Abstract
Intracranial hypotension is known to occur as a result of spinal cerebrospinal fluid (CSF) leaking, which may be iatrogenic, traumatic, or spontaneous. Headache is usually, but not always, orthostatic. Spontaneous cases are recognized more readily than in previous decades as a result of a greater awareness of clinical presentations and typical cranial magnetic resonance imaging findings. An underlying disorder of connective tissue that predisposes to weakness of the dura is implicated in spontaneous spinal CSF leaks. CT, MR, and digital subtraction myelography are the imaging modalities of choice to identify spinal CSF leakage. Spinal imaging protocols continue to evolve with improved diagnostic sensitivity. Epidural blood patching is the most common initial intervention for those seeking medical attention, and may be repeated several times. Surgery is reserved for cases that fail to respond or relapse after simpler measures. While the prognosis is generally good with intervention, serious complications do occur. More research is needed to better understand the genetics and pathophysiology of dural weakness as well as physiologic compensatory mechanisms, to continue to refine imaging modalities and treatment approaches, and to evaluate short- and long-term clinical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Orthostatic headache, which is a headache that occurs upon assuming an upright posture and improves with recumbency, is readily recognized following a diagnostic dural puncture. When this type of headache occurs spontaneously, the diagnosis may be delayed or missed altogether, although awareness amongst emergency room physicians, neurologists, and neurosurgeons has increased substantially over the last two decades as the clinical presentations, imaging findings, and treatment approaches have been described in the literature [1–5]. It is now well-established that spontaneous intracranial hypotension (SIH) arises from spontaneous cerebrospinal fluid (CSF) leaking from the spine, and rarely from the skull base [6]. Several lines of evidence suggest that spontaneous spinal CSF leaks are related to an underlying weakness of the dura.
Spontaneous intracranial hypotension resulting from spinal CSF leaks is an important cause of new daily persistent headache. The development and refinement of diagnostic criteria have been important in shortening the time to correct diagnosis of this headache syndrome. Many patients are significantly disabled by limited functional upright time, and not long ago, symptoms were often misdiagnosed with a variety of primary headache disorders, including migraine or tension headache, headache secondary to viral meningitis, or malingering.
The incidence and prevalence of spontaneous intracranial hypotension is not yet firmly established. Our current estimated annual incidence of 5/100,000 cases is based on a retrospective study of SIH diagnosis at an urban emergency room, where it was found to occur half as frequently as spontaneous subarachnoid hemorrhage [7]. Peak incidence is around the age of 40 years, but patients of all ages have presented. The female-to-male ratio is 1.5:1.
Clinical Presentation
The most common presenting symptom of intracranial hypotension is a headache that is worse when in an upright position and improves with recumbency. CFS leaks can be iatrogenic, resulting from a lumbar puncture, inadvertent dural puncture at the time of epidural injection or spinal surgery, intrathecal catheters, or from over-drainage of a CSF shunt. Traumatic spinal CSF leaks are also seen as a result of brachial plexus avulsion [8]. Occasionally, bony spurs or disc herniation may cause a dural tear. An orthostatic headache may also occur spontaneously as a result of single or multiple spontaneous spinal CSF leaks. Our discussion here focuses on spontaneously occurring spinal CSF leaks.
The headache related to spontaneous intracranial hypotension is usually orthostatic, arising within seconds, minutes, or even hours of assuming an upright position, and improving with recumbency over minutes to hours [1, 5]. Over time, the postural aspect of the headache often attenuates and can disappear completely, presumably as physiologic compensatory mechanisms develop, such as an up-regulated CSF production rate. The positional aspect of headache may also occasionally be reversed, improving when the individual is in an upright position. Headache severity ranges from mild to severe, and correlates poorly with degree of intracranial hypotension. Degree of disability is often underestimated. Location of headache is most often occipital or suboccipital, but may be diffuse, frontal, or temporal. Occasionally, the headache may be entirely absent or resolve despite persistence of other signs and symptoms.
Common associated symptoms include those suggestive of meningeal irritation such as neck stiffness or pain, nausea with or without vomiting, and photophobia, as well as hearing changes (phonophobia, muffled hearing, tinnitus), interscapular pain, upper limb radicular symptoms, sense of imbalance, and subtle cognitive dysfunction. While interscapular pain and upper limb radicular symptoms are common spinal manifestations, local back pain at the level of CSF leak, radiculopathy, and myelopathy, usually related to extrathecal CSF collection, have also been observed [9]. Less common symptoms or signs may be related to cranial nerve effects, including visual changes (blurring, visual field defects, diplopia), facial pain or numbness, facial weakness, and dysgeusia (Table 1). Other reported complications include subdural hematomas and cerebral dural sinus thrombosis [10–18].
Although inadequately studied to date, neuroendocrine dysfunction has been observed in association with SIH. Hyperprolactinemia has been reported occasionally, and we have also seen central diabetes insipidus, central hypocortisolism, and central hypothyroidism [unpublished]. More rarely, patients may present with Parkinsonism, ataxia, cerebellar hemorrhage, dementia, stupor and coma, or stroke; deaths have even been reported [19–28].
Clinical stigmata of heritable disorders of connective tissue may be noted, as discussed further below.
Pathophysiology
Dural Weakness
Spontaneous spinal CSF leaks appear to be related to an underlying structural weakness of the spinal dura. Mechanical factors, often relatively trivial, are reported in association with onset of symptoms in roughly one-third of patients, but onset appears to occur with no clear precipitant in the majority of cases [29]. Reported triggering events include lifting small or large items, straining, stretching, positional changes, sporting activities, roller coaster rides, falls, and rarely, chiropractic manipulation.
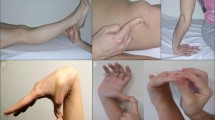
There is a substantial body of evidence of dural weakness in patients with SIH. Heritable disorders of connective tissue (HDCT) have been identified with higher frequency than in the general population. Well-characterized disorders that have been reported include Marfan syndrome, Ehlers–Danlos syndrome, autosomal dominant polycystic kidney disease, and joint hypermobility [1, 30, 31]. Many additional patients demonstrate manifestations of HDCT without meeting the criteria for any of the above disorders. These include blue/gray sclera, lens dislocation (personal or family history), retinal detachment, high-arched palate, joint hypermobility, joint dislocation, degenerative joint disease, tall stature, arachnodactyly, scoliosis, flat feet, skin hyperelasticity, soft/thin/transparent skin, easy bruising, slow wound healing, widened/thin scars, arterial tortuosity or aneurysm (aortic, intracranial, other arterial aneurysm – personal or family history), bicuspid aortic valve, mitral valve prolapse, and spontaneous pneumothorax.
A prospective series of 18 patients found stigmata of HDCT (Marfan syndrome, Ehlers–Danlos syndrome, and joint hypermobility syndrome) in seven of the patients in the cohort [32]. Another prospective study observed HDCT in 9 of 50 patients, including the classic and joint hypermobility subtypes of Ehlers–Danlos syndrome, Marfan syndrome, and unspecified HDCT [33]. An additional eight patients in this 50-patient series met criteria for benign joint hypermobility syndrome. In another study of 24 pediatric cases of SIH, 13 patients were found to have evidence of a generalized disorder of connective tissue, a notably higher prevalence compared with adults [34]. Intracranial aneurysm was found in 9 % of patients with SIH (n = 93) as compared to 1 % of controls (n = 291), also suggesting the presence of underlying connective tissue disorder [35•]. Bicuspid aortic valve was found in 3 of 273 SIH patients, but none of 506 controls [36]. Using echocardiographic screening, we recently found a higher prevalence of aortic dilatation in a prospective cohort of 50 patients with spontaneous CSF leak [37].
Common imaging findings of meningeal diverticula and surgical findings of dural defects, attenuated dura, meningeal diverticula, and nude nerve root sleeves are additional evidence of dural weakness [1].
The specific molecular pathways involved in these abnormalities of dural structure and strength are largely unknown. Electron microscopy of dura has shown severe and widespread variation in diameter of collagen fibers [38]. It has been shown that patients with SIH and with isolated skeletal features of Marfan syndrome do not harbor mutations in the fibrillin-1 gene (FBN1) despite the presence of abnormalities of fibrillin-1-containing microfibrils [38]. Because gene mutations involve the TGF-β receptor 2 (TGFBR2) in a type of Marfan syndrome as well as in patients with arterial tortuosity, we evaluated patients with SIH who also had skeletal features of Marfan syndrome, arterial tortuosity, and/or thoracic aortic aneurysm for TGFBR2 mutations and found them to be absent [39].
A genome-wide association study in patients with SIH may contribute insights regarding the correlation with heritable disorders of connective tissue as well as the mechanisms of the underlying dural abnormalities.
Mechanisms of Headache
Loss of CSF volume results in loss of brain buoyancy, which is more marked with upright positioning. It is thought that this descent of the brain produces traction on pain-sensitive structures such as the dura and blood vessels. The compensatory response of cerebral vasodilatation to the loss of CSF volume, evident as pachymeningeal enhancement on cranial MRI, may also contribute to pain.
Diagnostic Approach
Clinical presentation is often highly suggestive of the diagnosis. Imaging is used to confirm the diagnosis and to localize CSF leaking in order to target treatment. Since publication of the International Classification of Headache Disorders 2nd edition in 2004, the diagnostic criteria have been refined. The International Classification of Headache Disorders 3rd edition, beta version (ICHD-3 beta) [40•] describes three categories under “headache attributed to low cerebrospinal fluid pressure”: those occurring following a lumbar dural puncture; those following an iatrogenic or traumatic event; and those that occur spontaneously. The hallmark of headache that changes with position is noted in each of these categories. The ICHD-3 beta criteria refer to low CSF pressure of < 60 mmH2O or imaging evidence of CSF leak in spontaneous, traumatic, and iatrogenic cases exclusive of the post-LP situation.
Proposed diagnostic criteria for headache due to spontaneous intracranial hypotension, based on criteria published in 2008 and modified in 2011 [41, 42••], are as follows:
-
(a)
Orthostatic headache;
-
(b)
The presence of at least one of the following:
-
low opening pressure (≤60 mm H2O),
-
sustained improvement of symptoms after epidural blood patching,
-
demonstration of an active spinal cerebrospinal fluid leak,
-
cranial magnetic resonance imaging changes of intracranial hypotension (such as brain sagging or pachymeningeal enhancement);
-
-
(c)
No recent history of dural puncture; and
-
(d)
Not attributable to another disorder.
Note that these criteria recognize variability in clinical presentation and imaging findings. The orthostatic aspect of the headache may develop within minutes or hours, but it may also not be evident at all, particularly after some time has passed. While opening pressure below 60 mm H2O supports diagnosis of the disorder, a normal opening pressure does not rule it out. In addition, up to 25 % of patients with SIH may have normal cranial magnetic resonance imaging (MRI) [41].
Lumbar Puncture
While a lumbar puncture is not necessary for diagnosis, it may be performed following a negative cranial CT to rule out subarachnoid hemorrhage. The opening pressure will often be less than 60 mm H2O (reference, 65–195 mm H2O), and can be unmeasurable or subatmospheric, but normal pressures are common and do not rule out a spinal CSF leak. Analysis of CSF may show clear or xanthochromic color, normal or elevated protein, normal glucose, normal leukocyte count or lymphocytic pleocytosis, and normal or high erythrocyte count [1, 5].
Imaging
Cranial CT
is generally of limited value compared with cranial MRI, but may be ordered in the emergency room for symptoms that include headache, stiff neck, photophobia, phonophobia, nausea, and vomiting. Subdural fluid collections, cerebellar tonsillar herniation, or obliteration of subarachnoid cisterns and ventricular collapse may be found.
Cranial MRI
can be diagnostic of intracranial hypotension, as typical findings are present in about 80 % of cases. Conversely, it should be recognized that 20 % of cranial MRIs are normal [1]. This should be performed with gadolinium enhancement in all patients.
The mnemonic SEEPS is useful to remember the five main findings:
-
1.
Subdural fluid collections
-
2.
Enhancement of pachymeninges
-
3.
Engorgement of venous structures
-
4.
Pituitary hyperemia
-
5.
Sagging of the brain
Subdural hematomas are not uncommon, and may be the first manifestation of spontaneous intracranial hypotension; comments regarding their management are below [10–16]. Sagging of the brain may be evident from ventricular collapse, descent of the cerebellar tonsils mimicking a Chiari I malformation, effacement of the perichiasmatic cisterns with bowing of the optic chiasm over the pituitary fossa, and effacement of the prepontine cistern, with flattening of the pons against the clivus.
Spinal imaging may not be necessary in patients that respond to conservative measures or non-directed epidural blood patching, but it becomes necessary in many cases in order to localize the CSF leak to direct further treatment.
Spinal MRI
may reveal meningeal enhancement, meningeal diverticula, extrathecal fluid collections, and dilated epidural or intradural veins [1, 43].
Spinal MR with myelographic sequencing is increasingly being used, has good sensitivity, and obviates the need for a dural puncture to instill intrathecal contrast [44••]. Wang et al. compared heavily T2-weighted MR myelography with CT myelography in 19 patients; CT myelography was positive in 13, while MR myelography was positive in 15 [45].
Recent studies have compared intrathecal gadolinium-enhanced MR myelography, which involves off-label use of gadolinium, with conventional CT myelography. Neuroradiologists at the Mayo Clinic in Rochester, Minnesota, have reported that MR myelography with intrathecal gadolinium was useful for detecting suspected leaks that were undetected on CT myelography [46]. Chazen et al. also found spinal MR myelography with intrathecal gadolinium to be slightly more sensitive than CT myelography in detecting leaks [47].
CT Myelography
which is myelography with iodinated contrast followed by thin-cut CT of the entire spine, has been considered the gold standard in the detection and location of spinal CSF leaks. As discussed above, MR imaging has similar sensitivity using either heavily T2-weighted sequencing or intrathecal gadolinium enhancement. In addition to CSF leaks, meningeal diverticula or nerve root sleeve dilatations may also be visualized. Retrospinal collections of contrast at the C1–2 level occur in up to 10 % of patients, and are known to be a false localizing sign [48]. Delayed imaging or the addition of intrathecal saline may help to visualize slow leaks.
Dynamic CT Myelography
can be used to localize rapid leaks [49, 50].
Digital Subtraction Myelography
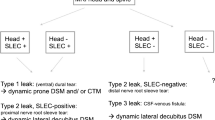
(DSM), which provides high-resolution images in real time, is seeing increasing adoption . At our center, it is usually performed under general anesthesia to remove movement artifact. We have found DSM to be helpful in three situations: 1) visualizing rapid and/or extensive leaks seen on other imaging without precise localization; 2) localizing leaks that are ventral to the spinal cord; and 3) identifying recently described CSF–venous fistulae between the subarachnoid space and spinal epidural veins that are not visualized on MRI or CT myelography [44••, 51–53, 54••].
Radioisotope Cisternography
may confirm the presence of a CSF leak, but is insensitive in localizing leaks [55].
Sensitivites of available imaging modalities areless than 100 %, so CSF leaks in some patients may be below the level of detection of current imaging.
Differential Diagnosis
It is important to keep in mind that not all orthostatic headaches are due to a CSF leak. Postural orthostatic tachycardia syndrome (POTS), which is a form of dysautonomia, can present with orthostatic headache as the most prominent symptom [56]. Diagnosis of POTS is made with autonomic testing. In practice, this may be challenging to sort out, since patients with spinal CSF leaks may have positional tachycardia to physiologically compensate for intracranial hypotension, may have preexisting POTS or can develop secondary POTS. In addition, there is an association between POTS and Ehlers–Danlos syndrome (EDS) in that a significant proportion of patients with POTS have EDS [57, 58]. Patients with Ehlers–Danlos syndrome appear to be at increased risk of both POTS and spinal CSF leaks, and can have both. Headaches may also have a positional component in diabetes insipidus, in cervicogenic headaches, and in patients post decompression surgery for Chiari malformation without CSF leak.
Management
Conservative
It is suspected that a percentage of spontaneous spinal CSF leaks will resolve without intervention, or it may be that the symptoms resolve with physiologic compensatory mechanisms. Bed rest, oral and IV hydration, and oral and IV caffeine are typical conservative approaches that are not unreasonable in the short term, but should not be expected to offer substantial or durable benefit. Steroids have been used to alleviate symptoms but have a high risk/benefit ratio. Some patients find symptomatic benefit from the use of an abdominal binder, which does increase intracranial pressure.
Epidural Patching (Autologous Blood, Fibrin Sealant)
The standard initial procedure for most patients seeking treatment is epidural blood patching (EBP) with autologous blood [1]. This may be undertaken if cranial MRI has been performed but spinal imaging may have been be deferred, as a substantial percentage of patients will respond to one or more EBPs. In this case, the EBP is placed at lumbar or thoracolumbar locations. The response offers some diagnostic information in that the diagnosis is supported. Volumes range from small (10 mL) to large (100 mL), but generally a small volume, 10–20 mL, is used for the first EBP. In patients that do not respond, or for those who respond but have relapsing symptoms, larger-volume EBP is recommended. We usually perform larger-volume patching at two levels, both the thoracolumbar junction and lower lumbar level. The volume of blood varies based on the patient’s anatomy, and is limited primarily by local or radicular pain. Success rates of EBP in SIH have not been well-studied, but are clearly much lower than those seen in post-dural puncture headaches [5]. Some patients require several EBPs, with a minimum of five days between procedures. When the level of the leak is known, patching can be directed to that level.
Epidural patching with fibrin sealant is usually guided via X-ray or CT to specific confirmed or suspected leak location(s).
Surgical
Surgical repair is used when simpler measures have been unsuccessful or lack durability, when the anatomy of the leak precludes non-surgical approaches, and when symptom severity justifies intervention. The specific procedure is tailored to the individual, depending upon the specific anatomy and location of the spinal CSF leak. Materials commonly used for dural repair include sutures, titanium aneurysm clips, muscle, Gelfoam, fibrin sealant, and artificial dura [1]. Posterior CSF leaks and leaks along nerve roots are often, although not always, easier to access surgically. The approach for ventral leaks is evolving. Currently, we approach ventral leaks at the cervical level with corpectomy and discectomy, ventral thoracic leaks with a transdural and transpedicular approach, and ventral lumbar leaks with a direct approach between the nerve roots. For CSF–venous fistulae with direct flow from the subarachnoid space to spinal epidural veins, we clip or cauterize the venous channel [54••].
In patients who have failed repeated EBPs and for whom imaging has been unsuccessful in locating the leak, an infusion of saline into the epidural space may offer symptomatic benefit. We have a small cohort of patients who have an implanted epidural catheter with subcutaneous port for regular infusions of saline.
A few patients with intractable symptoms have derived benefit from lumbar dural reduction surgery to decrease spinal CSF volume, and therefore increase cranial CSF volume and pressure; this can lead to a durable reduction in symptom severity, as compared with a temporary improvement following EBP [59].
Screening for Connective Tissue and Vascular Abnormalities
Due to the high prevalence of heritable disorders of connective tissue and related stigmata that may not currently meet diagnostic criteria for well-known disorders such as Marfan syndrome and Ehlers-Danlos syndrome, patients with SIH should be screened for HDCT.
Vascular abnormalities occur with higher frequency in this population; intracranial aneurysms have been reported in 9 % of SIH patients, compared to 1 % of controls, so consideration should be given to screening for intracranial aneurysms [35•]. Echocardiography should be considered to screen for aortic root dilatation, which is more prevalent in the spontaneous SIH patient population [37]. Individuals with Marfan syndrome, familial aortic aneurysms, and the vascular form of Ehlers–Danlos syndrome are at risk for large arterial aneurysms and should be screened with MRA of neck, chest, abdomen, and pelvis. This screening may be indicated for a subset of other SIH patients on the basis of clinical findings and family history.
Management of Serious Complications
Many of the clinical complications associated with SIH resolve with treatment, but commentary regarding some of the more serious complications is important.
Subdural hematomas are not an infrequent complication of SIH [10–16]. While surgical evacuation may be necessary, this may not always be the case, since many will resolve with treatment of the underlying spinal CSF leak. The subdural hematomas are almost always chronic, with or without an acute hemorrhage component. Craniotomy to evacuate the subdural hematoma may precipitate a worsening of the spinal CSF leak.
Cerebral sinus thrombosis is managed with anticoagulation.
An awareness that serious complications such as coma can arise from SIH is important so that urgent treatment of the CSF leak can be undertaken [24–27].
Outcomes
Prognosis for most patients with SIH is favorable. An unknown percentage of patients have symptoms that resolve spontaneously in a matter of hours, days, or weeks, without ever seeking medical care. Of those that do present for care, outcomes data remain somewhat limited.
Patients with a normal cranial MRI appear to have a poorer prognosis [60]. The reasons for this are unclear. These patients may have different mechanisms to compensate for loss of CSF volume than those with typical imaging findings, or a fraction of these patients may not have had a CSF leak.
A substantial percentage of patients respond favorably, with a durable result, to one or more EBPs. When EBPs are unsuccessful or lack durability, spinal imaging findings help to guide further treatment, often epidural patching with fibrin sealant directed at the leak location, or surgery.
Surgical repair of spinal CSF leaks have success rates of 80 % to 95 %, depending on the complexity and location of the leak. The highest success rate is seen for direct surgical repair of ventral CSF leaks or meningeal diverticula associated with a CSF leak on myelography. Short-term outcomes for repairs of recently reported CSF–venous fistulae between the subarachnoid space and spinal epidural veins have been favorable [54••].
Patients may notice a change in their headache pattern following successful epidural patching or surgical repair, typically waking from sleep with headache and improvement with upright positioning. The headache location and character are usually, but not always, distinct from the headache related to CSF leakage, and may be associated with nausea and emesis. This has been found to be indicative of elevated intracranial pressure, termed “rebound intracranial hypertension” [5, 61, 62]. It is suspected that within a short period of time from the onset of spinal CSF leaking, the CSF production rate is up-regulated; subsequently, following successful treatment, it may take days, weeks, or months for CSF production to return to normal. While it has not been well-studied to date, this rebound intracranial hypertension usually resolves uneventfully over the course of weeks to months, although we have seen symptoms persist for years. Acetazolamide is often used for treatment, although milder cases may not require treatment. Uncommonly, in severe cases that are left untreated, visual loss may occur. CSF shunting procedures are rarely recommended at our institution. Further study is needed to determine optimal management strategies for rebound intracranial hypertension.
In patients who have had a successful procedure, about 10 % will experience a recurrence over a period of 10 years, usually with a different leak location.
Research and Future Developments
While clinical research is ongoing, there is much need for additional study to better understand the genetics and pathophysiology of dural weakness as well as physiologic compensatory mechanisms, to continue to refine imaging modalities and treatment approaches, and to evaluate short- and long-term clinical outcomes.
A genome-wide association study may be able to clarify the role of genetics in spontaneous spinal CSF leaks. A better understanding of molecular pathways leading to dural weakness would provide targets for prevention and/or stabilization of dural pathology.
Epidural patching techniques may be broadened to compare outcomes using whole blood, platelet-rich plasma, fibrin sealant, or other novel preparations.
It is anticipated that surgical approaches will become less invasive and will result in superior outcomes.
Conclusions
Diagnosis of spontaneous intracranial hypotension should be considered on the basis of clinical presentation. While the most common presenting symptom is orthostatic headache, it is now known that the clinical presentation can vary substantially and that a range of serious complications can arise. An underlying weakness of the spinal dura is often found, with a significant proportion of patients meeting diagnostic criteria for heritable disorders of connective tissue. All patients should be screened for connective tissue abnormalities, and a subset of these patients may benefit from screening for vascular complications such as intracranial and large arterial aneurysms.
Cranial MRI with enhancement should be performed in all patients, and will reveal one or more of the typical findings in 80 % of cases. CT myelography, MR myelography, and digital subtraction myelography are the imaging modalities of choice for identifying spinal CSF leak. Imaging protocols continue to be refined, with increasing diagnostic sensitivity. Digital subtraction myelography can be helpful in identifying rapid leaks and leaks ventral to the spinal cord, as well as more recently appreciated CSF–venous fistulae between the subarachnoid space and spinal epidural veins that may not be evident on other imaging. Intrathecal gadolinium-enhanced MR myelography occasionally identifies a leak not seen on CT myelography.
Treatments for spinal CSF leaks include conservative approaches, epidural patching with autologous whole blood or fibrin sealant, and surgery. Approaches will continue to evolve and to achieve better outcomes.
Rebound intracranial hypertension following successful treatment is not uncommon but is usually self-limited.
While prognosis is generally favorable, the subset of patients with normal cranial MRI at diagnosis tends to have a poorer prognosis [60]. Both short-and long-term recurrence does occur in those who have had successful treatment.
A better understanding of the genetics and pathophysiology of dural weakness may lead to strategies for prevention of recurrent leaks or vascular complications. We may find more effective ways to support physiologic compensatory responses in order to reduce symptoms in refractory cases. Imaging modalities will become more sensitive and treatment approaches will continue to improve, leading to better short- and long-term clinical outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286–96.
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks. Cephalalgia. 2008;28:1345–56.
Amoozegar F, Guglielmin D, Hu W, Chan D, Becker WJ. Spontaneous intracranial hypotension: recommendations for management. Can J Neurol Sci. 2013;40:144–57.
Mokri B. Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Headache. 2013;53:1034–53.
Mokri B. Spontaneous CSF, leaks: low CSF volume syndromes. Neurol Clin. 2014;32:397–422.
Schievink WI, Schwartz MS, Maya MM, Moser FG, Rozen TD. Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks. J Neurosurg. 2012;116:749–54.
Schievink WI, Maya MM, Moser F, Tourje J, Torbati S. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8:325–8.
Hébert-Blouin MN, Mokri B, Shin AY, Bishop AT, Spinner RJ. Cerebrospinal fluid volume-depletion headaches in patients with traumatic brachial plexus injury. J Neurosurg. 2013;118:149–54.
Schievink WI, Chu RM, Maya MM, Johnson JP, Cohen HC. Spinal manifestations of spontaneous intracranial hypotension. J Neurosurg Spine. 2013;18:96–101.
Schievink WI, Maya MM, Moser FG, Tourje J. Spectrum of subdural fluid collections in spontaneous intracranial hypotension. J Neurosurg. 2005;103:608–13.
Lai TH, Fuh JL, Lirng JF, Tsai PH, Wang SJ. Subdural haematoma in patients with spontaneous intracranial hypotension. Cephalalgia. 2007;27:133–8.
Schievink WI, Maya MM, Pikul BK, Louy C. Spontaneous spinal cerebrospinal fluid leaks as the cause of subdural hematomas in elderly patients on anticoagulation. J Neurosurg. 2010;112:295–9.
Beck J, Gralla J, Fung C, Ulrich CT, Schucht P, Fichtner J, Andereggen L, Gosau M, Hattingen E, Gutbrod K, Z'Graggen WJ, Reinert M, Hüsler J, Ozdoba C, Raabe A. Spinal cerebrospinal fluid leak as the cause of chronic subdural hematomas in nongeriatric patients. J Neurosurg. 2014 Jul 18:1–8. [Epub ahead of print]
Chotai S, Kim JH, Kim JH, Kwon TH. Brain herniation induced by drainage of subdural hematoma in spontaneous intracranial hypotension. Asian J Neurosurg. 2013;8:112–5.
Hashizume K, Watanabe K, Kawaguchi M, Fujiwara A, Furuya H. Evaluation on a clinical course of subdural hematoma in patients undergoing epidural blood patch for spontaneous cerebrospinal fluid leak. Clin Neurol Neurosurg. 2013;115:1403–6.
Wang J, Zhang D, Gong X, Ding M. Rapid resolution of subdural hematoma after targeted epidural blood patch treatment in patients with spontaneous intracranial hypotension. Chin Med J. 2014;127:2063–6.
Schievink WI, Maya MM. Cerebral venous thrombosis in spontaneous intracranial hypotension. Headache. 2008;48:1511–9.
Costa P, Del Zotto E, Giossi A, Volonghi I, Poli L, Frigerio M, et al. Headache due to spontaneous intracranial hypotension and subsequent cerebral vein thrombosis. Headache. 2012;52:1592–6.
Mokri B. Movement disorders associated with spontaneous CSF leaks: A case series. Cephalalgia. 2014 Apr 11. [Epub ahead of print]
Schievink WI, Maya MM. Quadriplegia and cerebellar hemorrhage in spontaneous intracranial hypotension. Neurology. 2006;66:1777–8.
Schievink WI, Maya MM, Nuño M. Chronic cerebellar hemorrhage in spontaneous intracranial hypotension: association with ventral spinal cerebrospinal fluid leaks: clinical article. J Neurosurg Spine. 2011;15:433–40.
Tsai PH, Wang SJ, Lirng JF, Fuh JL. Spontaneous intracranial hypotension presenting as mental deterioration. Headache. 2005;45:76–80.
Wicklund MR, Mokri B, Drubach DA, Boeve BF, Parisi JE, Josephs KA. Frontotemporal brain sagging syndrome: an SIH-like presentation mimicking FTD. Neurology. 2011;76:1377–82.
Kashmere JL, Jacka MJ, Emery D, Gross DW. Reversible coma: a rare presentation of spontaneous intracranial hypotension. Can J Neurol Sci. 2004;31:565–8.
Schievink WI, Moser FG, Pikul BK. Reversal of coma with an injection of glue. Lancet. 2007;369:1402.
Schievink WI, Palestrant D, Maya MM, Rappard G. Spontaneous spinal cerebrospinal fluid leak as a cause of coma after craniotomy for clipping of an unruptured intracranial aneurysm. J Neurosurg. 2009;110:521–4.
Loya JJ, Mindea SA, Yu H, Venkatasubramanian C, Chang SD, Burns TC. Intracranial hypotension producing reversible coma: a systematic review, including three new cases. J Neurosurg. 2012;117:615–28.
Schievink WI. Stroke and death due to spontaneous intracranial hypotension. Neurocrit Care. 2013;18:248–51.
Schievink WI, Louy C. Precipitating factors of spontaneous spinal CSF leaks and intracranial hypotension. Neurology. 2007;69:700–2.
Mokri B, Maher CO, Sencakova D. Spontaneous CSF leaks: underlying disorder of connective tissue. Neurology. 2002;58:814–6.
Mokri B. Familial occurrence of spontaneous spinal CSF leaks: underlying connective tissue disorder. Headache. 2008;48:146–9.
Schievink WI, Gordon OK, Tourje J. Connective tissue disorders with spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension: a prospective study. Neurosurgery. 2004;54:65–70.
Reinstein E, Pariani M, Bannykh S, Rimoin DL, Schievink WI. Connective tissue spectrum abnormalities associated with spontaneous cerebrospinal fluid leaks: a prospective study. Eur J Hum Genet. 2013;21:386–90.
Schievink WI, Maya MM, Louy C, Moser FG, Sloninsky L. Spontaneous intracranial hypotension in childhood and adolescence. J Pediatr. 2013;163:504–10.
Schievink WI, Maya MM. Frequency of intracranial aneurysms in patients with spontaneous intracranial hypotension. J Neurosurg. 2011;115:113–5. Important to recognize higher prevalence of intracranial aneurysms in those with SIH.
Schievink WI, Raissi SS. Spontaneous intracranial hypotension in patients with bicuspid aortic valve. J Heart Valve Dis. 2012;21:714–7.
Pimienta AL, Rimoin DL, Pariani M, Schievink WI, Reinstein E. Echocardiographic findings in patients with spontaneous CSF leak. J Neurol. 2014 Jul 25. [Epub ahead of print]
Schrijver I, Schievink WI, Godfrey M, Meyer FB, Francke U. Spontaneous spinal cerebrospinal fluid leaks and minor skeletal features of Marfan syndrome: a microfibrillopathy. J Neurosurg. 2002;96:483–9.
Schievink WI, Gordon OK, Hyland JC, Ala-Kokko L. Absence of TGFBR2 mutations in patients with spontaneous spinal CSF leaks and intracranial hypotension. J Headache Pain. 2008;9:99–102.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808.
Schievink WI, Maya MM, Louy C, Moser FG, Tourje J. Diagnostic criteria for spontaneous spinal CSF leaks and intracranial hypotension. AJNR Am J Neuroradiol. 2008;29:853–6.
Schievink WI, Dodick DW, Mokri B, Silberstein S, Bousser MG, Goadsby PJ. Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache. 2011;51:1442–4. Most recent diagnostic criteria.
Watanabe A, Horikoshi T, Uchida M, Koizumi H, Yagishita T, Kinouchi H. Diagnostic value of spinal MR imaging in spontaneous intracranial hypotension syndrome. Am J Neuroradiol. 2009;30:147–51.
Schievink WI. Novel neuroimaging modalities in the evaluation of spontaneous cerebrospinal fluid leaks. Curr Neurol Neurosci Rep. 2013;13:358. Recent advances in imaging.
Wang YF, Lirng JF, Fuh JL, Hseu SS, Wang SJ. Heavily T2-weighted MR myelography vs CT myelography in spontaneous intracranial hypotension. Neurology. 2009;73:1892–8.
Akbar JJ, Luetmer PH, Schwartz KM, Hunt CH, Diehn FE, Eckel LJ. The role of MR myelography with intrathecal gadolinium in localization of spinal CSF leaks in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2012;33:535–40.
Chazen JL, Talbott JF, Lantos JE, Dillon WP. MR Myelography for Identification of Spinal CSF Leak in Spontaneous Intracranial Hypotension. Am J Neuroradiol. 2014 May 22. [Epub ahead of print]
Schievink WI, Maya MM, Tourje J. False localizing sign of C1–2 cerebrospinal fluid leak in spontaneous intracranial hypotension. J Neurosurg. 2004;100:639–44.
Luetmer PH, Schwartz KM, Eckel LJ, Hunt CH, Carter RE, Diehn FE. When should I do dynamic CT myelography? Predicting fast spinal CSF leaks in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2012;33:690–4.
Luetmer PH, Mokri B. Dynamic CT myelography: a technique for localizing high-flow spinal cerebrospinal fluid leaks. AJNR Am J Neuroradiol. 2003;24:1711–4.
Hoxworth JM, Trentman TL, Kotsenas AL, Thielen KR, Nelson KD, Dodick DW. The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. Am J Roentgenol. 2012;199:649–53.
Carstensen M, Chaudhary N, Leung A, Ng W. Supine digital subtraction myelography for the demonstration of a dorsal cerebrospinal fluid leak in a patient with spontaneous intracranial hypotension: a technical note. J Radiol Case Rep. 2012;6:1–9.
Schievink WI, Maya MM. Ventral spinal cerebrospinal fluid leak as the cause of persistent post-dural puncture headache in children. J Neurosurg Pediatr. 2013;11:48–5.
Schievink W, Moser F, Maya M. CSF–venous fistula in spontaneous intracranial hypotension: demonstration by digital subtraction myelography. Neurology. 2014;83:472–3. Recently described type of spinal CSF leak.
Mokri B. Radioisotope Cisternography in Spontaneous CSF Leaks: Interpretations and Misinterpretations. Headache. 2014 Jul 4. [Epub ahead of print]
Mokri B, Low PA. Orthostatic headaches without CSF leak in postural tachycardia syndrome. Neurology. 2003;61:980–2.
Wallman D, Weinberg J, Hohler AD. Ehlers–Danlos Syndrome and Postural Tachycardia Syndrome: A relationship study. J Neurol Sci. 2014;340:99–102.
Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87:1214–25.
Schievink WI. A novel technique for treatment of intractable spontaneous intracranial hypotension: lumbar dural reduction surgery. Headache. 2009;49:1047–51.
Schievink WI, Maya MM, Louy C. Cranial MRI predicts outcome of spontaneous intracranial hypotension. Neurology. 2005;64:1282–4.
Mokri B. Intracranial hypertension after treatment of spontaneous cerebrospinal fluid leaks. Mayo Clin Proc. 2002;77:1241–6.
Kranz PG, Amrhein TJ, Gray L. Rebound Intracranial Hypertension: A Complication of Epidural Blood Patching for Intracranial Hypotension. AJNR Am J Neuroradiol. 2014;35:1237–40.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Wouter I. Schievink and Dr. Constance R. Deline each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Secondary Headache
Rights and permissions
About this article
Cite this article
Schievink, W.I., Deline, C.R. Headache Secondary to Intracranial Hypotension. Curr Pain Headache Rep 18, 457 (2014). https://doi.org/10.1007/s11916-014-0457-9
Published:
DOI: https://doi.org/10.1007/s11916-014-0457-9