understand the underlying mechanisms of neuromodulation with this technology. The earliest proposed concept was that the continuous stimulation of Aβ fibers in the dorsal column of the spinal cord causes the release of neurotransmitters which inhibits C fiber responses in the dorsal horn neurons with the resultant closure of the gate and reduced ascending transmission of pain signals. More recently, electrical stimulation of the dendrites of dorsal horn islet neurons in the spinal cord has been identified as one of the primary mechanisms by which spinal cord stimulation provides relief of neuropathic pain [86].
Stepwise algorithm for treatment of refractory meralgia recommends spinal cord stimulation for patients who failed conservative management and subsequently failed to respond to local injection of local anesthetic and steroid as well as PRF [87]. The only report of spinal cord stimulation was published by Barna et al. in 2005 for the treatment of intractable refractory meralgia [84]. The patient reported 100% pain relief lasting 8 months after the implant, significant functional improvement, and no adverse effects. The authors report that spinal cord stimulation may be a better alternative to surgery since the former is not destructive and does not worsen the pain; success can be predicted by the trial before permanent implant and the stimulator can be explanted without long-term complications.
Surgical Treatment
The most common surgical options for the treatment of meralgia include decompression or neurolysis and nerve section or neurectomy. Neurolysis involves the technique of incising the inguinal ligament to decompress the LFCN, and neurectomy is the technique of transecting the LFCN at the area of the inguinal ligament [88]. Neurectomy is often reserved in patients who failed to respond favorably to surgical decompression, with the major concern of numbness in the distribution of LFCN after nerve resection. A review of Cochrane database published in 2012 revealed that there was a large variation reported among observational studies on the range of improvement after surgical decompression from 60 to 99%. Surgical neurectomy had a smaller range of reported improvement between 85 and 100%, although the sample size was much smaller in the neurectomy group compared to decompression [70]. The review found that surgery was often used in meralgia refractory to previous conservative treatment and injections. A retrospective study by Benezis et al. revealed that 78% of patients reported improvement (61% complete pain relief and 17% partial relief) after neurolysis, while only 35.7% reported improvement after neurectomy [89].
De Ruiter et al. in a retrospective cohort study reported a higher fraction of patients (75%) who underwent primary neurectomy who had complete pain relief compared to 60% of patients who underwent primary neurolysis [90]. A subsequent prospective cohort study by the same author revealed a much striking difference in pain improvement between the two surgical techniques, that is, 93.3% patients after primary neurectomy vs 37.5% patients after primary neurolysis [91••]. While Benezis reported a lower success rate after neurectomy, it is important to note that this study included patients with prior surgery or traumatic injury, unlike De Ruiter’s study. A retrospective cohort study by Antoniadis et al. revealed complete or partial symptomatic relief in 72% of patients after neurolysis and 82% after neurectomy [92].
Van Eerten et al. reported 60% symptomatic relief in the neurolysis group (mean follow-up at 46 months) compared to 100% in the neurectomy group (mean follow-up at 116 months) [93]. While all patients who underwent neurolysis had a recurrence of symptoms within 9 months in Emamhadi’s study, all patients who underwent neurectomy had complete relief of pain without recurrence [94]. Sui et al. reported 73% of patients had complete symptomatic relief, while 20% had partial relief after neurolysis, while there was no recurrence. While the duration of preoperative symptoms did not influence the rate of complete symptomatic relief after surgery, obese patients were six times more likely to have partial relief on long-term follow-up [95]. Haim et al. performed surgery on three patients with positive LFCN block test who failed conservative therapy (1 neurolysis, 2 neurectomy), with complete resolution of symptoms and lack of recurrence during follow-up (mean 3.3 years) [96]. Morimoto reported symptomatic improvement in all patients who underwent microsurgical deep decompression, with 75% reporting complete symptom resolution and no cases of recurrence at 19-month mean follow-up [97]. Benini favors decompression over neurectomy for surgical treatment of meralgia [98].
While studies comparing neurolysis and neurectomy show mixed results and large variability in the efficacy of each procedure, there is a lack of conclusive evidence to guide clinical decision-making on surgical treatment options [70, 88].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roth WK. Meralgia paraesthetica. Meralgia paraesthetica. 1895;3–24. https://doi.org/10.1159/000405004.
Weng W-C, Wei Y-C, Huang W-Y, Chien Y-Y, Peng T-I, Wu C-L. Risk factor analysis for meralgia paresthetica: a hospital-based study in Taiwan. J Clin Neurosci. 2017;43:192–5.
Ivins GK. Meralgia paresthetica: the elusive diagnosis. Ann Surg. 2000;232:281–6.
Parisi TJ, Mandrekar J, Dyck PJB, Klein CJ. Meralgia paresthetica. Neurology. 2011;77:1538–42.
Haładaj R. Anatomic variations of the lateral femoral cutaneous nerve: remnants of atypical nerve growth pathways revisited by intraneural fascicular dissection and a proposed classification. World Neurosurg. 2018;118:e687–98.
Aszmann OC, Dellon ES, Dellon AL. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg. 1997;100:600–4.
Grothaus MC. Lateral femoral cutaneous nerve - an anatomic study. Clin Orthop Relat Res. 2005;164–8.
Tomaszewski KA, Popieluszko P, Henry BM, Roy J, Sanna B, Kijek MR, et al. The surgical anatomy of the lateral femoral cutaneous nerve in the inguinal region: a meta-analysis. Hernia. 2016;20:649–57.
Hanna AS. Lateral femoral cutaneous nerve transposition: renaissance of an old concept in the light of new anatomy. Clin Anat (New York, NY). 2017;30:409–12.
Kokubo R. Anatomic variation in patient with lateral femoral cutaneous nerve entrapment neuropathy. World Neurosurg. 2018;115:274–6.
de Ruiter GCW, Wesstein M, Vlak MHM. Preoperative ultrasound in patients with meralgia paresthetica to detect anatomical variations in the course of the lateral femoral cutaneous nerve. World Neurosurg. 2021;149:e29-35.
Becciolini M, Pivec C, Riegler G. Ultrasound of the Lateral Femoral Cutaneous Nerve: A Review of the Literature and Pictorial Essay. J Ultrasound Med. 2022 May;41(5):1273-1284. https://doi.org/10.1002/jum.15809. Epub 2021 Aug 13. PMID: 34387387.
Omichi Y, Tonogai I, Kaji S, Sangawa T, Sairyo K. Meralgia paresthetica caused by entrapment of the lateral femoral subcutaneous nerve at the fascia lata of the thigh: a case report and literature review. J Med Invest. 2015;62:248–50.
de Ridder VA, de Lange S, Popta JV. Anatomical variations of the lateral femoral cutaneous nerve and the consequences for surgery. J Orthop Trauma. 1999;13:207–11.
Goel A. Meralgia paresthetica secondary to limb length discrepancy: case report. Arch Phys Med Rehabil. 1999;80:348–9.
Bierma-Zeinstra S, Ginai A, Prins A, Geleijnse M, van den Berge H, Bernsen R, et al. Meralgia paresthetica is related to degenerative pubic symphysis. J Rheumatol. 2000;27:2242–5.
Suber DA, Massey EW. Pelvic mass presenting as meralgia paresthetica. Obstet Gynecol. 1979;53:257–8.
Tharion G, Bhattacharji S. Malignant secondary deposit in the iliac crest masquerading as meralgia paresthetica. Arch Phys Med Rehabil. 1997;78:1010–1.
Boyce JR. Meralgia paresthetica and tight trousers. JAMA. 1984;251:1553.
Korkmaz N, Ozçakar L. Meralgia paresthetica in a policeman: the belt or the gun. Plast Reconstr Surg. 2004;114:1012–3.
Karwa KA, Patel D, Tavee JO. Smart device neuropathy. J Neurol Sci. 2016;370:132–3.
Harney D, Patijn J. Meralgia paresthetica: diagnosis and management strategies. Pain Med. 2007;8:669–77.
Williams PH, Trzil KP. Management of meralgia paresthetica. J Neurosurg. 1991;74:76–80.
Ostrominski JW, Huang Q, Kamenker-Orlov Y. Beneath the surface: massive retroperitoneal liposarcoma masquerading as meralgia paresthetica. Fed Pract. 2021;38:S61–7.
Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336–44.
Pearce JMS. Meralgia paraesthetica (Bernhardt-Roth syndrome). J Neurol Neurosurg Psychiatry. 2006;77:84.
Antunes PE, Antunes MJ. Meralgia paresthetica after aortic valve surgery. J Heart Valve Dis. 1997;6:589–90.
Reddy YM, Singh D, Chikkam V, Bommana S, Atkins D, Verma A, et al. Postprocedural neuropathy after atrial fibrillation ablation. J Interv Card Electrophysiol. 2013;36:279–85.
Jellish WS, Oftadeh M. Peripheral nerve injury in cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32:495–511.
Parsonnet V, Karasakalides A, Gielchinsky I, Hochberg M, Hussain SM. Meralgia paresthetica after coronary bypass surgery. J Thorac Cardiovasc Surg. 1991;101:219–21.
Butler R, Webster MWI. Meralgia paresthetica: an unusual complication of cardiac catheterization via the femoral artery. Catheter Cardiovasc Interv. 2002;56:69–71.
Kvarnström N, Järvholm S, Johannesson L, Dahm-Kähler P, Olausson M, Brännström M. Live donors of the initial observational study of uterus transplantation-psychological and medical follow-up until 1 year after surgery in the 9 cases. Transplantation. 2017;101:664–70.
Peters G, Larner AJ. Meralgia paresthetica following gynecologic and obstetric surgery. Int J Gynaecol Obstet. 2006;95:42–3.
Chung KH, Lee JY, Ko TK, Park CH, Chun DH, Yang HJ, et al. Meralgia paresthetica affecting parturient women who underwent cesarean section -a case report-. Korean J Anesthesiol. 2010;59(Suppl):S86-89.
Macgregor AM, Thoburn EK. Meralgia paresthetica following bariatric surgery. Obes Surg. 1999;9:364–8.
Grace DM. Meralgia paresthetica after gastroplasty for morbid obesity. Can J Surg. 1987;30:64–5.
Polidori L, Magarelli M, Tramutoli R. Meralgia paresthetica as a complication of laparoscopic appendectomy. Surg Endosc. 2003;17:832.
Yamout B, Tayyim A, Farhat W. Meralgia paresthetica as a complication of laparoscopic cholecystectomy. Clin Neurol Neurosurg. 1994;96:143–4.
Broin EO, Horner C, Mealy K, Kerin MJ, Gillen P, O’Brien M, et al. Meralgia paraesthetica following laparoscopic inguinal hernia repair. An anatomical analysis. Surg Endosc. 1995;9:76–8.
Eubanks S, Newman L, Goehring L, Lucas GW, Adams CP, Mason E, et al. Meralgia paresthetica: a complication of laparoscopic herniorrhaphy. Surg Laparosc Endosc. 1993;3:381–5.
Atamaz F, Hepgüler S, Karasu Z, Kilic M. Meralgia paresthetica after liver transplantation: a case report. Transplant Proc. 2005;37:4424–5.
DePasse JM, Palumbo MA, Haque M, Eberson CP, Daniels AH. Complications associated with prone positioning in elective spinal surgery. World J Orthop. 2015;6:351–9.
Yoshida S, Oya S, Matsui T. Risk factors of meralgia paresthetica after prone position surgery: possible influence of operating position, laminectomy level, and preoperative thoracic kyphosis. J Clin Neurosci. 2021;89:292–6.
Bhargava T, Goytia RN, Jones LC, Hungerford MW. Lateral femoral cutaneous nerve impairment after direct anterior approach for total hip arthroplasty. Orthopedics. 2010;33.
Bellinghausen AL, LaBuzetta JN, Chu F, Novelli F, Rodelo AR, Owens RL. Lessons from an ICU recovery clinic: two cases of meralgia paresthetica after prone positioning to treat COVID-19-associated ARDS and modification of unit practices. Crit Care. 2020;24:580.
Juhl CS, Ballegaard M, Bestle MH, Tfelt-Hansen P. Meralgia paresthetica after prone positioning ventilation in the intensive care unit. Case Reports in Critical Care. 2016;2016: e7263201.
Marinelli L, Mori L, Avanti C, Cotellessa F, Fabbri S, Schenone C, et al. Meralgia Paraesthetica after prone position ventilation in a patient with COVID-19. Eur J Case Rep Intern Med. 2020;7:002039.
Kitchen C, Simpson J. Meralgia paresthetica a review of 67 patients. Acta Neurol Scand. 1972;48:547–55.
Seror P, Seror R. Meralgia paresthetica: clinical and electrophysiological diagnosis in 120 cases. Muscle Nerve. 2006;33:650–4.
Chhuttani PN, Chawla LS, Sharma TD. Meralgia paraesthetica. Acta Neurol Scand. 1966;42:483–90.
Ecker A, Woltman H. Meralgia paraesthetica: a report of 150 cases. JAMA. 1938;110:1650–2.
Musser HH, Sailer J. Meralgia paresthetica (Roth), with the report of ten cases. J Nerv Ment Dis. 1900;27:16–40.
Dharmasaroja P, Dharmasaroja P. Meralgia paresthetica-like syndrome may be caused by transient lumbar nerve root injury without definite compression: a case report. J Med Assoc Thai. 2010;93(Suppl 7):S307-310.
Trummer M, Flaschka G, Unger F, Eustacchio S. Lumbar disc herniation mimicking meralgia paresthetica: case report. Surg Neurol. 2000;54:80–1.
Rho M, Camacho-Soto A, Cheng A, Havran M, Morone NE, Rodriguez E, et al. Deconstructing chronic low back pain in the older adult—step by step evidence and expert-based recommendations for evaluation and treatment. Part VIII: Lateral Hip and Thigh Pain. Pain Med. 2016;17:1249–60.
Travell JG, Simons DG. Myofascial pain and dysfunction: the trigger point manual. Lippincott Williams & Wilkins, 1983.
Williams BS, Cohen SP. Greater trochanteric pain syndrome: a review of anatomy, diagnosis and treatment. Anesth Analg. 2009;108:1662–70.
Cho W, Son B. Delayed diagnosis of meralgia paresthetica: a case report. Nerve. 2018;4:82–5.
Shetty VD, Shetty GM. Persistent bilateral anterior hip pain in a young adult due to meralgia paresthetica: a case report. Cases J. 2008;1:396.
Ahuja V, Thapa D, Patial S, Chander A, Ahuja A. Chronic hip pain in adults: current knowledge and future prospective. J Anaesthesiol Clin Pharmacol. 2020;36:450–7.
Beltran LS, Bencardino J, Ghazikhanian V, Beltran J. Entrapment neuropathies III: lower limb. Semin Musculoskelet Radiol. 2010;14:501–11.
Madiraca Glasović D, Šlaus N, Šitum M, Pećina M. Meralgia paresthetica – lateral femoral cutaneous nerve entrapment. Rad Hrvatske akademije znanosti i umjetnosti. 2021;54–55:56–63.
Moy WL. A cook with ‘burning in the thigh’ and a ‘hotspot’ in the groin!. Oxf Med Case Reports. 2021;2021:omab112.
Seror P. Lateral femoral cutaneous nerve conduction v somatosensory evoked potentials for electrodiagnosis of meralgia paresthetica. Am J Phys Med Rehabil. 1999;78:313–6.
El-Tantawi GAY. Reliability of sensory nerve-conduction and somatosensory evoked potentials for diagnosis of meralgia paraesthetica. Clin Neurophysiol. 2009;120:1346–51.
Seror P. Somatosensory evoked potentials for the electrodiagnosis of meralgia paresthetica. Muscle Nerve. 2004;29:309–12.
Suh DH, Kim DH, Park JW, Park BK. Sonographic and electrophysiologic findings in patients with meralgia paresthetica. Clin Neurophysiol. 2013;124:1460–4.
• Ally RM. Meralgia paresthetica: now showing on 3T magnetic resonance neurography. S.A. J Radiol. 2019;23:1745–1745. This study showed a new imaging technique for meralgia.
Chhabra A, Del Grande F, Soldatos T, Chalian M, Belzberg AJ, Williams EH, et al. Meralgia paresthetica: 3-Tesla magnetic resonance neurography. Skeletal Radiol. 2013;42:803–8.
Khalil N, Nicotra A, Rakowicz W. Treatment for meralgia paraesthetica. Cochrane Database Syst Rev. 2012;2012:CD004159.
Massey EW. Meralgia paresthetica secondary to trauma of bone graft. J Trauma. 1980;20:342–3.
Stevens H. Meralgia paresthetica. AMA. Arch Neurol Psychiatry. 1957;77:557–74.
Puig L, Alegre M, de Moragas JM. Treatment of meralgia paraesthetica with topical capsaicin. Dermatology. 1995;191:73–4.
Fisher AP, Hanna M. Transcutaneous electrical nerve stimulation in meralgia paraesthetica of pregnancy. Br J Obstet Gynaecol. 1987;94:603–4.
Dureja GP, Gulaya V, Jayalakshmi TS, Mandal P. Management of meralgia paresthetica: a multimodality regimen. Anesth Analg. 1995;80:1060–1.
Su Y-C, Lee C-Y, Chang C-Y, Chen L-C, Wu Y-T. Efficacy of nerve hydrodissection with 5% dextrose in chronic meralgia paresthetica. Pain Pract. 2020;20:566–7.
Kapural L, Deering JP. A technological overview of cooled radiofrequency ablation and its effectiveness in the management of chronic knee pain. Pain Manag. 2020;10(3):133–40.
Khalil N, Nicotra A, Rakowicz W. Treatment for meralgia paraesthetica. Cochrane Database Syst Rev. 2008 Jul 16;(3):CD004159. https://doi.org/10.1002/14651858.CD004159.pub2. Update in: Cochrane Database Syst Rev. 2012;12:CD004159. PMID: 18646102.
Philip CN, Candido KD, Joseph NJ, Crystal GJ. Successful treatment of meralgia paresthetica with pulsed radiofrequency of the lateral femoral cutaneous nerve. Pain Physician. 2009;12(5):881–5. PMID: 19787014.
Dalmau-Carolà J. Treatment of meralgia paresthetica with pulsed radiofrequency of the lateral femoral cutaneous nerve. Pain Physician. 2009;12(6):1025–6, 1026–7. PMID: 19935989.
Lee JJ, Sohn JH, Choi HJ, Yang JS, Lee KH, Do HJ, et al. Clinical efficacy of pulsed radiofrequency neuromodulation for intractable meralgia paresthetica. Pain Physician. 2016;19(3):173–9. PMID: 27008291.
Thompson S. Challenges of peripheral nerve stimulator implantation in a patient with new onset thrombocytopenia. J Neurol Neurosci. 2015;S1.
Perryman LT, Kubias J, Stockli S, Herschkowitz D. Wireless peripheral nerve stimulation in the management of debilitating extremity pain from meralgia paresthetica and complex regional pain syndrome: report of two case illustrations. Biomed J Sci Tech Res. 2018;9:3.
Barna SA, Hu MM, Buxo C, Trella J, Cosgrove GR. Spinal cord stimulation for treatment of meralgia paresthetica. Pain Physician. 2005;8(3):315–8. PMID: 16850089.
Chmiela MA, Hendrickson M, Hale J, Liang C, Telefus P, Sagir A, et al. Direct peripheral nerve stimulation for the treatment of complex regional pain syndrome: a 30-year review. neuromodulation. 2021;24(6):971–982. https://doi.org/10.1111/ner.13295. Epub 2020 Oct 24. PMID: 33098229.
Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: still in the dark after 50 years. Eur J Pain. 2019;23(4):652–659. https://doi.org/10.1002/ejp.1336. Epub 2018 Dec 3. PMID: 30407696; PMCID: PMC6491991.
Patijn J, Mekhail N, Hayek S, Lataster A, van Kleef M, Van Zundert J. Meralgia paresthetica. Pain Pract. 2011;11(3):302–8. https://doi.org/10.1111/j.1533-2500.2011.00458.x. PMID: 21435164.
Payne R, Seaman S, Sieg E, Langan S, Harbaugh K, Rizk E. Evaluating the evidence: is neurolysis or neurectomy a better treatment for meralgia paresthetica? Acta Neurochir (Wien). 2017;159(5):931–6. https://doi.org/10.1007/s00701-017-3136-x. Epub 2017 Mar 10. PMID: 28283866.
Benezis I, Boutaud B, Leclerc J, Fabre T, Durandeau A. Lateral femoral cutaneous neuropathy and its surgical treatment: a report of 167 cases. Muscle Nerve. 2007;36(5):659–63. https://doi.org/10.1002/mus.20868. PMID: 17657804.
de Ruiter GC, Wurzer JA, Kloet A. Decision making in the surgical treatment of meralgia paresthetica: neurolysis versus neurectomy. Acta Neurochir (Wien). 2012;154(10):1765–72. https://doi.org/10.1007/s00701-012-1431-0. Epub 2012 Jul 6. PMID: 22766927.
•• de Ruiter GC, Kloet A. Comparison of effectiveness of different surgical treatments for meralgia paresthetica: results of a prospective observational study and protocol for a randomized controlled trial. Clin Neurol Neurosurg. 2015;134:7–11. https://doi.org/10.1016/j.clineuro.2015.04.007. Epub 2015 Apr 11. PMID: 25911497. Great study that compared the different surgical treatment for meralgia.
Antoniadis G, Braun V, Rath S, Moese G, Richter HP. Die Meralgia paraesthetica und ihre operative Behandlung [Meralgia paraesthetica and its surgical treatment]. Nervenarzt. 1995;66(8):614–7. German. PMID: 7566273.
van Eerten PV, Polder TW, Broere CA. Operative treatment of meralgia paresthetica: transection versus neurolysis. Neurosurgery. 1995;37(1):63–5. https://doi.org/10.1227/00006123-199507000-00009. PMID: 8587692.
Emamhadi M. Surgery for meralgia paresthetica: neurolysis versus nerve resection. Turk Neurosurg. 2012;22(6):758–62. https://doi.org/10.5137/1019-5149.JTN.6068-12.4. PMID: 23208909.
Siu TL, Chandran KN. Neurolysis for meralgia paresthetica: an operative series of 45 cases. Surg Neurol. 2005 Jan;63(1):19-23; discussion 23. https://doi.org/10.1016/j.surneu.2004.07.035. PMID: 15639511.
Haim A, Pritsch T, Ben-Galim P, Dekel S. Meralgia paresthetica: a retrospective analysis of 79 patients evaluated and treated according to a standard algorithm. Acta Orthop. 2006;77(3):482–6. https://doi.org/10.1080/17453670610046433. PMID: 16819689.
Morimoto D, Kim K, Kokubo R, Kitamura T, Iwamoto N, Matsumoto J, et al. Deep decompression of the lateral femoral cutaneous nerve under local anesthesia. World Neurosurg. 2018;118:e659–65. https://doi.org/10.1016/j.wneu.2018.06.252. Epub 2018 Jul 11. PMID: 30017766.
Benini A. Die Meralgiaparaesthetica. Pathogenese, Klinik und Therapie der Kompression des Nervus cutaneus femoris lateralis [Meralgia paresthetica. Pathogenesis, clinical aspects and therapy of compression of the lateral cutaneous nerve of the thigh]. Schweiz Rundsch Med Prax. 1992 Feb 18;81(8):215–21. German. PMID: 1539116.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors of this article, Jane Nithya Tolson Solomons M.D. ; Afrin Sagir, MD; and Cyrus Yazdi, MD, declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Chronic Pain Medicine
Rights and permissions
About this article
Cite this article
Solomons, J.N.T., Sagir, A. & Yazdi, C. Meralgia Paresthetica. Curr Pain Headache Rep 26, 525–531 (2022). https://doi.org/10.1007/s11916-022-01053-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-022-01053-7