Abstract
The increased rate of fractures associated with epilepsy has been long recognised but remains incompletely understood. Study quality and study results have varied, with some but not all studies showing bone diseases including osteoporosis and/or osteomalacia, and a high prevalence of vitamin D insufficiency and deficiency are also noted. Falls risk can also be higher in patients with epilepsy taking anti-epileptic medications, potentially leading to fracture. Larger research collaborations are recommended to further advance understanding in this field, particularly to examine underlying genetic and pharmacogenomic associations of epilepsy and anti-epileptic medication usage and its association with bone diseases and fractures, as well as further investigation into optimal management of bone health in epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical problem of fractures in patients with epilepsy has been long recognised [1, 2]. The association of fractures with epilepsy appears to be multifactorial in aetiology; however, the direct mechanisms linking epilepsy and impaired bone health still require further investigation to fully define this association and to establish best management guidelines [3]. Achieving optimum control of seizures remains the primary goal of therapy, ideally in partnership with monitoring for side effects and recognised comorbidities, including monitoring and maintenance of bone health.
Epilepsy was recently redefined from the previous practically applied definition of having two unprovoked seizures more than 24 h apart, to include a single unprovoked (or reflex) seizure where the probability of further seizures is >60 %, i.e. similar to the risk of further seizures occurring after two unprovoked seizures; however, anti-epileptic medication (AEM) treatment decisions after a first seizure remain individualised [4]. Osteoporosis is defined by the WHO as a bone mineral density (BMD) 2.5 standard deviations or more below the average value for a young adult (T-score < −2.5 SD) while osteopenia is defined as a T-score between −1 and 2.5 SD below the young adult mean [5, 6]. Dual-energy x-ray absorptiometry (DXA) is the most frequently utilised clinical investigation [7], but it should also be noted that testing BMD can have high specificity but low sensitivity, as many osteoporotic fractures may occur with osteopenia or a normal test [8]. Changes in bone quality may not be reflected in DXA results [9] but are likely to affect fracture risk. Risk factors for osteoporosis—all of which may also occur in patients with epilepsy, should also be taken into consideration [8–10].
A number of studies have reported an increased risk of fractures in association with epilepsy [11], with fractures both during seizures and at other times; some but not all studies have reported reduced bone mineral density [12], and further longitudinal studies of bone microarchitecture are warranted to explore bone quality in association with epilepsy and its treatment. Falls risk and balance impairment likely contribute to the risk of fracture at times other than during seizures. The expansion of indications for use of AEMs, including migraine, chronic pain, bipolar affective disorder and other psychiatric indications, increases the need to better understand the association of fracture risk and impaired bone health in epilepsy and to determine whether this is at least in part a result of AEM side effects [13]; however, studies of bone health relating to the non-epilepsy indications of AEMs are not reviewed here.
Several reviews have been written on the topic of bone disease in association with epilepsy and its therapy [14–20]. A recent review provides detailed comparison of data on prevalence of bone disease in epilepsy and examines general and specific risk factors [14]. Other reviews provide detailed data up to the year 2010 on individual AEMs [21], and others highlight proposed mechanisms for the multiple abnormalities seen in bone metabolism in associated with AEM usage [18, 22]; therefore, this article will review key aspects of past and current research regarding fractures and osteoporosis associated with epilepsy and anti-epileptics and identify suggested areas of further research.
Methods
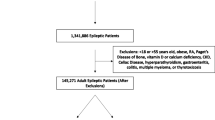
Databases searched included Ovid MEDLINE, PsycINFO (Ovid), CINAHL Plus, EMBASE, Informit Health Collection & Informit Humanities & Social Sciences Collection and Cochrane Library. Search strategy is shown in Fig. 1. In total, 635 results remained after duplicates were removed: 305 remained after abstracts were screened for relevance, and 43 secondary references were identified from reference lists. Therefore, 348 abstracts were further assessed and 209 excluded (e.g. conference references, papers unrelated to topic). Reviews and case studies were retained in addition to full text original articles (total 139 papers). Results were limited to English language (except for secondary references) but not by date.
Discussion
While the association between anti-epileptic medication usage and bone disease was first reported in the 1960s [18], the precise nature and aetiology of the problem still requires further explanation. The explanation for the observed increase in fracture risk in patients with epilepsy is likely multifactorial, including fractures during seizures, and fractures at other times, including as a result of falls and impaired balance due to medication side effects, or underlying neurological lesions [23]. In the case of fractures due to impaired bone quality or reduced bone density, multiple factors are again likely, including metabolism of vitamin D from cytochrome p450 enzyme-inducing AEMs [24•], endocrine factors and potentially direct side effects from some AEMs which still require further investigation. Further research is required to determine whether type of epilepsy is a risk factor or epilepsy itself for bone disease. In addition, known osteoporosis risk factors in the general population such as family history [25], smoking, glucocorticoid usage, early menopause and low physical activity may also occur in patients with epilepsy. Underlying causes directly linking dual pathology causing both seizures and bone deficits have not been published at this time, but potential dual mechanisms of interest would include genetics of vitamin D receptor type and ion channelopathies.
Bone Disease and Epilepsy—History
Earlier studies reported rickets in children [26], as well as bone mineral loss [27], and osteomalacia in adults with epilepsy who were residing in an institutionalised setting [28] likely due to low vitamin D secondary to cytochrome p450 liver enzyme inducer medication usage and potentially lifestyle factors including poor nutrition and low sun exposure [16]. Impairments in mobility were also associated with bone disease [16, 29] and increased fracture risk [28]. The relevance of these early studies to the modern ambulatory epilepsy patient [30] and the limitations of the available studies [31] may account at least in part for the previously noted under-recognition of bone disease in epilepsy by neurologists [32] and by patients [33]. Initially, metabolism of vitamin D to inactive forms induced by AEMs which induce the cytochrome p450 system was proposed as the mechanism underlying AEM-associated bone disease. However, studies emerged showing no difference in fracture rates between those on inducers and non-inducers [34, 35], a placebo-controlled RCT of phenytoin, which showed no effect on 25(OH)D levels over 2 years [36] and reduced BMD in patients taking valproate [37, 38]. Despite recent research attention to the issue of bone health in epilepsy, particularly after Valmadrid’s 2001 paper, there still appears to be a bone health detection and treatment lag in applying the research findings in clinical practice [33], with suboptimal screening of vitamin D, PTH and DXA scanning even in patients taking inducer AEMs [39], which is therefore likely to result in under-management of bone health [40] and limited opportunity to intervene and potentially reduce fracture risk in higher risk patients. Some patients with epilepsy continue to live in institutionalised settings, and their bone health may be affected by general and specific risk factors for osteoporosis in association with epilepsy, for instance inducer AEM use and immobility [41]; recent studies of institutionalised patients with epilepsy (many with comorbidities such as intellectual disability) have found reduced BMD and vitamin D levels, osteopenia and osteoporosis [42, 43]; therefore, monitoring and management of vitamin D and bone health should be included as standard of care in this population. Quantitative ultrasonography may be a useful tool to monitor for osteoporosis in this population, with QUS showing strong positive correlations with DXA, and relative ease of use [44]. In patients with developmental disorders and immobility as well as epilepsy, fracture risk is also elevated, with immobility likely to be a major contributor to fracture risk [29] as well as severity of seizures and underlying disorders [45]; however, an analysis of literature examining bone health in individual developmental disorders is outside the scope of this review.
Fractures
Types of fractures reported during seizures most commonly include fractures of thoracic and lumbar vertebrae [46]; therefore, fractures should be examined for in the acute setting [47], particularly to avoid late detection and management [48]. Potentially serious complications have been published including case reports of a patient with a burst lumbar vertebral fracture during seizure which resulted in acute cauda equina/conus medullaris syndrome [46] and bilateral acetabular fractures and left proximal humerus fracture [49].
A meta-analysis was performed by Shen et al. examining the association of anti-epileptic medications and fracture risk noted statistically significantly increased relative risk of fractures RR 1.86, (CI 1.62–2.12) utilising 22 studies (1,222,910 participants) using the random effects model due to the high degree of heterogeneity [2]. Shen et al. sought to exclude studies which did not adjust for potential confounders, did not supply specific risk estimates or used only benzodiazepines. As with any meta-analysis, this drew on both the quality of the studies included as well as the limitations. These authors calculated pooled estimates of specific drug effects on fracture risk, including increases in fracture risk of phenobarbiturate (78 %), phenytoin (70 %) and topiramate (39 %). The finding that carbamazepine had a non-significant effect on fracture risk was noted to not be consistent with previous studies, and the exclusion of a single study of patients with Rett syndrome (which is associated with a recognised increased fracture risk [50]) was found to increase the relative risk. Shen et al. chose an approach of AEM exposure for their inclusion criteria, rather than underlying epilepsy diagnosis, upon which they based their aim that this assessed the potential effects of the medications; the inclusion of a study of patients with Rett syndrome however appeared to affect the results of the meta-analysis, which raises the issue of confounding by indication. While the authors noted that a study by Reyes et al. [51] met their inclusion criteria, this study primarily examines for any association of proton pump inhibitors in a retrospective case–control multicentre study of 358 patients and 698 age and gender-matched controls, using a ratio of two controls per patient to achieve adequate power; of the patients with hip fracture, only 9 (2.5 %) listed epilepsy as a diagnosis versus 7 (1 %) of controls, and 11 (3 %) patients with hip fracture recorded taking an AEM versus 9 (1.3 %) of controls; fractures at other sites and vitamin D levels were not included. The adjusted conditional logistic regression gave an odds ratio for hip fractures in users of anti-epileptic medications of 3.36 (CI 1.13–9.96), although due to the limited numbers in this subgroup, the study was not well-powered to examine for effects of AEM and it was not a primary outcome measure [51]; this rate is similar to a Southern European study of men aged over 50 years where hip fracture relative risk with use of AEM was 3.16 [52], but higher than other studies such as a UK cohort study where the adjusted hazard ratio with use of inducer AEMs was 1.22 for fracture (95 % CI 1.12–1.34; p < 0.001) and 1.49 for hip fracture (1.15–1.94; p = 0.002) in women, for fracture 1.09 (0.98–1.20; p = 0.123) and hip fracture 1.53 (1.10–2.12; p = 0.011) in men [53], and a Danish study of men over 50 years discharged from hospital with a fracture, where the odds ratio for fracture associated with prescription of AEM was 1.6 [1.4–1.8] for any fracture and 1.7 [1.5–2.0] at the hip [54].
Other limitations referred to by the authors of the meta-analysis [2] included relatively fewer numbers of patients taking newer generation AEMs included in the studies used in the meta-analysis, and lingering effects of previous or concurrent older AEMs, and mode of fracture such as trauma due to seizures exaggerating effects ascribed to AEMs - factors which broadly reflect some of the limitations encountered in clinical research in the field. To add to the debate, a study of ambulatory patients with unprovoked seizures reported that the incidence of hip fractures was increased, but this was not thought to be related to AEM usage, and a consistent pattern between fracture sites, and AEM duration could not be established [55]. There has been variation in the findings of previous studies in terms of fracture risk, site and mechanism [56]. In one Australian study, 69 % of cumulative fractures were non-seizure-related [33], which appears similar to another study where 34 % of fractures occurred during seizures [25], compared to 43 % of the fractures were classed as definitely or possibly seizure-related, 22 % were not seizure-related and the remaining 35 % were not able to be classified due to incomplete records [57]; the description and severity of falls as well as retrospective reporting have limited some studies [58], and whether the fractures are due to trauma and falls, effects of AEM, or both remains to be completely understood. Fractures occurring at times other than during a seizure may potentially be due to an increased risk of falls, reduced balance and bone disease either associated with epilepsy or independent of this if there are other risk factors.
Bone Health
Cumulative exposure to AEDs [59], duration of therapy and female gender have been associated with fracture risk [34, 60•] and reduced bone mineral density [61]. Alteration of bone quality has also been suggested as a possible reason for the increased fracture risk [62]. Examination of regions of interest clinically relevant for fracture risk is recommended for assessing bone health in patients with epilepsy [10, 63]; ideally, DXA should be performed at hip, femur and lumbar spine, as reduced BMD may not be restricted to one site and the diagnosis may be missed if only lumbar spine is assessed [64]. Recently, genetic variation has been proposed to explain some of the variation in study findings regarding bone disease in epilepsy [65••], and where available, this should now be included in studies. In some studies, it has been difficult to conclude whether bone density deficits seen are due to AEMs, or the high rates of vitamin D deficiency in association with, or independent of AEM usage [66, 67].
Further data regarding newer AEMs are required [68], although some preliminary studies suggest these may have lesser association with bone disease than older agents [69]. However, some data are available showing alterations of bone metabolism with oxcarbazepine [70], gabapentin [71] and for levetiracetam [72] (in preclinical studies). In a 1-year prospective clinical study of patients newly treated with levetiracetam, no significant decreases in BMD were found [70], and similarly, a prospective 1-year study of premenopausal women taking lamotrigine did not show any detectable adverse effect on BMD [73].
Paediatric Studies
Further information is still required regarding bone health in the paediatric age group, and prospective studies which include assessment of age, seizure diagnosis, duration of AEM usage, vitamin D levels, physical activity, growth [74] and pharmacogenetics are now required, as is an assessment of any effects on attaining optimal peak bone mass in paediatric patients required to take AEM and to establish best practice for treatment [15].
Examining available literature, a Turkish study of children (mean age of approximately 9 years) taking carbamazepine or valproate for more than 1 year (mean monotherapy duration for carbamazepine was 2.6 years and valproate 2.4 years) compared to controls reported no significant difference in BMD (measured at L2-L4), no difference in calcium intake or calcium levels [75]. In contrast, a study of 13 children treated with valproate (for mean duration 3.1 years) had a reduction in BMD at L2-L4 of 14 % (p = 0.003) and a reduction of 10 % (p = 0.005) at the distal 1/3 of radius site compared to controls; the 13 children taking carbamazepine (for mean duration 3.9 years), a reduction in BMD of less than 5 % was not statistically significant. The patients and controls had similar dietary intakes and physical activity levels [38]. A Taiwanese study measured L1-L4 BMD, comparing 21 children, aged 5–18 years, and noted that there was increased frequency of low BMD in children treated with carbamazepine compared to valproate; no control participants were included [76] which is a limitation. A Korean paediatric study evaluated 143 epilepsy patients taking AEMs for a minimum of 1 year, with a mean (SD) age of 6.25 (±4.24) years (90 boys and 43 girls), and included 62 (43 %) participants with developmental delay; patients with immobility or multivitamin usage before the study were excluded. Vitamin D was measured during summer and autumn, and for those 53 patients whose 25 (OH)D3 level was lower than <30 ng/mL, DXA scanning was performed in 32 cases. The levels of 25(OH)D3 were lower in patients with mental retardation or developmental delay compared to those with normal IQ levels and also low where AEMs had been prescribed for longer than (versus shorter than) 2 years. Those taking oxcarbazepine had significantly lower 25(OH)D3 levels than those taking valproate, demonstrating a difference between an inducer and non-inducer AEMs. AEM polytherapy did not show an effect on 25(OH)D3 levels, but specific polytherapy combinations were not listed for the 53 (37 %) of patients taking polytherapy. High rates of osteopenia and osteoporosis (72.3 %) were found in association with the insufficient or deficient vitamin D levels [77]; however, due to the methodology, DXA results for those with vitamin D levels above 30 ng/mL were not available.
A Polish study [78] compared 126 children with epilepsy to 132 healthy controls, aged between 7 and 16, and examined DXA, calcium and phosphate, finding that mean BMD measured at spine and proximal femur was significantly lower in patients: 9 patients had osteoporosis and 29 patients had osteopenia, whereas no healthy controls had low BMD. There was a history or fracture in 34 % of patients, compared to 14 % of controls. In this study, 35 of 43 patients with fracture were taking valproate either as monotherapy (10 patients) or as part of polytherapy (33 patients). Analysis of serum and urine also showed lower calcium levels in blood and urine, lower serum phosphate level and higher urine phosphate levels in patients compared to controls. Vitamin D levels and pubertal staging and the mechanism of fracture were not included.
A paediatric study which included measuring height in 27 boys and 26 girls with epilepsy, aged between 3 and 17 years with mean (SD) age 9.2 (3.9) years, and treated with valproate and/or lamotrigine for two or more years found that long-term treatment with valproate and lamotrigine (particularly as polytherapy) were associated with short stature, low BMD and reduced bone formation, which they assessed to be mediated primarily through reduced physical activity rather than directly due to AEM [74].
An Italian study of 50 male and 46 female patients with epilepsy (age range 3–25 years, mean age 11) compared to a healthy control group found increased rates of osteopenia and osteoporosis, with reduced BMD in 58 % of epileptic patients (75 % of which was osteopenia and 25 % osteoporosis); the study included children with either epilepsy as a sole diagnosis or in combination with cerebral palsy and/or mental retardation. Factors which the authors found correlated to reduced BMD included lack of autonomous gait, severe mental retardation, increased duration of AEM, topiramate as adjunctive therapy and less physical activity. The finding of the correlation of reduced BMD with adjunctive topiramate is novel, and potentially may relate to renal tubular acidosis [79], but requires further investigation; their study findings related to severity of underlying developmental disorder supports findings of other studies [77]. It is noted that the ketogenic diet has also been associated with an increased risk of osteopenia and osteoporosis with progressive loss of bone mineral content particularly in children who were non-ambulant and had low body mass index [80] (although the mechanism is unclear); fractures have been observed in 6/28 patients with a median time to fracture after commencing the ketogenic diet of 1.5 years. Hence, DXA monitoring has been recommended [81].
Longitudinal Studies
A number of longitudinal studies of varying design and follow-up periods have now been published and show progressive bone loss in association with some but not all AEMs. A study of risk factors for fractures in 9516 postmenopausal white females, where 1.1 % took AEM found that women taking anticonvulsants had a higher risk of hip fractures, none of which occurred during a seizure [56].
Andress examined bone health in 81 male patients aged under 55 (mean age 45, range 25–54 years) who were attending a Veteran’s clinic [82] and showed reduction in BMD over time, and interestingly, no association of low BMD with vitamin D deficiency, hypogonadism, cigarette smoking or excess alcohol intake after correcting for age and time on AEMs [82].
A study of osteoporotic fractures in community dwelling men reported increased bone loss in those taking non-inducer AEMs, of −0.60 % per year at the hip, over a mean follow-up period of 4.6 years, compared to control participants who lost −0.35 % per year, p = 0.04, after adjusting for multiple confounders [71]. A single-practice prospective longitudinal study of women aged over 70 years in the UK found that epilepsy was a significant predictor of hip fracture over the next 3 years [83].
A Turkish study of 50 adults with epilepsy treated with valproate (the patient group consisted of 24 males and 26 females, aged 20–40 years) and 60 healthy controls reported reduced BMD compared to controls at the first visit at both lumbar spine and femur, and a reduction of BMD when rescanning was performed at a 6-month follow-up visit in the patient group of 4.9 % at the lumbar [84] spine and 4.6 % at the femur [85]. However, limitations included that vitamin D levels, sunlight exposure and vitamin D supplementation were not included in the study, and these together with further longer-term follow-up would be of interest.
Another prospective longitudinal Turkish study of patients taking valproate was performed in children (38 girls, 28 boys; mean age 6.8 ± 3.7 years; range 1 to 14 years) with serum markers and DXA performed both before valproate treatment and after 1 year (excluding from analysis patients who required polytherapy for seizure control). The authors noted that two of 61 (3.3 %) participants had developed osteoporosis, but the overall mean BMD (measured using Z-scores) did not decrease in the group over that time period [86]; limitations included that pubertal staging, vitamin D levels, patients requiring polytherapy and healthy controls were not assessed.
Further longitudinal prospective studies compared to control data would be of interest, including also longitudinal assessment of bone health optimisation and treatment strategies.
Bone Biopsy and Pathophysiology
A small number of published studies have included bone biopsy samples. A study of institutionalised patients where a subgroup of 7/13 of patients who had sustained a fracture in the preceding year underwent bone biopsy, and results showed increased resorptive activity of trabecular bone compared to controls, a degree of osteoporosis which the authors attributed to reduced mobility, as well as increased osteoid, suggestive of osteomalacia [28]. A histological study which included 11 patients with epilepsy, compared to control samples showed reduced bone formation and resorption as well as an increase in size of haversian canals [84].
Feldkamp [87] detailed a clinical study of 59 patients with epilepsy taking carbamazepine or valproate, who had significantly reduced BMD at lumbar spine compared to 55 age-matched controls, and that duration of AEM therapy was a significant factor. They also examined human osteoblast-like cells and found that with both carbamazepine and phenytoin, there were changes suggestive of inhibition of cell growth at clinically relevant drug concentrations.
Theories previously linking epilepsy and AEM to bone disease include induction of vitamin D metabolism by liver cytochrome p450 system inducer AEM [88–90], secondary hyperparathyroidism [22, 73, 91–94], increased bone turnover [37, 42, 95, 96], vitamin K inhibition due to AEM leading to bone disease [93, 97], hormonal factors in epilepsy [98], calcitonin deficiency [22, 93, 94, 99, 100] and inhibition of intestinal calcium absorption [101]. Homocysteine levels may also be of relevance to bone health in patients with epilepsy and require further investigation [102]. Nuclear pregnane-X-receptor (PXR) may also be implicated in the development of osteomalacia in association with phenobarbital use, with one study reporting that phenobarbital upregulates 25-hydroxyvitamin D(3)-24-hydroxylase (CYP24) gene expression in vitro via this mechanism [103]. Bone density and vitamin D levels are not always correlated [67, 104], and AEMs which do not induce the liver cytochrome p450 system and therefore metabolism of vitamin D have also been associated with reduction of bone mineral density (BMD) [37] in some studies. Other potential mechanisms, such as a role for ion channels, and inflammation require further investigation. Recent studies of polymorphisms of the vitamin D receptor (VDR) [105–107] have appeared promising and may explain some of the variation in results seen across the studies. VDR genotype studies to date have reported that the BsmI restriction fragment polymorphism of the VDR B allele was associated with reduced BMD in patients with epilepsy [108], and in another study of young adults with epilepsy in an ambulatory setting, the BsmI polymorphism was associated with lower BMD in patients taking phenytoin. Some early evidence links low vitamin D as having a role in immune modulation and potentially setting the stage for later development of neurological disorders potentially including types of epilepsy [109]. In a Thai study examining temporal lobe epilepsy but not bone health, the VDR genotype GAT (BsmI/ApaI/TaqI) was associated with an increased risk of temporal lobe epilepsy [110]; studies examining for direct links between epilepsy, bone health and pharmacogenomics are required.
Treatment Studies
In a study of male veterans with epilepsy, calcium, vitamin D and risedronate reduced new onset vertebral fractures, compared to the placebo group where calcium and vitamin D were taken, without risedronate [111••]. Five new vertebral fractures were detected in the placebo group (taking calcium and vitamin D) and none in the risedronate group (p = 0.0229) who also took calcium and vitamin D during the 2-year study where fractures were measured as a secondary endpoint.
The finding of calcium and vitamin D alone not preventing fractures was supported in another study of 80 male veterans with epilepsy on long-term AEMs, where these supplements with or without bisphosphonates decreased rate of bone loss and increased bone mass associated with long-term treatment with phenytoin, phenobarbital, sodium valproate or carbamazepine; however, new fractures were not prevented by supplementation with calcium and vitamin D alone [112••].
From these preliminary data, it seems that further study of specific bone therapies is required, as initial data do not support fracture prevention with calcium and vitamin D alone and also in order to establish whether this finding may be at least partly related to limitations in study power. Results of clinical trials of other bone treatments such as strontium [113] have commenced but are yet to be fully published; to our knowledge, no trials of efficacy and safety of denosumab have been published specifically examining patients with epilepsy. Larger studies of treatment are required, both in males and females, as well as consideration of safety of treatment and fracture prevention options in younger patients. Fractures occurring at a younger age in patients with epilepsy place added importance on assessment of safety, efficacy and optimal timing of treatment with bone therapies.
Management
The development of clinical guidelines would be greatly facilitated by a better understanding of the underlying causes of bone disease associated with epilepsy.
Empirically, optimum control of seizures is recommended firstly to manage epilepsy and to prevent fracture-related seizures [68]. A significant proportion of the fracture risk in epilepsy can be potentially attributed to seizures [114]. Therefore, this would ideally reduce seizure-related injuries including fractures and reduce the impact and cost of fractures on the individual and the health system [63, 96, 115]. However, results of studies have suggested variation in the mechanisms of fracture, including that there is also higher risk of fracture through mechanisms other than seizures and that monitoring for neurotoxicity is also important [116].
In the acute setting in post-ictal patients, the possibility of fracture should be considered on history and examination [47], and if there is clinical suspicion of fracture, relevant investigation, management and referrals should be requested, to avoid late detection [48].
Physician and patient education regarding bone disease and fracture risk in association with epilepsy will be important [32, 33], as well as education regarding bone protective strategies [117, 118], and being aware of (and successfully navigating) barriers to receiving appropriate screening and management of bone health in epilepsy care [119]. Modification of lifestyle factors to optimise bone health is also recommended [120], including consideration of sources of vitamin D through either sensible sunlight exposure (and/ or vitamin D supplementation), adequate calcium intake, avoidance of other bone-depleting medications, weight-bearing exercise within appropriate abilities and avoidance of both smoking and excessive alcohol intake [63, 121].
Specific study and guidelines for monitoring bone health in children, including indications, dosage and effect for vitamin D supplementation in prevention and management of bone disease in children with epilepsy, are still required [122, 123]. However, vitamin D deficiency is common in epilepsy and can be found not only with inducer AEM usage, but also with non-inducers, and therefore, monitoring is recommended [124].
Particularly in older patients, where seizures are increasingly due to underlying causes such as stroke and tumour, and where age is also an independent risk factor for osteoporosis and falls risk is important [125], monitoring for AEM toxicity, clinical consideration of bone health and falls prevention strategies are recommended [126]. In peri- and postmenopausal women taking long-term AEM, bone health and falls risk require attention alongside optimization of control of seizures [127–130]. Protecting bone health in patients with epilepsy and who are menopausal is important in management [96, 131].
Falls risk [132, 133] and balance impairment [4, 23] are increased in patients with epilepsy, potentially relating to both underlying neurologic lesions and AEM side effects or toxicities and should be included in assessment; studies of effectiveness of intervention are still required.
DXA screening is recommended [134]. All patients should have adequate calcium and vitamin D intake including supplementation where required [135], vitamin D screening and BMD screening for prolonged AED usage especially if other risk factors are present [96]. Monitoring of non-specific alkaline phosphatase is not useful for monitoring bone turnover clinically [136]. Strategies for prevention of bone loss should also be developed [137, 138]. Treatment with bisphosphonates may also be required to aim to prevent fractures in epileptic patients with osteoporosis [111••, 112••]; however, specialist advice and management is recommended, particularly noting the often younger age group, and further research is required [72, 98].
Conclusions
Ideally, international collaboration and larger studies are now required to design comparable and more standardised methodology. Adoption of an international methodology for trials to better define the problem would be of practical use, given the number of smaller studies available, which have had conflicting results [89].
Studies should be designed with collaboration between neurologists and bone specialists to be adequately powered, prospective and controlled and consider inclusion of comparable techniques such as DXA scanning, bone turnover markers, PTH, vitamin D (and vitamin D metabolite) levels, as well as screening for pharmacogenomics and vitamin D receptor (VDR) status [65••]. General osteoporosis risk factor information such as dietary intake, sunlight exposure, family history information and types and levels of physical activity should be included. The applicability of FRAX score requires further investigation as a potentially useful screening tool in epilepsy patients, and if required, the development of epilepsy-specific FRAX score should be examined and utilised with newer technologies such as DXA lateral vertebral assessment [139]. The inclusion of high resolution peripheral quantitative computed tomography (pQCT) and other techniques for studying bone microarchitecture has commenced and results will be useful to identify changes in bone quality in addition to what has been deduced by the DXA studies. Falls and balance interventions should also be included in future research planning to assess whether the rate of fractures can be reduced at least in part by balance retraining to prevent non-seizure-related falls. Tissue and laboratory studies are still required to further understand the mechanisms underlying bone fragility. Further studies examining for specific links between the development of both epilepsy and bone disease, as well as pharmacogenomics associated with epilepsy and AEM usage [106], would be of great interest. Treatment trials in this population require more data, including best pharmacotherapy for bone health, acknowledging that bone disease can be found in younger patients in this group.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Kruse R. Osteopathies, calcium- and vitamin D metabolism errors during anti-epileptic long term therapy. Bibl Psychiatr. 1975;151:114–43.
Shen C, Chen F, Zhang Y, Guo Y, Ding M. Association between use of antiepileptic drugs and fracture risk: a systematic review and meta-analysis. Bone. 2014;64:246–53. doi:10.1016/j.bone.2014.04.018.
Ali II, Schuh L, Barkley GL, Gates JR. Antiepileptic drugs and reduced bone mineral density. Epilepsy and Behavior. 2004;5(3):296–300.
Fife TD, Blum D, Fisher RS. Measuring the effects of antiepileptic medications on balance in older people. Epilepsy Res. 2006;70(2–3):103–9. doi:10.1016/j.eplepsyres.2006.03.004.
WHO Study Group, Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporosis International. 1994;4(6):368–81.
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton 3rd LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75. doi:10.1016/j.bone.2007.11.001.
Elliott ME, Binkley N. Evaluation and measurement of bone mass. Epilepsy & Behavior. 2004;5(Suppl2):S16–23. doi:10.1016/j.yebeh.2003.11.027.
Kanis J. Assessment of osteoporosis at the primary health care level. Sheffield: World Health Organization; 2007.
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129.
Salimipour H, Kazerooni S, Seyedabadi M, Nabipour I, Nemati R, Iranpour D, et al. Antiepileptic treatment is associated with bone loss: difference in drug type and region of interest. Journal of Nuclear Medicine Technology. 2013;41(3):208–11.
Pickrell WO, Lacey AS, White CP, Rees MI, Natarajan J. Fractures in people with a diagnosis of epilepsy: a population based study. Epilepsia2014. 29–30.
Mintzer S. Metabolic consequences of antiepileptic drugs. Curr Opin Neurol. 2010;23(2):164–9. doi:10.1097/WCO.0b013e32833735e7.
Arora SK, Bubb C, Karam J, McFarlane SI. Expanding use of anti-epileptic therapy: implications on bone disease. Therapy. 2007;4(1):79–89.
Beerhorst K, van der Kruijs SJ, Verschuure P, Tan IY, Aldenkamp AP. Bone disease during chronic antiepileptic drug therapy: general versus specific risk factors. Journal of the Neurological Sciences. 2013;331(1–2):19–25. doi:10.1016/j.jns.2013.05.005.
Gissel T, Poulsen CS, Vestergaard P. Adverse effects of antiepileptic drugs on bone mineral density in children. Expert Opin Drug Saf. 2007;6(3):267–78.
Hahn TJ. Bone complications of anticonvulsants. Drugs. 1976;12(3):201–11.
Svalheim S, Roste LS, Nakken KO, Tauboll E. Bone health in adults with epilepsy. Acta Neurologica Scandinavica. 2011;191:89–95. doi:10.1111/j.1600-0404.2011.01551.x.
Khanna S, Pillai KK, Vohora D. Insights into liaison between antiepileptic drugs and bone. Drug Discov Today. 2009;14(7–8):428–35. doi:10.1016/j.drudis.2009.01.004.
Miziak B, Blaszczyk B, Chroscinska-Krawczyk M, Danilkiewicz G, Jagiello-Wojtowicz E, Czuczwar SJ. The problem of osteoporosis in epileptic patients taking antiepileptic drugs. Expert Opin Drug Saf. 2014;13(7):935–46. doi:10.1517/14740338.2014.919255.
Samaniego EA, Sheth RD. Bone consequences of epilepsy and antiepileptic medications. Semin Pediatr Neurol. 2007;14(4):196–200.
Verrotti A, Coppola G, Parisi P, Mohn A, Chiarelli F. Bone and calcium metabolism and antiepileptic drugs. Clin Neurol Neurosurg. 2010;112(1):1–10. doi:10.1016/j.clineuro.2009.10.011.
Fitzpatrick LA. Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy & behavior : E&B. 2004;5 Suppl 2:S3–15. doi:10.1016/j.yebeh.2003.11.026.
Petty SJ, Hill KD, Haber NE, Paton LM, Lawrence KM, Berkovic SF, et al. Balance impairment in chronic antiepileptic drug users: a twin and sibling study. Epilepsia. 2010;51(2):280–8. doi:10.1111/j.1528-1167.2009.02254.x.
Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;54(1):11–27. doi:10.1111/j.1528-1167.2012.03671.x. An important review for neurologists and prescribers of enzyme-inducing AEMs and the multiple associated comorbidities.
Vestergaard P, Tigaran S, Rejnmark L, Tigaran C, Dam M, Mosekilde L. Fracture risk is increased in epilepsy. Acta neurologica Scandinavica 1999.
Latorre H, Kenny FM. High dosage intravenous calcium therapy for osteoporosis and osteomalacia in anticonvulsant therapy with hypomobilization. Pediatrics. 1974;53(1):100–5.
Lidgren L, Nilsson BE, Walloe A. Bone mineral content in epileptics. Calcified Tissue International. 1979;28(2):99–102.
Nilsson OS, Lindholm TS, Elmstedt E, Lindback A, Lindholm TC. Fracture incidence and bone disease in epileptics receiving long-term anticonvulsant drug treatment. Arch Orthop Trauma Surg. 1986;105(3):146–9.
Murchison LE, Bewsher PD, Chesters M. Effects of anticonvulsants and inactivity on bone disease in epileptics. Postgraduate Medical Journal. 1975;51(1):18–21.
Weinstein RS, Bryce GF, Sappington LJ. Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. Journal of Clinical Endocrinology and Metabolism. 1984;58(6):1003–9.
Petty S. Epilepsy and bone health. Neurology Asia. 2011;16(1):63–4.
Valmadrid C, Voorhees C, Litt B, Schneyer CR. Practice patterns of neurologists regarding bone and mineral effects of antiepileptic drug therapy. Arch Neurol. 2001;58(9):1369–74.
Ahmad BS, Hill KD, O’Brien TJ, Gorelik A, Habib N, Wark JD. Falls and fractures in patients chronically treated with antiepileptic drugs. Neurology. 2012;79(2):145–51.
Souverein P, Webb D, Weil J, Van Staa T, Egberts A. Use of antiepileptic drugs and risk of fractures: case–control study among patients with epilepsy. Neurology. 2006;66(9):1318–24. doi:10.1212/01.wnl.0000210503.89488.88.
Stephen LJ, McLellan AR, Harrison JH, Shapiro D, Dominiczak MH, Sills GJ, et al. Bone density and antiepileptic drugs: a case-controlled study. Seizure. 1999;8(6):339–42.
Wark JD, Larkins RG, Perry-Keene D, Peter CT, Ross DL, Sloman JG. Chronic diphenylhydantoin therapy does not reduce plasma 25-hydroxy-vitamin D. Clin Endocrinol (Oxf). 1979;11(3):267–74.
Sato Y, Kondo I, Ishida S, Motooka H, Takayama K, Tomita Y, et al. Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology. 2001;57(3):445–9.
Sheth RD, Wesolowski CA, Jacob JC, Penney S, Hobbs GR, Riggs JE, et al. Effect of carbamazepine and valproate on bone mineral density. J Pediatr. 1995;127(2):256–62.
Cheah J, Stacpoole S, Heaney D, Duncan J. Are we measuring the bone health of epilepsy patients? Rheumatology. 2010;49:i84.
Adler R, Semla T, Cunningham F, Pogach L. Potential under-treatment of male veterans with or at risk for osteoporotic fractures. J Bone Mineral Res 2010. S473.
Tohill C. A study into the possible link between anti-epileptic drugs and the risk of fractures in Muckamore Abbey Hospital. Journal of Intellectual and Developmental Disability. 1997;22(4):281–92. doi:10.1080/13668259700033501.
Lyngstad-Brechan MA, Tauboll E, Nakken KO, Gjerstad L, Godang K, Jemtland R, et al. Reduced bone mass and increased bone turnover in postmenopausal women with epilepsy using antiepileptic drug monotherapy. Scand J Clin Lab Invest. 2008;68(8):759–66. doi:10.1080/00365510802233442.
Beerhorst K, Tan IY, De Krom M, Verschuure P, Aldenkamp AP. Antiepileptic drugs and high prevalence of low bone mineral density in a group of inpatients with chronic epilepsy. Acta Neurologica Scandinavica. 2013;128(4):273–80. doi:10.1111/ane.12118.
Beerhorst K, Tan J, Tan IY, Verschuure P, Aldenkamp AP. Dual-energy X-ray absorptiometry versus quantitative ultrasonography in diagnosing osteoporosis in patients with refractory epilepsy and chronic antiepileptic drug use. Ther. 2013;5(2):59–66. doi:10.1177/1759720X13475851.
Vestergaard P. Changes in bone turnover, bone mineral and fracture risk induced by drugs used to treat epilepsy. Curr Drug Saf. 2008;3(3):168–72.
Roohi F, Fox A. Burst fracture of the first lumbar vertebra and conus-cauda syndrome complicating a single convulsive seizure: a challenge of diagnosis in the emergency department. Journal of Emergency Medicine. 2006;31(4):381–5.
Napier R, Nolan PC. Diagnosis of vertebral fractures in post-ictal patients. Emergency Medicine Journal. 2011;28(2):169–70.
Copuroglu C, Ozcan M, Dulger H, Yalniz E. Late-diagnosed bilateral intertrochanteric femur fracture during an epileptic seizure. Ulus Travma Acil Cerrahi Derg. 2012;18(1):92–4. doi:10.5505/tjtes.2011.76402.
Alenazi B, Rana AQ, Vaid HM. The importance of imaging for status epilepticus patients to rule out fractures—a case report. Journal of Taibah University Medical Sciences. 2013;8(2):120–2.
Caffarelli C, Hayek J, Tomai Pitinca MD, Nuti R, Gonnelli S. A comparative study of dual-X-ray absorptiometry and quantitative ultrasonography for the evaluating bone status in subjects with Rett syndrome. Calcified Tissue International. 2014;95(3):248–56. doi:10.1007/s00223-014-9888-x.
Reyes C, Formiga F, Coderch M, Hoyo J, Ferriz G, Casanovas J, et al. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone. 2013;52(2):557–61. doi:10.1016/j.bone.2012.09.028.
Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporosis International. 1999;9(1):45–54.
Nicholas JM, Ridsdale L, Richardson MP, Grieve AP, Gulliford MC. Fracture risk with use of liver enzyme inducing antiepileptic drugs in people with active epilepsy: cohort study using the general practice research database. Seizure. 2013;22(1):37–42. doi:10.1016/j.seizure.2012.10.002.
Abrahamsen B, Brixen K. Mapping the prescriptiome to fractures in men—a national analysis of prescription history and fracture risk. Osteoporosis International. 2009;20(4):585–97. doi:10.1007/s00198-008-0711-2.
Annegers JF, Melton 3rd LJ, Sun CA, Hauser WA. Risk of age-related fractures in patients with unprovoked seizures. Epilepsia. 1989;30(3):348–55.
Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–73. doi:10.1056/NEJM199503233321202.
Persson HB, Alberts KA, Farahmand BY, Tomson T. Risk of extremity fractures in adult outpatients with epilepsy. Epilepsia. 2002;43(7):768–72.
Lapi F, Simonetti M, Michieli R, Pasqua A, Brandi ML, Frediani B, et al. Assessing 5-year incidence rates and determinants of osteoporotic fractures in primary care. Bone. 2012;50(1):85–90. doi:10.1016/j.bone.2011.09.048.
Tsiropoulos I, Andersen M, Nymark T, Lauritsen J, Gaist D, Hallas J. Exposure to antiepileptic drugs and the risk of hip fracture: a case–control study. Epilepsia. 2008;49(12):2092–9. doi:10.1111/j.1528-1167.2008.01640.x.
Beerhorst K, Schouwenaars FM, Tan IY, Aldenkamp AP. Epilepsy: fractures and the role of cumulative antiepileptic drug load. Acta Neurologica Scandinavica. 2012;125(1):54–9. doi:10.1111/j.1600-0404.2011.01509.x. Introduces the concept of cumulative AEM load and effects on bone health.
Petty SJ, Paton LM, O’Brien TJ, Makovey J, Erbas B, Sambrook P, et al. Effect of antiepileptic medication on bone mineral measures. Neurology. 2005;65(9):1358–63.
Badcock LJ, Smith JA, Price T, Mulherin DM. Bone mineral density in post-menopausal women on anticonvulsant therapy. Journal of the Irish Colleges of Physicians and Surgeons. 2000;29(3):138–40.
Petty SJ, O’Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporosis International. 2007;18(2):129–42.
Moro-Alvarez MJ, Diaz Curiel M, de la Piedra C, Marinoso ML, Carrascal MT. Bone disease induced by phenytoin therapy: clinical and experimental study. Eur Neurol. 2009;62(4):219–30. doi:10.1159/000229309.
Pack AM. Genetic variation may clarify the relationship between epilepsy, antiepileptic drugs, and bone health. European Journal of Neurology. 2011;18(1):3–4. doi:10.1111/j.1468-1331.2010.03137.x. A review of new studies examining vitamin D receptor genetic variation and the association between epilepsy and bone health.
Hamed SA, Moussa EM, Youssef AH, Abd ElHameed MA, NasrEldin E. Bone status in patients with epilepsy: relationship to markers of bone remodeling. Front Neurol. 2014;5:142. doi:10.3389/fneur.2014.00142.
Farhat G, Yamout B, Mikati MA, Demirjian S, Sawaya R, El-Hajj FG. Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology. 2002;58(9):1348–53.
Mattson RH, Gidal BE. Fractures, epilepsy, and antiepileptic drugs. Epilepsy & Behavior. 2004;5 Suppl 2:S36–40.
Lee R, Lyles K, Sloane R, Colon-Emeric C. The association between newer anticonvulsants and bone mineral density. J Bone Mineral Res 2011.
Koo DL, Joo EY, Kim D, Hong SB. Effects of levetiracetam as a monotherapy on bone mineral density and biochemical markers of bone metabolism in patients with epilepsy. Epilepsy Res. 2013;104(1–2):134–9. doi:10.1016/j.eplepsyres.2012.09.002.
Ensrud KE, Walczak TS, Blackwell TL, Ensrud ER, Barrett-Connor E, Orwoll ES, et al. Antiepileptic drug use and rates of hip bone loss in older men: a prospective study. Neurology. 2008;71(10):723–30. doi:10.1212/01.wnl.0000324919.86696.a9.
Meier C, Kraenzlin ME. Antiepileptics and bone health. Ther. 2011;3(5):235–43. doi:10.1177/1759720X11410769.
Pack AM, Morrell MJ, Randall A, McMahon DJ, Shane E. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology. 2008;70(18):1586–93. 8p.
Guo CY, Ronen GM, Atkinson SA. Long-term valproate and lamotrigine treatment may be a marker for reduced growth and bone mass in children with epilepsy. Epilepsia. 2001;42(9):1141–7.
Akin RI, Okutan V, Sarici U, Altunbaş A, Gökçay E. Evaluation of bone mineral density in children receiving antiepileptic drugs. Pediatric Neurology. 1998;19(2):129–31.
Chou IJ, Lin KL, Wang HS, Wang CJ. Evaluation of bone mineral density in children receiving carbamazepine or valproate monotherapy. Acta Paediatr Taiwan. 2007;48(6):317–22.
Baek JH, Seo YH, Kim GH, Kim MK, Eun BL. Vitamin D levels in children and adolescents with antiepileptic drug treatment. Yonsei Med J. 2014;55(2):417–21. doi:10.3349/ymj.2014.55.2.417.
Gniatkowska-Nowakowska A. Fractures in epilepsy children. Seizure. 2010;19(6):324–5. doi:10.1016/j.seizure.2010.04.013.
Mirza N, Marson AG, Pirmohamed M. Effect of topiramate on acid–base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009;68(5):655–61. doi:10.1111/j.1365-2125.2009.03521.x.
Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008;88(6):1678–84. doi:10.3945/ajcn.2008.26099.
Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Developmental Medicine and Child Neurology. 2006;48(12):978–81.
Andress DL, Ozuna J, Tirschwell D, Grande L, Johnson M, Jacobson AF, et al. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch Neurol. 2002;59(5):781–6.
McGrother CW, Donaldson MM, Clayton D, Abrams KR, Clarke M. Evaluation of a hip fracture risk score for assessing elderly women: the Melton Osteoporotic Fracture (MOF) study. Osteoporosis International. 2002;13(1):89–96. doi:10.1007/s198-002-8343-6.
Broulik P, Kragstrup J, Mosekilde L, Melsen F. Osteon cross-sectional size in the iliac crest: variation in normals and patients with osteoporosis, hyperparathyroidism, acromegaly, hypothyroidism and treated epilepsia. Acta Pathol Microbiol Immunol Scand [A]. 1982;90(5):339–44.
Boluk A, Guzelipek M, Savli H, Temel I, Ozisik HI, Kaygusuz A. The effect of valproate on bone mineral density in adult epileptic patients. Pharmacol Res. 2004;50(1):93–7.
Bostancioǧlu M, Öner N, Küçükuǧurluoǧlu Y, Kaya M, Aladaǧ N, Çeltik C, et al. Does valproate therapy decrease the bone mineral density in one-year follow-up in children? Trakya Universitesi Tip Fakultesi Dergisi. 2009;26(1):24–8.
Feldkamp J, Becker A, Witte OW, Scharff D, Scherbaum WA. Long-term anticonvulsant therapy leads to low bone mineral density—evidence for direct drug effects of phenytoin and carbamazepine on human osteoblast-like cells. Exp Clin Endocrinol Diabetes. 2000;108(1):37–43.
Pack AM, Morrell MJ. Epilepsy and bone health in adults. Epilepsy & Behavior. 2004;5 Suppl 2:S24–9.
Fraser LA, Burneo JG, Fraser JA. Enzyme-inducing antiepileptic drugs and fractures in people with epilepsy: a systematic review. Epilepsy Res. 2015;116:59–66. doi:10.1016/j.eplepsyres.2015.07.003.
Voudris KA, Attilakos A, Katsarou E, Garoufi A, Dimou S, Skardoutsou A, et al. Early alteration in bone metabolism in epileptic children receiving carbamazepine monotherapy owing to the induction of hepatic drug-metabolizing enzymes. J Child Neurol. 2005;20(6):513–6.
Pack A. Bone health in people with epilepsy: is it impaired and what are the risk factors? Seizure. 2008;17(2):181–6. doi:10.1016/j.seizure.2007.11.020.
Babacan O, Karaoglu A, Vurucu S, Yesilyurt O, Yesilkaya E, Cayci T et al. May long tem oxcarbazepine therapy be leading to secondary hyperparathyroidism? Eur J Paediatric Neurology 2011. S90.
Hamed SA. Influences of bone and mineral metabolism in epilepsy. Expert Opin Drug Saf. 2011;10(2):265–80. doi:10.1517/14740338.2011.534455.
Kruse K, Bartels H, Ziegler R, Dreller E, Kracht U. Parathyroid function and serum calcitonin in children receiving anticonvulsant drugs. Eur J Pediatr. 1980;133(2):151–6.
Verrotti A, Greco R, Latini G, Morgese G, Chiarelli F. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia. 2002;43(12):1488–92.
Pack AM, Walczak TS. Bone health in women with epilepsy: clinical features and potential mechanisms. Int Rev Neurobiol. 2008;83:305–28. doi:10.1016/S0074-7742(08)00018-4.
Pack AM. Antiepileptic drugs and bone disease. Clinical Reviews in Bone and Mineral Metabolism. 2004;2(2):159–65.
Pack AM. The impact of long-term antiepileptic drug use on bone health. Advanced Studies in Medicine. 2005;5(6 C):S567–S71.
Kruse K. On the pathogenesis of anticonvulsant-drug-induced alterations of calcium metabolism. Eur J Pediatr. 1982;138(3):202–5.
Kruse K, Suss A, Busse M, Schneider P. Monomeric serum calcitonin and bone turnover during anticonvulsant treatment and in congenital hypothyroidism. J Pediatr. 1987;111(1):57–63.
Mosekilde L, Hansen HH, Christensen MS, Lund B, Sorensen OH, Melsen F, et al. Fractional intestinal calcium absorption in epileptics on anticonvulsant therapy. Short-term effect of 1,25-dihydroxycholecalciferol and 25-hydroxycholecalciferol. Acta Med Scand. 1979;205(5):405–9.
Elliott JO, Jacobson MP, Haneef Z. Homocysteine and bone loss in epilepsy. Seizure. 2007;16(1):22–34.
Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115(1):177–86. doi:10.1172/JCI21867.
Kulak CA, Borba VZ, Bilezikian JP, Silvado CE, Paola L, Boguszewski CL. Bone mineral density and serum levels of 25 OH vitamin D in chronic users of antiepileptic drugs. Arq Neuropsiquiatr. 2004;62(4):940–8.
Phabphal K, Geater A. The association between BsmI polymorphism and risk factors for atherosclerosis in patients with epilepsy taking valproate. Seizure. 2013;22(9):692–7. doi:10.1016/j.seizure.2013.05.003.
Phabphal K, Geater A, Limapichart K, Sathirapanya P, Setthawatcharawanich S, Witeerungrot N, et al. The association between BsmI polymorphism and bone mineral density in young patients with epilepsy who are taking phenytoin. Epilepsia. 2013;54(2):249–55. doi:10.1111/epi.12049.
De Miguel-Elízaga I, Martínez-Villanueva M, Villegas-Martínez I, Carrasco-Torres R, Martínez-Ruíz A, Cebreiros-López I, et al. Bone loss associated with use of antiepileptic drugs and genetic predisposition. Clinical Chemistry and Laboratory Medicine. 2015;53:S445.
Lambrinoudaki I, Kaparos G, Armeni E, Alexandrou A, Damaskos C, Logothetis E, et al. BsmI vitamin D receptor’s polymorphism and bone mineral density in men and premenopausal women on long-term antiepileptic therapy. European Journal of Neurology. 2011;18(1):93–8. doi:10.1111/j.1468-1331.2010.03103.x.
de Abreu DA F, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34 Suppl 1:S265–77. doi:10.1016/j.psyneuen.2009.05.023.
Jiang P, Zhu WY, He X, Tang MM, Dang RL, Li HD, et al. Association between vitamin D receptor gene polymorphisms with childhood temporal lobe epilepsy. Int J Environ Res Public Health. 2015;12(11):13913–22. doi:10.3390/ijerph121113913.
Lazzari AA, Dussault PM, Thakore-James M, Gagnon D, Baker E, Davis SA, et al. Prevention of bone loss and vertebral fractures in patients with chronic epilepsy—antiepileptic drug and osteoporosis prevention trial. Epilepsia. 2013. doi:10.1111/epi.12351. Treatment studies—calcium and vitamin D alone do not appear to prevent fractures compared to bisphosphonate.
Dussault P, Davis Jr S, Lazzari AA. The effect of calcium and vitamin D on bone loss in an epileptic population. Arthritis and rheumatism 2012. p. S842. Treatment studies—calcium and vitamin D alone do not appear to prevent fractures compared to bisphosphonate.
Pepe I, Campisi G, Scozzari F, Napoli N, Rini G, Di Fede G. Bone loss caused by anticonvulsants: BMD improvement using strontium ranelate. J Bone Mineral Res 2011.
Vestergaard P. Epilepsy, osteoporosis and fracture risk—a meta-analysis. Acta Neurologica Scandinavica. 2005;112(5):277–86.
Davidson DL, Macdonald S. The costs of trauma caused by seizures: can they be reduced? Seizure. 2002;11(5):344–7.
Koppel BS, Harden CL, Nikolov BG, Labar DR. An analysis of lifetime fractures in women with epilepsy. Acta Neurologica Scandinavica. 2005;111(4):225–8.
Elliott JO, Seals BF, Jacobson MP. Osteoprotective knowledge in a multiethnic epilepsy population. J Neurosci Nurs. 2008;40(1):14–24. 39.
Elliott JO, Seals BF, Jacobson MP. Use of the Precaution Adoption Process Model to examine predictors of osteoprotective behavior in epilepsy. Seizure. 2007;16(5):424–37.
Elliott JO, Jacobson MP. Bone loss in epilepsy: barriers to prevention, diagnosis, and treatment. Epilepsy & Behavior. 2006;8(1):169–75.
Gross RA, Gidal BE, Pack AM. Patient page. Antiseizure drugs and reduced bone density. Neurology. 2004;62(11):E24–5.
Nakken KO, Tauboll E. Bone loss associated with use of antiepileptic drugs. Expert Opin Drug Saf. 2010;9(4):561–71. doi:10.1517/14740331003636475.
Harijan P, Khan A, Hussain N. Vitamin D deficiency in children with epilepsy: do we need to detect and treat it? Journal of Pediatric Neurosciences. 2013;8(1):5–10. doi:10.4103/1817-1745.111413.
Marcuccilli CJ. Vitamin D, deficiency in pediatric epilepsy. Journal of Pediatric Epilepsy. 2013;2(3):199–208.
Teagarden DL, Meador KJ, Loring DW. Low vitamin D levels are common in patients with epilepsy. Epilepsy Res. 2014;108(8):1352–6. doi:10.1016/j.eplepsyres.2014.06.008.
Cloyd J, Hauser W, Towne A, Ramsay R, Mattson R, Gilliam F, et al. Epidemiological and medical aspects of epilepsy in the elderly. Epilepsy Res. 2006;68 Suppl 1:S39–48.
Cohen A, Lancman M, Mogul H, Marks S, Smith K. Strategies to protect bone mass in the older patient with epilepsy. Geriatrics. 1997;52(8):5–8. 81.
Ziemba KS, Noe KH. Treatment of epilepsy in postmenopausal women. Aging Health. 2010;6(1):87–96.
Khan SA. Women, epilepsy and anti-epileptic drugs. Neurosciences. 2004;9(2):74–9.
Crawford P. Best practice guidelines for the management of women with epilepsy. Epilepsia. 2005;46 Suppl 9:117–24.
Erel T, Guralp O. Epilepsy and menopause. Arch Gynecol Obstet. 2011;284(3):749–55. doi:10.1007/s00404-011-1936-4.
Adis Medical Writers. Be aware of the potential effects of menopause on epilepsy and its treatment. Drugs and Therapy Perspectives. 2015;31(5):161–3.
Homann B, Plaschg A, Grundner M, Haubenhofer A, Griedl T, Ivanic G, et al. The impact of neurological disorders on the risk for falls in the community dwelling elderly: a case-controlled study. BMJ Open. 2013;3(11), e003367. doi:10.1136/bmjopen-2013-003367.
Stolze H, Klebe S, Zechlin C, Baecker C, Friege L, Deuschl G. Falls in frequent neurological diseases—prevalence, risk factors and aetiology. J Neurol. 2004;251(1):79–84. doi:10.1007/s00415-004-0276-8.
Lee L, Wagner M, Wu B. Epilepsy patients should receive DXA screening. Epilepsy Currents. 2013;13:92–3.
Drezner MK. Treatment of anticonvulsant drug-induced bone disease. Epilepsy & Behavior. 2004;5 Suppl 2:S41–7.
Kurth C, Keller L, Steinhoff BJ. Determination of alkaline phosphatase without isozymes is not appropriate for monitoring of increased bonemass turnover in patients on inducing antiepileptic drugs. Epilepsia. 2009;50:49.
Elliott JO. Possible methods for the prevention of bone loss in persons with epilepsy. Expert rev. 2009;9(6):797–812. doi:10.1586/ern.09.35.
Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Current Treatment Options in Neurology. 2014;16(5):288. doi:10.1007/s11940-014-0288-3.
Lazzari AA, Dussault P, Davis S. Improvement of treatment decisions in epileptic patients by performing lateral vertebral assessment (LVA). Journal of Clinical Densitometry. 2010;13:118.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Helen Wilding declares no conflict of interest. Sandra J. Petty reports grants from UCB Pharma, grants from Novartis, other from UCB Pharma, outside the submitted work. John D. Wark reports grants from UCB Pharma, grants from Novartis, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors. All studies performed by the authors’ references in this paper were approved by the relevant institutional human research ethics committee.
Additional information
This article is part of the Topical Collection on Secondary Causes of Osteoporosis
Rights and permissions
About this article
Cite this article
Petty, S.J., Wilding, H. & Wark, J.D. Osteoporosis Associated with Epilepsy and the Use of Anti-Epileptics—a Review. Curr Osteoporos Rep 14, 54–65 (2016). https://doi.org/10.1007/s11914-016-0302-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-016-0302-7