Abstract
Purpose of Review
Type 2 diabetes mellitus (T2DM) is associated with an increased fracture risk. Weight loss in T2DM management may result in lowering of bone mass. In this systematic literature review, we aimed to investigate how exercise affects bone health in people with T2DM. Furthermore, we examined the types of exercise with the potential to prevent and treat bone fragility in people with T2DM.
Recent Findings
Exercise differs in type, mechanical load, and intensity, as does the osteogenic response to exercise. Aerobic exercise improves metabolic health in people with T2DM. However, the weight-bearing component of exercise is essential to bone health. Weight loss interventions in T2DM induce a loss of bone mass that may be attenuated if accompanied by resistance or weight-bearing exercise.
Summary
Combination of weight-bearing aerobic and resistance exercise seems to be preventive against excessive bone loss in people with T2DM. However, evidence is sparse and clinical trials investigating the effects of exercise on bone health in people with T2DM are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) and osteoporosis are major public health concerns associated with increased morbidity and mortality globally [1, 2]. T2DM is related to chronic metabolic derangements that may affect multiple organs and lead to serious complications [3]. The International Diabetes Federation (IDF) estimated in 2017 that 425 million (8.8%) adults had diabetes mellitus (DM), 90% of which had T2DM [1]. Osteoporosis is characterized as a state of low bone mineral density (BMD) as well as weakened bone microstructure resulting in reduced bone strength and elevated fracture risk [4]. Osteoporosis is estimated to affect over 200 million people worldwide [2].
In patients with T2DM, the risk of fractures is increased and not sufficiently predicted by BMD estimated by dual-energy X-ray absorptiometry (DXA) scans [5,6,7]. The fracture risk may be triggered by a deficit in bone quality, though the bone abnormalities in diabetes are not fully clarified [8].
Exercise is recommended as prevention and treatment in both osteoporosis and T2DM and accounts for a great number of health benefits, e.g., preventing cardiovascular events and muscle and bone loss. Exercise is most often referred to as “aerobic” and addressed by cardiorespiratory fitness (maximal oxygen consumption, VO2max) [2, 9,10,11]. Aerobic exercise includes weight-bearing, e.g., jogging, brisk walking, tennis, and soccer, and non-weight-bearing, e.g., swimming and cycling [11]. Resistance exercise refers to strengthening of muscle groups by the use of resistance machines, free weights, or bands, and flexibility exercise refers to stretching or yoga [11]. Weight-bearing and resistance exercises provide a fundamental and beneficial mechanical load to the skeleton [12].
The terms “physical activity” and “exercise” differ slightly in meaning. “Physical activity” refers to any movement of the body resulting in an increase in energy expenditure, whereas “exercise” is planned or structured physical activity [13, 14]. Strong evidence supports that exercise can prevent and treat T2DM [9, 15,16,17]. IDF states that “physical activity is most effective when it includes a combination of both aerobic exercise and resistance training, as well reduction of sedentary time” [1]. Conversely, bone tissue responds differentially to varying types of exercise. Thus, knowledge about tissue-specific metabolic alterations elicited by exercise in patients with T2DM is essential.
A combination of resistance and weight-bearing aerobic exercise is shown to increase BMD of the femoral neck and lumbar spine in postmenopausal women [18]. Strain exercise of high amplitude and low frequency, e.g., locomotion, running, and jumping [19], or of low amplitude and high frequency, e.g., vibration [20], has been shown to stimulate BMD in bone segments exposed to the strain.
This review aims to analyze and critically evaluate the literature regarding optimal exercise strategies for promoting bone health as well as preventing bone fragility and fractures in people suffering from T2DM. Firstly, the regulatory aspects of bone metabolism in normal conditions and in patients with T2DM will be examined. Secondly, we will evaluate current knowledge on bone adaptation to different types of exercise. Lastly, the content of the systematic literature search regarding the effects of exercise on bone health in patients with T2DM will be discussed.
Methodology
The PRISMA guidelines were followed [21]. A systematic literature search was performed in the database Medline via PubMed using the terms “diabetes mellitus,” “insulin resistance,” “exercise,” “physical activity,” “bones,” “osteoporosis,” and “fractures” (full search string schematization in Supplementary Table 1). Additional relevant articles referenced in the included records were also reviewed for eligibility. The inclusion criteria were studies examining correlations and associations between exercise and bone health in people with T2DM or prediabetes/insulin resistance. Only human studies were eligible. Studies in which the presence of T2DM or exercise was merely adjusted for statistically were not included. Osteoarthritis and joint pain were not considered relevant bone-related outcomes. Records on children (age < 18 years) were excluded. All records were included regardless of the language of the record. The search was limited to records from 2004 or later. Case reports and series, posters, commentaries, and conference abstracts were excluded. The final literature search was performed on February 25, 2020.
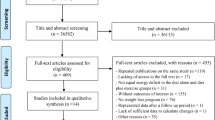
In total, 389 records were identified, and 24 articles (Table 1) were included based on the inclusion criteria (see Supplementary Fig. 1 for detailed exclusion process).
Bone Metabolism and Regulation
The relationship between body weight and bone size was acknowledged in the seventeenth century by Galileo [45]. The ability of bone mass to adjust metabolic need and physical strain is pivotal in order to prevent bone fragility and fractures. The following section describes the regulation of bone metabolism and outlines the changes in people with T2DM.
Biochemical Regulation of Bone Metabolism
Optimal skeletal structure is dependent on balanced bone remodeling, i.e., strict control of bone resorption and formation [46]. Regulation of bone metabolism includes several metabolically active agents, both endocrine and paracrine, but is highly influenced by exogenous factors as well, such as mechanical load and strain [12].
The number of circulating markers of bone turnover has increased over the last decade and some are used in the clinical setting in addition to BMD, such as C-terminal telopeptide of type 1 collagen (CTX) and N-terminal propeptide of type 1 procollagen (P1NP), which serve to monitor the activity of bone resorption and formation, respectively [47].
Osteocytes are strain-sensitive cells that release controlling factors of bone formation and resorption in response to external mechanical stimuli [12]. Exposure to mechanical forces is crucial in maintaining bone mass equilibrium [48]. The absence of mechanical strain increases osteocyte gene expression of the glycoprotein sclerostin in rodents [49]. Sclerostin reduces bone formation by inhibition of the Wnts; small proteins are secreted from osteocytes to stimulate osteoblast differentiation and promote bone formation [50]. Furthermore, sclerostin signals through increased osteoblastic expression of receptor activator of nuclear factor-kappa beta ligand (RANKL) to stimulate osteoclasts and promote bone resorption [12]. Osteoclasts initialize bone resorption by adhering to the underlying bone and secreting acidic proteases that degrade bone tissue and generate circulating products of resorption, e.g., CTX and Isoform 5b of tartrate resistant acid phosphatase (TRACP5b) [51].
Osteoblasts are primers of bone formation at the resorption site and perform bone mineralization which releases products of formation, e.g., P1NP [52]. In addition to RANKL and sclerostin as regulators of bone, osteoprotegerin (OPG) is produced by osteoblasts and functions as a decoy receptor for RANKL [46, 53].
Osteocalcin (OC) is another osteoblast-secreted protein. OC undergoes decarboxylation and activation by the acidic environment created by osteoclasts in the extracellular matrix [54]. Undercarboxylated OC (ucOC) has been suggested to perform endocrine functions, e.g., inducing insulin expression [54].
Bone-Specific Alterations in T2DM
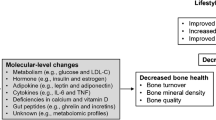
Impaired bone quality appears to be multifactorial and affected by several modifiable and non-modifiable factors [55]. Compromised insulin pathways in T2DM are assumed to cause a deficit in bone structure, reduced osteoblast activity, and a lower number of osteoclasts [56]. The increased fracture risk in T2DM predominantly pertains to the hip (relative risks approx. 1.4–2.0) [5, 6, 57], but vertebral and humerus fractures are also increased [5, 6, 58, 59]. A central diagnostic criterion of osteoporosis is based on BMD measurements by DXA scan and is defined as a T-score below − 2.5 SD [60]. Despite an increased fracture risk, people with T2DM have 5–10% higher BMD than people without T2DM [5, 61]. Thus, diagnosis of fragile bones in people with T2DM is underestimated by BMD and by FRAX [62], which emphasizes the importance of prevention strategies.
Meta-analyses have shown decreased bone turnover in people with T2DM compared with people without DM [63, 64]. This involves decreased circulating levels of CTX, P1NP, and OC and increased OPG and sclerostin [64]. However, bone-specific alkaline phosphatase (ALP) is reported as normal or increased, suggesting that the bone matrix may become hypermineralized in patients with T2DM [65].
Exercise-Induced Metabolic Responses in Bone Tissue
Bone is a metabolically active tissue similar to muscle and adipose tissue [66] and requires flexibility to promote adaptations during mechanical load and increased energy demand [67]. Chronic and acute changes in whole-body metabolism are reported to affect bone turnover [68]. This indicates that bone tissue provides more than just mechanical strength to the body. However, the exercise-induced metabolic response in bone tissue seems to differ from muscle and adipose tissue, mainly depending on mechanical loading [69]. In the following, we evaluate how bone tissue responds when exposed to different types of exercise.
Exercise-Induced Changes in Bone Turnover
Bone tissue is commonly believed to respond to mechanical load by stimulation of the mechanosensors of the osteocytes. However, short-term studies report bone turnover markers to be affected in response to not only acute weight-bearing [19, 70,71,72] but also non-weight-bearing [73, 74] and resistance [26•, 75] exercise.
It is generally accepted that sclerostin expression in rodents decreases in response to loading and increases in response to inactivity [49, 76, 77]. One study reports increases in CTX and sclerostin 5 min after both weight-bearing and non-weight-bearing exercise based on intensity-matched interval exercise on either bike or treadmill in healthy young men [78]. The same study also reported a return of both sclerostin and CTX to baseline after 1 and 28 h of rest, respectively. However, the increase in sclerostin observed 5 min after exercise may reflect a release of stored sclerostin rather than increased production (Fig. 1). In humans, the majority of long-term studies report decreasing sclerostin levels in response to exercise, e.g., based on self-reported physical activity (minutes per week), after 2 months of moderate exercise 120 min four times per week [79], after 12 months of resistance or jump exercise three times a week [70], or after 12 weeks of cardio exercise [80]. All of these studies collected blood samples in the fasted state and after a minimum of 12 h following the last exercise session.
Exercise-induced biochemical bone response. (1) Exercise induces load and decreases sclerostin expressed by osteocytes. (2) Immediately after high-intensity exercise, stored sclerostin may be released to the circulation. (3) Due to temporary RANK binding, bone resorption increases and CTX is released. (4) However, after time Wnt binding by sclerostin may be reduced, leading to more free Wnt. (5) Wnt leads to increased osteoblast activation, increasing bone formation and P1NP release. (6) Bone formation leads to increased OC and ucOC levels. (7) Insulin sensitivity increases, inhibiting OPG. (8) Inhibition of OPG leads to less RANKL inhibition, facilitating increased resorption. (9) Moderate exercise favors bone formation, increasing OPG and P1NP and decreasing CTX, suggesting RANKL binding by OPG
An acute aerobic exercise session, e.g., 80–90% of maximal capacity, seems to increase the circulating bone resorption marker CTX with no significant response in the formation marker P1NP in healthy participants [71, 73, 81, 82]. However, circulating P1NP is also found to increase in response to exercise [81, 83] (Fig. 1). Alkahtani et al. reported increased levels of P1NP after running at 60% of maximal capacity for 40 min independent of running flat or downhill. P1NP levels returned to baseline after 24 h [83•]. No differences in CTX were observed immediately or 24 h after exercise.
The high-intensity exercise study did not observe any difference in P1NP [78], suggesting that high-intensity exercise favors immediate bone resorption, whereas moderate-intensity exercise favors bone formation (Fig. 1). This is supported by increased resting levels of P1NP 1 month after moderate endurance exercise for 90 min 3–4 times a week compared with before exercise and compared with sedentary controls [84].
A randomized controlled trial (RCT) including healthy men with osteopenia (T-score between − 1.0 and − 2.5) estimated bone turnover markers after 6 months of supervised jumping or resistance exercise [19]. The authors reported increased OC after 6 months and a further increase after 12 months in both interventions [19] (Fig. 1). In addition, the study reported a reduction in CTX after 6 months and a return to baseline after 12 months.
Findings concerning the impact of exercise on bone turnover markers are conflicting and depending on exercise type and duration of exercise intervention as well as timing of the sample collection. However, the majority or studies suggest that bone resorption markers respond to acute and high-intensity exercise and swiftly return to baseline. In contrast, the long-term effects of exercise reported are in favor of bone formation possibly by decreased sclerostin levels (Fig. 1).
Exercise-Induced Effects on Bone Structure
The International Osteoporosis Foundation states that a 10% loss of spine BMD doubles the risk of vertebral fractures and a 10% loss of hip BMD increases fracture risk in the hip by a factor of 2.5 [2]. Walking is shown to prevent age-related reduction in hip BMD, although it does not affect spine BMD [85, 86]. The previously cited RCT by Hinton et al. found a 0.6% increase in whole-body BMD and a 1.3% increase in spine BMD after 6 months, which was maintained after 12 months in both exercise groups (jumping or resistance) [19]. Total hip BMD was only increased (0.8%) in the resistance exercise group after 6 months with no further improvement after 12 months [19].
It is estimated that a combination of aerobic and resistance exercise reduces BMD loss in both spine and hip by 3.2% and 1.0%, respectively [11]. Approximately 60% of the variation in bone strength can be attributed to variation in BMD, measured by DXA [87]. More recent measuring methods have reported the natural age-related bone loss to be located in cortical bone rather than trabecular bone: a distinction which cannot be made by DXA [88, 89]. Resistance exercise is reported to increase cortical bone density measured by peripheral quantitative computed tomography (pQCT) [90, 91]. The high-resolution pQCT (HRpQCT) enables more accurate estimation of trabecular and cortical bone structure. However, the use of HRpQCT in exercise intervention studies is sparse. Thus, RCTs estimating changes in bone microstructure in response to exercise are warranted.
Exercise-Induced Effects on Bone Compartments
Haddock et al. investigated the metabolic and hemodynamic responses to exercise in healthy volunteers using whole-body positron emission tomography (PET) and magnetic resonance imaging (MRI) [92•]. They measured bone [18F]NaF-uptake and fitted time-activity curves to a three-compartment (one of which represents fluoride binding into the bone matrix) tracer kinetic model, whereby they could estimate bone mineralization using non-linear regression. They reported a higher resting mineralization in trabecular than in cortical bone. However, after a stepping exercise producing a higher strain on the left than on the right leg, bone mineralization increased in cortical bone and decreased in trabecular bone with no difference between left and right leg.
Bone marrow is another important facilitator of bone remodeling. Heinonen et al. found increased bone marrow glucose uptake during exercise using PET and tracer technique [93•]. Additionally, they found increasing glucose uptake in bone marrow with increasing exercise intensity but with no significant difference between moderate and high intensity. Furthermore, the ratio between glucose uptake in bone marrow and muscle tissue decreased significantly with higher exercise intensity from less than a half to less than one-third, indicating glucose uptake in favor of muscles rather than bone during higher-intensity exercise.
The ability of tracer techniques to reveal direct bone metabolism in response to exercise may enable further knowledge of the bone-specific metabolic differences or inconsistent response between trabecular and cortical components. These changes may identify regional differences or improper responses to external stimuli inside bone that may not be detectable by neither circulating markers nor structural imaging.
The Potential of Bone-Induced Effects on Glucose Metabolism
OC and ucOC are found to increase in men in response to acute high-intensity exercise [23] as well as after long-term exercise [33, 94] (Fig. 1). Some studies report that ucOC, but not total OC, increases acutely after exercise and correlates positively with insulin sensitivity [23, 35], although another study found no change in OC after 12 weeks of exercise [32]. Insulin may also inhibit expression of OPG in osteoblasts and thereby stimulate bone resorption resulting in lower pH at the resorption sites [12] (Fig. 1). Low pH allows decarboxylation and activation of OC (Fig. 1). Still, the evidence for an effect of ucOC on glucose metabolism in humans is very limited and the hypothesis is based on an animal model [95] which is not sufficiently supported by human trials.
The Effect of Exercise on T2DM and Bones
Low cardiorespiratory fitness is an independent predictor of mortality in people with T2DM [15, 96]. Today, weight loss achieved through diet and exercise is a standard recommendation when encountering a newly diagnosed T2DM patient [97]. A common goal is 150 min of exercise per week [98]. However, exercise per se is not necessarily beneficial for all metabolic compartments, e.g., muscle, adipose, and bone tissue. Contrarily, patients with osteoporosis are recommended to ensure adequate nutrient intake and specifically weight-bearing exercise, as the osteoporosis phenotype is often quite different from the patient with T2DM. The effect of exercise on bone health is not only determined by the mechanical load and type of exercise but also by substrate metabolism, insulin sensitivity, and glucose disposal. In the following, effects of exercise on bone health in patients with T2DM will be discussed.
Exercise as a Hindrance to or Facilitator of Bone Loss in T2DM
Low lifelong physical activity appears to be associated with lower BMD, higher fracture risk, and T2DM in one study [43], but another study reports no effect of physical activity on BMD in T2DM based on WHO questionnaires [41]. Large cohort studies not only confirmed the increased fracture risk among men and women with T2DM but also reported that those with higher physical activity had lower fracture risk [37, 42]. A cross-sectional study on former rugby players found lower prevalence of T2DM but higher prevalence of osteoporosis [39]. A small RCT (n = 14) did not find any difference in BMD after 32 weeks of aerobic or resistance exercise in postmenopausal women with prediabetes [31]. Another study reported that healthy exercise habits were associated with higher BMD in patients with T2DM [44]. However, Nilsson et al. confirmed higher BMD (DXA-scan) and cortical porosity (HRpQCT) among elderly women with T2DM but interestingly found reduced physical performance, i.e., walking speed and one-leg standing, compared with people without T2DM [40]. In addition, Nilsson et al. only included elderly women aged over 75 years and found a significantly higher BMI among participants with T2DM. Paccou et al. estimated volumetric BMD (vBMD) by HRpQCT in people with and without T2DM with equal self-reported physical activity [8]. They reported higher cortical bone density, pore volume, and porosity in participants with T2DM compared with those without T2DM [8].
Skoradal et al. performed a mixed-gender RCT investigating the effect of soccer on bone health in elderly people with prediabetes [24••]. Femoral (neck, trochanter, and shaft) and lumbar spine BMD increased by 2.5–3.9% after soccer training 30–60 min twice a week for 16 weeks. As a combination of aerobic exercise and mechanical load, soccer may be an easily accessible exercise type to improve bone health in weight-bearing sites, i.e., spine and hip. However, it is possible that the majority of patients with diagnosed T2DM have physical limitations. Thus, soccer may be related to greater risk of falling and injuries, overturning the beneficial effects on bone structure.
Vibration may be an effective exercise form in T2DM patients with physical limitations. It is found to effectively improve lumbar spine and femoral neck BMD in people without DM [20]. In patients with T2DM, vibration exercise has been suggested to enhance glycemic control and decrease HbA1c [99]. However, studies on humans are sparse and the evidence is mostly based on animal trials [100].
These conflicting results may be caused by different methods of assessment of physical performance in (e.g., questionnaires [44] versus tests [40]). Even though BMD loss can be reduced in elderly, it is possible that physical activity during adolescence is an important factor to prevent BMD loss during adulthood.
Prevention of Bone Loss During Weight Loss
In 2005, Daly et al. reported that progressive resistance training in patients with T2DM should be combined with dietary modification in order to prevent a decrease in BMD [36].
An RCT by Courteix et al. reported beneficial and protective effects of exercise in participants with metabolic syndrome (MetS) who were compliant to the exercise program [29]. This was quantified by a reduction of bone loss during weight loss in participants compliant with the exercise intervention compared with those who were non-compliant [29]. An RCT by Beavers et al. investigated structural and biochemical bone alterations in people with MetS exposed to weight loss alone or in combination with either aerobic or resistance exercise [25••]. The planned weight loss was 0.3 kg/week with a total of 7–10% body weight loss over 18 months. The exercise sessions were either aerobic by walking or resistance exercise for 45 min four times a week. They observed a greater weight loss when dietary intervention was combined with exercise. Furthermore, they reported a significant reduction in total hip BMD by 2% in all groups after 18 months of intervention. However, after 30 months, they observed an increased lumbar spine BMD and attenuated BMD loss in the hip in the resistance exercise group but not in the aerobic exercise group. Estimation of vBMD by computed tomography (CT) scan suggested a beneficial effect in the resistance exercise group by a smaller reduction in vBMD [25••] which was supported by another RCT [34]. They did not observe any differences in bone turnover markers [25•]. Thus, resistance exercise enabled bone tissue to adapt to whole-body requirements and maintain bone mass despite whole-body weight loss. However, it seems that a significant loss of BMD persists in the first year following a weight loss intervention in obese adult [101]. It is possible that bone loss in obese people after diet-induced weight loss can be prevented by GLP-1 receptor agonists, a commonly used drug in the treatment of T2DM [102].
The Look AHEAD trial has previously confirmed a significant bone loss at the hip and spine in both men and women with T2DM after 1 year of intensive lifestyle intervention including weight loss and physical activity [27••]. After 3 years of weight maintenance, the bone loss proceeded in the hip in men but not in women [27••]. Further analyses revealed a 39% increased risk of frailty fracture but no difference in incident fracture risk [28]. This observed increase in risk of frailty fractures was mainly driven by pelvic and hip fractures and may be due to falls related to the physical activity. However, the absolute number of frailty fractures was relatively low [28].
Obesity is a major burden in T2DM, but it seems that intensive weight loss induces bone loss years after the intervention and may even increase the risk of fragility fractures. However, the addition of weight-bearing exercise may induce an osteogenic response, adaptation, and the ability to maintain bone mass and prevent fractures despite whole-body weight loss.
Exercise-Induced Biochemical Bone Responses in T2DM
Human studies on the acute effect of exercise on bone turnover markers in subjects with T2DM are sparse [22, 38]. Borer et al. designed a study on postmenopausal women with T2DM to reveal the effects of meals on the osteogenic response before and after up- and downhill treadmill exercise [22••]. They did not find any differences in CTX. However, they found higher circulating levels of C-terminal propeptide of type I collagen (C1CP), a marker of bone formation, after up- or downhill exercise 60 min after a standardized meal compared with the same exercise in the fasting state: with the highest levels measured after the uphill exercise. Rᾰska et al. reported that women with T2DM and daily walking activity less than 2 h had higher sclerostin levels compared with those walking more than 2 h daily [38].
Exercise enhances insulin sensitivity and improves glycemic control in patients with T2DM [103,104,105], both after aerobic [106] and resistance exercise [107]. However, the effect is most pronounced when a combination is used, suggesting a synergistic effect between aerobic and resistance exercise when treating people with T2DM [105, 108]. Hur et al. reported increased circulating levels of OPG in women with MetS after moderate resistance exercise 3 days a week for 5 weeks with an additional decrease in HOMA-IR [26•] (Fig. 1). Borer et al. reported a lower HOMA-IR after uphill exercise following a meal compared with an equivalent downhill exercise [22••]. Furthermore, they discovered a delayed rise in the ratio of C1CP/CTX when exercising uphill compared with downhill. This finding may be an expression of increased muscle energy expenditure in the beginning of uphill exercise, compared with the lower cardio-respiratory intensity and higher mechanical load on the skeleton during downhill exercise. The rise in cardio-respiratory requirement may favor muscle energy supply compared with bone [93•]. However, a T2DM rat model suggests that non-weight-bearing exercise, e.g., swimming, may mitigate suppressed bone turnover based on higher RANKL/OPG levels [109] (Fig. 1).
In addition to higher BMD after 16 weeks of soccer training in participants with prediabetes, Skoradal et al. found 23–52% increased bone turnover markers, i.e., osteocalcin, CTX, and P1NP, after the intervention period [24••]. The ratio between P1NP and CTX also increased with no changes observed in the control group. This indicates a stimulated bone formation relative to bone resorption and an anabolic response to the combined weight-bearing and aerobic exercise intervention.
In summary, the biochemical bone response to exercise is poorly investigated in T2DM. However, it seems that sclerostin decreases after exercise along with increasing formation markers. In addition, aerobic exercise with high cardio-respiratory requirement may benefit muscle tissue in favor of bone remodeling compared with a bone-protective effect after low-intensity or combined aerobic and resistance exercise.
Discussion
Exercise has proven pivotal in the prevention and treatment of both T2DM and osteoporosis. A study including 450 participants with T2DM identified that over 70% had knowledge about the effect of exercise in prevention of osteoporosis [110]. However, only half of the population identified weight-bearing exercise as important [110]. Based on the current evidence, the optimal osteogenic stimulus in people without T2DM seems to be obtainable with a combination of both resistance and aerobic exercise. This is similar to the effect on glycemic control, suggesting a synergistic effect [105, 108]. However, clinical trials investigating effects of exercise on bone-specific outcomes in people with T2DM are sparse. Thus, this review is limited by the number of published studies.
Bone resorption appears to be stimulated shortly after exercise by increased levels of sclerostin and CTX (both aerobic and weight-bearing) in healthy adults. Knowledge about the short-term effects of exercise in T2DM is sparse. However, two studies [22, 23] report increased bone formation compared with resorption. The overall long-term effects of exercise in people with T2DM seem to be driven by an increase in bone turnover in favor of formation, e.g., decreased sclerostin, increased P1NP/CTX-ratio, and increased BMD.
The combination of increased hip fracture risk and weight loss–associated BMD loss at the hip is troublesome in patients with T2DM. Hip BMD benefits from resistance exercise and walking more than spine BMD does. This may explain why physical activity (and not exercise per se), e.g., walking, has been shown to reduce hip fractures in both men and women [2]. Intensive weight loss in patients with T2DM may benefit from accompanying resistance exercise to reduce bone loss.
The presented studies include data on both men and women and on both structural and biochemical bone outcomes. Most of the current knowledge on bone health is based on BMD measurements by DXA scans. Only one of the presented intervention studies measured bone microstructure [34], and only one study reported data on biochemical bone measurements in participants diagnosed with T2DM after an exercise intervention [22••]. However, increased cortical porosity may be an important estimate when appraising bone health in patients with T2DM [8]. Thus, studies investigating the long-term effects of exercise on bone microstructure, e.g., by HRpQCT, are warranted. Currently, results from an RCT comparing standard T2DM care with a supervised exercise program for 2 years regarding bone-related outcomes are awaited [111].
The exercise protocols differ greatly among the included studies, e.g., exercise intensity, duration, and mechanical load, making it difficult to compare study results. Meta-analyses of graded exercise intensities in T2DM patients found that both aerobic and resistance exercise with higher intensity resulted in greater reduction in HbA1c compared with lower intensity exercise studies [17, 112, 113]. However, the presented results mainly include participants with prediabetes or MetS and cannot conclude if current weight loss recommendations and exercise strategies are sufficient in order to prevent bone loss in T2DM. Future studies investigating the effect of exercise on bone health in T2DM could simplify the exercise modality by focusing on the known beneficial effects on glycemic control and test if this applies to bone outcomes. Hence, exercise intervention RCTs on T2DM patients including measurements of bone markers, e.g., CTX, P1NP, and sclerostin, and bone microstructure are of great interest. The measurements of bone markers should be performed in a standardized steady state along with microstructural measurements before and after a minimum of 3-month intervention period. Lastly, it would be interesting to test if anti-diabetic drugs, e.g., GLP-1 receptor agonists and metformin, impact the potential exercise-induced protective effects on bones in T2DM.
In conclusion, the evidence behind the beneficial effects of aerobic exercise and weight loss on physical health and glycemic control in people with T2DM is persuasive. Weight-bearing exercise during weight loss is paramount in the prevention of bone fragility and fractures. Thus, when guiding patients with T2DM, it may be favorable to encourage a combination of weight-bearing aerobic and resistance exercise, e.g., downhill running, jumping, or alternating mechanical loading sessions, as well as ensuring adequate nutrition supply prior to the exercise session. Personalized diet and exercise strategies that favor both metabolic and bone health are advisable in order to reduce bone loss and fracture risk in people with T2DM.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
International Diabetes Federation (2017) IDF Diabetes Atlas, 8th ed. IDF Diabetes Atlas, 8th Ed. https://doi.org/10.1016/S0140-6736(16)31679-8.
International Osteoporosis Foundation. The global burden of osteoporosis: a factsheet. Found: Int. Osteoporos; 2014.
Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017. https://doi.org/10.1016/S2213-8587(17)30097-9.
Compston JE, Mcclung MR, Leslie WD. Seminar osteoporosis. Lancet. 2019;393:364–76. https://doi.org/10.1016/S0140-6736(18)32112-3.
Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes - a meta-analysis. Osteoporos Int. 2007;18:427–44. https://doi.org/10.1007/s00198-006-0253-4.
Forsén L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag health survey. Diabetologia. 1999;42:920–5. https://doi.org/10.1007/s001250051248.
Baleanu F, Bergmann P, Hambye AS, Dekelver C, Iconaru L, Cappelle SI, et al. Assessment of bone quality with trabecular bone score in type 2 diabetes mellitus: a study from the FRISBEE cohort. Int J Clin Pract. 2019;73:e13347. https://doi.org/10.1111/ijcp.13347.
Paccou J, Ward KA, Jameson KA, Dennison EM, Cooper C, Edwards MH. Bone microarchitecture in men and women with diabetes: the importance of cortical porosity. Calcif Tissue Int. 2016;98:465–73. https://doi.org/10.1007/s00223-015-0100-8.
Hayes C, Kriska A. Role of physical activity in diabetes management and prevention. J Am Diet Assoc. 2008;108:S19–23. https://doi.org/10.1016/j.jada.2008.01.016.
Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254–62.
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011. https://doi.org/10.1002/14651858.cd000333.pub2.
Bilezikian JP (ed) (2019) Primer on the metabolic bone diseases and disorders of mineral metabolism, Ninth Edi. https://doi.org/ISBN: 978–1–119-26656-3.
Howell J. The 1996 surgeon general’s report on physical activity and health. Nurse Pract Forum. 1996;7:104.
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–79.
Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132:605–11.
Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. In: Diabetes Care; 2006. p. 1433–8.
Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia. 2003;46:1071–81.
Marín-Cascales E, Alcaraz PE, Ramos-Campo DJ, Rubio-Arias JA. Effects of multicomponent training on lean and bone mass in postmenopausal and older women: a systematic review. Menopause. 2018;25:346–56. https://doi.org/10.1097/GME.0000000000000975.
Hinton PS, Nigh P, Thyfault J. Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: a 12-month randomized, clinical trial. Bone. 2015;79:203–12.
Marín-Cascales E, Alcaraz PE, Ramos-Campo DJ, Martinez-Rodriguez A, Chung LH, Rubio-Arias J. Whole-body vibration training and bone health in postmenopausal women: a systematic review and meta-analysis. Med (United States). 2018;97:e11918. https://doi.org/10.1097/MD.0000000000011918.
Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. J Clin Epidemiol. 2016;70:68–89. https://doi.org/10.1016/j.jclinepi.2015.09.001.
•• Borer KT, Zheng Q, Jafari A, Javadi S, Kernozek T. Nutrient intake prior to exercise is necessary for increased osteogenic marker response in diabetic postmenopausal women. Nutrients. 2019. https://doi.org/10.3390/nu11071494C1CP levels were higher after exercise sessions preceded by a meal compared with the same exercise in the fasting state. The highest levels were found after the uphill exercise.
Levinger I, Jerums G, Stepto NK, Parker L, Serpiello FR, McConell GK, et al. The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. J Bone Miner Res. 2014;29:2571–6.
•• Skoradal MB, Helge EW, Jørgensen NR, Mortensen J, Weihe P, Krustrup P, et al. Osteogenic impact of football training in 55- to 70-year-old women and men with prediabetes. Scand J Med Sci Sport. 2018. https://doi.org/10.1111/sms.13252Soccer training increased BMD after 16 weeks at lumbar spine and femur in elderly people with prediabetes.
•• Beavers KM, Walkup MP, Weaver AA, et al. Effect of exercise modality during weight loss on bone health in older adults with obesity and cardiovascular disease or metabolic syndrome: a randomized controlled trial HHS public access. J Bone Min Res. 2018;33:2140–9 Greater weight loss when dietary intervention was combined with exercise along with bone loss. Possible long-term attenuation of weight loss induced bone loss when combined with resistance exercise and not aerobic exercise.
• Hur S, Cho SH, Song BK, Cho BJ. Effect of resistance exercise on serum osteoprotegerin levels and insulin resistance in middle-aged women with metabolic syndrome. Med Sci Monit. 2018. https://doi.org/10.12659/MSM.911548Resistance exercise induced decreased insulin resistance and higher OPG levels in women with metabolic syndrome.
•• Lipkin EW, Schwartz AV, Anderson AM, et al. The Look AHEAD trial: bone loss at 4-year follow-up in type 2 diabetes. Diabetes Care. 2014. https://doi.org/10.2337/dc14-0762Bone loss at the hip and spine in both men and women with T2DM after one year of intensive lifestyle intervention including weight loss and physical activity. After three years the bone loss proceeded with increased risk of frailty fractures.
Johnson KC, Bray GA, Cheskin LJ, Clark JM, Egan CM, Foreyt JP, et al. The effect of intentional weight loss on fracture risk in persons with diabetes: results from the Look AHEAD randomized clinical trial. J Bone Miner Res. 2017;32:2278–87.
Courteix D, Valente-Dos-Santos J, Ferry B, et al. Multilevel approach of a 1-year program of dietary and exercise interventions on bone mineral content and density in metabolic syndrome - the RESOLVE randomized controlled trial. PLoS One. 2015;10:e0136491. https://doi.org/10.1371/journal.pone.0136491.
Al-Shreef FM, Al-Jiffri OH, Abd El-Kader SM. Bone metabolism and hand grip strength response to aerobic versus resistance exercise training in non-insulin dependent diabetic patients. Afr Health Sci. 2015;15:896–901. https://doi.org/10.4314/ahs.v15i3.25.
Bello M, Sousa MC, Neto G, Oliveira L, Guerras I, Mendes R, et al. The effect of a long-term, community-based exercise program on bone mineral density in postmenopausal women with pre-diabetes and type 2 diabetes. J Hum Kinet. 2014;43:43–8.
Jiang J, Boyle LJ, Mikus CR, Oberlin DJ, Fletcher JA, Thyfault JP, et al. The effects of improved metabolic risk factors on bone turnover markers after 12 weeks of simvastatin treatment with or without exercise. Metabolism. 2014;63:1398–408. https://doi.org/10.1016/j.metabol.2014.07.011.
Hinton PS, Rector RS, Linden MA, Warner SO, Dellsperger KC, Chockalingam A, et al. Weight-loss-associated changes in bone mineral density and bone turnover after partial weight regain with or without aerobic exercise in obese women. Eur J Clin Nutr. 2012;66:606–12. https://doi.org/10.1038/ejcn.2011.212.
Kemmler W, Bebenek M, von Stengel S, Engelke K, Kalender WA. Effect of block-periodized exercise training on bone and coronary heart disease risk factors in early post-menopausal women: a randomized controlled study. Scand J Med Sci Sport. 2013;23:121–9. https://doi.org/10.1111/j.1600-0838.2011.01335.x.
Fernández-Real JM, Izquierdo M, Ortega F, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009;94:237–45. https://doi.org/10.1210/jc.2008-0270.
Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE, Zimmet PZ. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int. 2005;16:1703–12.
Huang H-L, Pan C-C, Hsiao Y-F, Chen M-C, Kung C-Y, Kung P-T, et al. Associations of body mass index and diabetes with hip fracture risk: a nationwide cohort study. BMC Public Health. 2018;18:1325.
Raška I, Rašková M, Zikán V, Škrha J. Prevalence and risk factors of osteoporosis in postmenopausal women with type 2 diabetes mellitus. Cent Eur J Public Health. 2017. https://doi.org/10.21101/cejph.a4717.
Davies MAM, Judge AD, Delmestri A, Kemp SPT, Stokes KA, Arden NK, et al. Health amongst former rugby union players: a cross-sectional study of morbidity and health-related quality of life. Sci Rep. 2017;7:11786.
Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellström D, Rudäng R, et al. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res. 2017;32:1062–71. https://doi.org/10.1002/jbmr.3057.
Kamalanathan S, Nambiar V, Shivane V, Bandgar T, Menon P, Shah N. Bone mineral density and factors influencing it in Asian Indian population with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2014;18:831–7. https://doi.org/10.4103/2230-8210.140268.
Melton LJ, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res. 2008;23:1334–42. https://doi.org/10.1359/jbmr.080323.
Korpelainen R, Korpelainen J, Heikkinen J, Vaananen K, Keinanen-Kiukaanniemi S. Lifelong risk factors for osteoporosis and fractures in elderly women with low body mass index--a population-based study. Bone. 2006;39:385–91.
De Luis Román DA, Aller R, Perez Castrillon JL, De Luis J, Gonzalez Sagrado M, Izaola O, et al. Effects of dietary intake and life style on bone density in patients with diabetes mellitus type 2. Ann Nutr Metab. 2004;48:141–5. https://doi.org/10.1159/000078376.
(1648) Discorsi e Dimostrazioni Matematiche. Discorsi e Dimostrazioni Mat. https://doi.org/10.1016/c2013-0-13317-x
Tyrovola JB, Odont XX. The “mechanostat theory” of frost and the OPG/RANKL/RANK system. J Cell Biochem. 2015;116:2724–9. https://doi.org/10.1002/jcb.25265.
Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28:2541–56.
Rosa N, Simoes R, Magalhães FD, Marques AT. From mechanical stimulus to bone formation: a review. Med Eng Phys. 2015;37:719–28. https://doi.org/10.1016/j.medengphy.2015.05.015.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. https://doi.org/10.1074/jbc.M705092200.
Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007;21:2605–14.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42.
Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011;343:289–302.
Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5.
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord. 2015;16:93–8.
Jilka RL, O’Brien CA. The role of osteocytes in age-related bone loss. Curr Osteoporos Rep. 2016;14:16–25. https://doi.org/10.1007/s11914-016-0297-0.
Al-Hariri M. Sweet bones: the pathogenesis of bone alteration in diabetes. J Diabetes Res. 2016;2016:1–5. https://doi.org/10.1155/2016/6969040.
Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the nurses’ health study. Diabetes Care. 2006;29:1573–8. https://doi.org/10.2337/dc06-0440.
Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–8. https://doi.org/10.1210/jcem.86.1.7139.
Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: the Tromsø study. Osteoporos Int. 2006;17:495–500. https://doi.org/10.1007/s00198-005-0013-x.
(1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Heal Organ - Tech Rep Ser 843:1–129.
Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, et al. Risk of fracture in women with type 2 diabetes: the women’s health initiative observational study. J Clin Endocrinol Metab. 2006;91:3404–10.
Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27:301–8. https://doi.org/10.1002/jbmr.556.
Starup-Linde J, Eriksen SA, Lykkeboe S, Handberg A, Vestergaard P. Biochemical markers of bone turnover in diabetes patients - a meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporos Int. 2014;25:1697–708. https://doi.org/10.1007/s00198-014-2676-7.
Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. Diabetes mellitus, a state of low bone turnover-a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137–57. https://doi.org/10.1530/EJE-16-0652.
Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus - a systematic review. Bone. 2016;82:69–78. https://doi.org/10.1016/j.bone.2015.02.019.
De Paula FJA, Rosen CJ. Bone remodeling and energy metabolism: new perspectives. Bone Res. 2013;1:72–84. https://doi.org/10.4248/BR201301005.
Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;25:1027–36. https://doi.org/10.1016/j.cmet.2017.04.015.
Fuglsang-Nielsen R, Starup-Linde J, Gregersen S, Vestergaard P. The effect of meals on bone turnover - a systematic review with focus on diabetic bone disease. Expert Rev Endocrinol Metab. 2018;13:233–49. https://doi.org/10.1080/17446651.2018.1518131.
Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–54.
Hinton PS, Nigh P, Thyfault J. Serum sclerostin decreases following 12 months of resistance- or jump-training in men with low bone mass. Bone. 2017;96:85–90.
Rantalainen T, Heinonen A, Linnamo V, Komi PV, Takala TES, Kainulainen H. Short-term bone biochemical response to a single bout of high-impact exercise. J Sport Sci Med. 2009;8:553–9.
Scott JPR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J Appl Physiol. 2011;110:423–32.
Guillemant J, Accarie C, Peres G, Guillemant S. Acute effects of an oral calcium load on markers of bone metabolism during endurance cycling exercise in male athletes. Calcif Tissue Int. 2004;74:407–14.
Barry DW, Hansen KC, van Pelt RE, Witten M, Wolfe P, Kohrt WM. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med Sci Sports Exerc. 2011;43:617–23.
Pasqualini L, Ministrini S, Lombardini R, Bagaglia F, Paltriccia R, Pippi R, et al. Effects of a 3-month weight-bearing and resistance exercise training on circulating osteogenic cells and bone formation markers in postmenopausal women with low bone mass. Osteoporos Int. 2019;30:797–806. https://doi.org/10.1007/s00198-019-04908-9.
Galea GL, Lanyon LE, Price JS. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone. 2017;96:38–44. https://doi.org/10.1016/j.bone.2016.10.008.
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–34. https://doi.org/10.1007/s00198-011-1656-4.
Kouvelioti R, LeBlanc P, Falk B, Ward WE, Josse AR, Klentrou P. Effects of high-intensity interval running versus cycling on sclerostin, and markers of bone turnover and oxidative stress in young men. Calcif Tissue Int. 2019;104:582–90.
Ardawi MSM, Rouzi AA, Qari MH. Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab. 2012;97:3691–9. https://doi.org/10.1210/jc.2011-3361.
Janik M, Stuss M, Michalska-Kasiczak M, Jegier A, Sewerynek E. Effects of physical activity on sclerostin concentrations. Endokrynol Pol. 2018. https://doi.org/10.5603/EP.a2018.0008.
Scott JPR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running. J Clin Endocrinol Metab. 2010;95:3918–25.
Dolan E, Varley I, Ackerman KE, Pereira RMR, Elliott-Sale KJ, Sale C. The bone metabolic response to exercise and nutrition. Exerc Sport Sci Rev. 2019;1.
• Alkahtani SA, Yakout SM, Reginster JY, Al-Daghri NM. Effect of acute downhill running on bone markers in responders and non-responders. Osteoporos Int. 2019;30:375–81 Higher P1NP after running at 60% of maximal capacity for 40 minutes independent of running flat or downhill. P1NP levels returned to baseline after 24 hours.
Adami S, Gatti D, Viapiana O, Fiore CE, Nuti R, Luisetto G, et al. Physical activity and bone turnover markers: a cross-sectional and a longitudinal study. Calcif Tissue Int. 2008;83:388–92.
Kachroo S, Kawabata H, Colilla S, Shi L, Zhao Y, Mukherjee J, et al. Association between hypoglycemia and fall-related events in type 2 diabetes mellitus: analysis of a U.S. commercial database. J Manag care Spec Pharm. 2015;21:243–53.
Moreira LDF, de Oliveira ML, Lirani-Galvão AP, Marin-Mio RV, dos Santos RN, Lazaretti-Castro M. Physical exercise and osteoporosis: effects of different types of exercises on bone and physical function of postmenopausal women. Arq Bras Endocrinol Metabol. 2014;58:514–22. https://doi.org/10.1590/0004-2730000003374.
Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13–8.
Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–36.
Zebaze R, Seeman E. Cortical bone: a challenging geography. J Bone Miner Res. 2015;30:24–9. https://doi.org/10.1002/jbmr.2419.
Polidoulis I, Beyene J, Cheung AM. The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women - a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2012;23:39–51. https://doi.org/10.1007/s00198-011-1734-7.
Hong AR, Kim SW. Effects of resistance exercise on bone health. Endocrinol Metab. 2018;33:435. https://doi.org/10.3803/EnM.2018.33.4.435.
• Haddock B, Fan AP, Uhlrich SD, Jørgensen NR, Suetta C, Gold GE, et al. Assessment of acute bone loading in humans using [18F]NaF PET/MRI. Eur J Nucl Med Mol Imaging. 2019. https://doi.org/10.1007/s00259-019-04424-2Possible novel use of a tracer kinetic model to estimate bone mineralization in trabecular and cortical bone during and after exercise.
• Heinonen I, Kemppainen J, Fujimoto T, Knuuti J, Kalliokoski KK. Increase of glucose uptake in human bone marrow with increasing exercise intensity. Int J Sport Nutr Exerc Metab. 2019. https://doi.org/10.1123/ijsnem.2018-0094PET and tracer technique enables measurement of glucose uptake in bone marrow, which was reported higher when exercise intensity rises with no significant difference between moderate and high intensity.
Kim YS, Nam JS, Yeo DW, Kim KR, Suh SH, Ahn CW. The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin Endocrinol. 2015;82:686–94. https://doi.org/10.1111/cen.12601.
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–75. https://doi.org/10.1016/j.bone.2011.04.017.
Blair SN, Kohl HW, Barlow CE, Gibbons LW, Paffenbarger RS, Macera CA. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. JAMA J Am Med Assoc. 1995;273:1093–8.
Johnson EL, Feldman H, Butts A, et al. Standards of medical care in diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11–34.
Who WHO. Global recommendations on physical activity for health. Geneva World Heal Organ. 2010;48:57. https://doi.org/10.1080/11026480410034349.
Baum K, Votteler T, Schiab J. Efficiency of vibration exercise for glycemic control in type 2 diabetes patients. Int J Med Sci. 2007;4:159–63. https://doi.org/10.7150/ijms.4.159.
Yin H, Berdel HO, Moore D, Davis F, Liu J, Mozaffari M, et al. Whole body vibration therapy: a novel potential treatment for type 2 diabetes mellitus. Springerplus. 2015;4:578. https://doi.org/10.1186/s40064-015-1373-0.
Kammire DE, Walkup MP, Ambrosius WT, Lenchik L, Shapses SA, Nicklas BJ, Houston DK, Marsh AP, Rejeski WJ, Beavers KM (2019) Effect of weight change following intentional weight loss on bone health in older adults with obesity. Obesity 27:1839–1845.
Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jørgensen NR, et al. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. 2015;100:2909–17. https://doi.org/10.1210/jc.2015-1176.
Conn VS, Koopman RJ, Ruppar TM, Phillips LJ, Mehr DR, Hafdahl AR. Insulin sensitivity following exercise interventions: systematic review and meta-analysis of outcomes among healthy adults. J Prim Care Community Heal. 2014;5:211–22. https://doi.org/10.1177/2150131913520328.
Madsen SM, Thorup AC, Overgaard K, Jeppesen PB. High intensity interval training improves glycaemic control and pancreatic β cell function of type 2 diabetes patients. PLoS One. 2015;10:e0133286. https://doi.org/10.1371/journal.pone.0133286.
Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–69. https://doi.org/10.7326/0003-4819-147-6-200709180-00005.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–6. https://doi.org/10.2337/dc10-1548.
Lee JH, Kim DH, Kim CK. Resistance training for glycemic control, muscular strength, and lean body mass in old type 2 diabetic patients: a meta-analysis. Diabetes Ther. 2017;8:459–73. https://doi.org/10.1007/s13300-017-0258-3.
Pan B, Ge L, Xun Y qin, et al (2018) Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act https://doi.org/10.1186/s12966-018-0703-3, 15, 72.
Pezhman L, Sheikhzadeh Hesari F, Ghiasi R, Alipour MR. The impact of forced swimming on expression of RANKL and OPG in a type 2 diabetes mellitus rat model. Arch Physiol Biochem. 2019;125:195–200. https://doi.org/10.1080/13813455.2018.1446178.
Abdulameer SA, Sahib MN, Syed Sulaiman SA, Hassali MA. A comprehensive view of knowledge and osteoporosis status among type 2 diabetes mellitus in Malaysia: a cross sectional study. Pharm Pract (Granada). 2019. https://doi.org/10.18549/PharmPract.2019.4.1636.
Balducci S, Conti F, Sacchetti M, et al. Study to Weigh the Effect of Exercise Training on BONE quality and strength (SWEET BONE) in type 2 diabetes: study protocol for a randomised clinical trial. BMJ Open. 2019. https://doi.org/10.1136/bmjopen-2018-027429.
Liubaoerjijin Y, Terada T, Fletcher K, Boulé NG. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769–81. https://doi.org/10.1007/s00592-016-0870-0.
Liu Y, Ye W, Chen Q, Zhang Y, Kuo CH, Korivi M. Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: a systematic review and meta-analysis. Int J Environ Res Public Health. 2019;16. https://doi.org/10.3390/ijerph16010140.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors have received funding by Steno Collaborative grant, Novo Nordisk Foundation Denmark (Grant no. NNF18OC0052064). Author 1 (RV) reports personal travel fees from AstraZeneca Denmark outside the submitted work. Author 3 (JSL) reports personal fees from GSK A/S and Gilead Sciences Denmark outside the submitted work. Author 4 (SG) reports personal fees from Novo Nordisk Denmark A/S non-related to the work presented here.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Bone and Diabetes
Rights and permissions
About this article
Cite this article
Viggers, R., Al-Mashhadi, Z., Fuglsang-Nielsen, R. et al. The Impact of Exercise on Bone Health in Type 2 Diabetes Mellitus—a Systematic Review. Curr Osteoporos Rep 18, 357–370 (2020). https://doi.org/10.1007/s11914-020-00597-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00597-0