Abstract
Purpose of Review
The neuro-oncology team faces a unique challenge when assessing treatment response in patients diagnosed with glioblastoma. Magnetic resonance imaging (MRI) remains the standard imaging modality for measuring therapeutic response in both clinical practice and clinical trials. However, even for the neuroradiologist, MRI interpretations are not straightforward because of tumor heterogeneity, as evidenced by varying degrees of enhancement, infiltrating tumor patterns, cellular densities, and vasogenic edema. The situation is even more perplexing following therapy since treatment-related changes can mimic viable tumor. Additionally, antiangiogenic therapies can dramatically decrease contrast enhancement giving the false impression of decreasing tumor burden. Over the past few decades, several approaches have emerged to augment and improve visual interpretation of glioblastoma response to therapeutics. Herein, we summarize the state of the art for evaluating the response of glioblastoma to standard therapies and investigational agents as well as challenges and future directions for assessing treatment response in neuro-oncology.
Recent Findings
Monitoring glioblastoma responses to standard therapy and novel agents has been fraught with many challenges and limitations over the past decade. Excitingly, new promising methods are emerging to help address these challenges. Recently, the Response Assessment in Neuro-Oncology (RANO) working group proposed an updated response criteria (RANO 2.0) for the evaluation of all grades of glial tumors regardless of IDH status or therapies being evaluated. In addition, advanced neuroimaging techniques, such as histogram analysis, parametric response maps, morphometric segmentation, radio pharmacodynamics approaches, and the integrating of amino acid radiotracers in the tumor evaluation algorithm may help resolve equivocal lesion interpretations without operative intervention. Moreover, the introduction of other techniques, such as liquid biopsy and artificial intelligence could complement conventional visual assessment of glioblastoma response to therapies.
Summary
Neuro-oncology has evolved over the past decade and has achieved significant milestones, including the establishment of new standards of care, emerging therapeutic options, and novel clinical, translational, and basic research. More recently, the integration of histopathology with molecular features for tumor classification has marked an important paradigm shift in brain tumor diagnosis. In a similar manner, treatment response monitoring in neuro-oncology has made considerable progress. While most techniques are still in their inception, there is an emerging body of evidence for clinical application. Further research will be critically important for the development of impactful breakthroughs in this area of the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroimaging using MRI remains the standard imaging modality in the management of patients with glioblastoma. MRI is useful for establishing the initial diagnosis and for assessing response to conventional therapies (i.e., surgery, radiation, chemotherapy) and experiential agents. However, response assessments following treatment can be troublesome because of tumor heterogeneity, which is characterized by varying degrees of enhancement, infiltrating tumor patterns, cellular densities, distinct and indistinct borders, necrotic core regions, and vasogenic edema on neuroimaging. Additionally, treatment-related changes on MRI often mimic progressive disease, making interpretations particularly challenging for even the most experienced radiologists. Moreover, supportive therapies such as antiangiogenic medications and corticosteroids can dramatically decrease contrast enhancement due to changes in vascular permeability, suggesting a reduction in tumor burden radiographically when in reality that may not be the case. In this scenario, clinical decision-making requires the treating physician to use judgment to reconcile a potential discrepancy between improved imaging findings and clinical progression. These limitations of MRI assessment have spurred investigations of novel ancillary imaging biomarkers and nonimaging approaches to augment and improve visual interpretation of glioblastoma responses to standard and novel therapies. Herein, we summarize and discuss the general framework for response assessment to therapies in adults with glioblastoma as well as complementary and emerging techniques in the era of precision medicine that are poised to modernize our methods for measuring disease response.
Response Assessment in Neuro-Oncology
The expansion of novel efficacious therapies for patients with glioblastoma requires reliable criteria for objectively assessing response to treatment. This requisite has been particularly difficult in neuro-oncology because contrast enhancement on MRI is an imprecise surrogate marker for tumor viability and volume. Additionally, the intensity of enhancement is influenced by medications that decrease brain tumor-associated vascular permeability and may give a false impression of response to therapy. Furthermore, glioblastoma is poorly marginated and characterized by histopathological heterogeneity, posing a significant challenge when assessing infiltrative non-enhancing tumor [1]. Historically, the gold standard for assessing treatment response was the Macdonald criteria from 1990 [2]. The Macdonald criteria incorporated two-dimensional tumor measurements on computed tomography (CT) in conjunction with neurological findings and corticosteroid dose but were later extrapolated to MRI-based diagnostic imaging. Objective response to treatment included four response categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [2]. These criteria allowed comparisons of response rates in clinical trials. Still, they fell out of favor a decade later when MRI became the standard imaging modality for assessing glioblastoma.
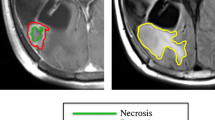
The Macdonald criteria had several limitations, including challenges with measuring irregularly shaped lesions, substantial interobserver variability, lack of assessment of the non-enhancing tumor component, lack of guidelines for assessing multifocal disease, and the difficulty in measuring enhancing lesions in the wall of cystic or surgical cavities [3,4,5]. The RANO working group was created to address these pitfalls and harmonize the criteria used to assess different central nervous system (CNS) tumors, specifically in the clinical trial context [6]. This multidisciplinary, international working group includes representatives from varied disciplines, including neuro-oncology, medical oncology, neuroradiology, neurosurgery, radiation oncology, neuropsychology, and experts in clinical outcomes assessments, all working in collaboration with government and industry to integrate a fundamental framework of radiographic parameters to classify therapeutic outcome in patients with glioblastoma. The RANO criteria defined measurable disease as bidimensional contrast-enhancing lesions with clearly defined margins, with two perpendicular diameters of at least 10 mm, visible on ≥ 2 axial slices [6]. Nonmeasurable disease included unidimensional measurable lesions, masses with margins not clearly defined as frequently noted in the surgical margins, or lesions with maximal perpendicular diameters of < 10 mm [6]. T2/FLAIR hyperintense lesions were also considered nonmeasurable and typically represented infiltrating tumor extending from the tumor core into adjacent tissue. When multiple contrast-enhancing lesions exist, a minimum of two target lesions must be selected, representing the tumor burden. In addition, the sum of the products of the perpendicular diameters of these lesions must be determined [6]. The RANO criteria defined radiographic progression as a greater than or equal to 25% growth in the contrast-enhancing tumor burden when compared to the baseline MRI (i.e., pre-therapy or best imaging response timepoint) and/or the appearance of a new lesion(s). The working group defined CR as the complete disappearance of all enhancing measurable and nonmeasurable disease sustained for at least 4 weeks and PR as a greater than or equal to 50% decrease in the contrast-enhancing tumor burden when relative to the baseline assessment MRI. As a final point, the time between the completion of the radiation therapy and the imaging acquisition should also be considered when interpreting posttreatment imaging [7]. The group acknowledged the high incidence of increased contrast enhancement and perilesional edema on posttreatment (i.e., chemoradiotherapy) imaging simulating progressive disease, a phenomenon referred to as pseudoprogression (PsP). Because of this, the RANO criteria defined radiographically progressive disease within the first 12 weeks after completion of chemoradiation as new enhancement outside the radiation field (beyond the high-dose region or 80% isodose line) [6]. Pseudoprogression occurs, on average, in 36% of patients with glioblastoma following chemoradiotherapy with the alkylating agent temozolomide [8]. However, the phenomenon occurs more frequently in patients with hypermethylation of the O [6]-methylguanine DNA methyltransferase (MGMT) promoter gene [9]. The RANO working group also considered the pseudoresponse observed with antiangiogenic therapies, such as bevacizumab, that rapidly reduce vascular permeability and contrast enhancement [6]. These agents target and neutralize secreted vascular endothelial growth factor (VEGF) and block its signal transduction through both the vascular endothelial growth factor receptor 1 (VEGFR-1) and vascular endothelial growth factor receptor 2 (VEGFR-2), and dramatically decrease contrast enhancement after the initiation of therapy, masquerading as a reduction of tumor burden [10].
Generally, the efficacy of antineoplastic agents is determined by the drug’s ability to improve overall survival (OS) in large randomized, phase III trials. However, the use of OS as a primary clinical endpoint is often impractical due to several drawbacks. OS is limited by long trial times and confounding effects of post protocol events, such as salvage therapies [11]. As a result, clinical trials rely heavily on surrogate endpoints, such as objective response rates (ORR) and progression-free survival (PSF) to gauge efficacy. Several studies have demonstrated that ORR and PFS correlate with OS [11,12,13]. These radiographic endpoints can be assessment quickly and are vitally important in glioblastoma response assessment because they are not confounded by salvage therapies and other variables that may affect OS [14, 15]. Moreover, PSF offers greater statistical power at the time of analysis [11]. Validation of the surrogacy of PFS in glioblastoma clinical trials has been established [11].
While the RANO criteria addressed the limitations of the Macdonald criteria, significant drawbacks of these guidelines were identified. For instance, the RANO criteria require bidirectional measurement of contrast-enhancing tumor size that overestimates MRI defined tumor size [16]. Additionally, thresholds for defining response and progression were arbitrarily assigned [17]. Furthermore, the RANO criteria defines the post-operative MRI as the baseline for treatment response evaluation; however, this scan is not a reliable reference for accurately determining radiographic changes for several reasons. First, post-surgical MRI is generally obtained prior to histomolecular confirmation, a fundamental prerequisite for clinical trial participation. Therefore, the baseline post-surgical MRI technique may not be consistent with the clinical trial imaging protocol leading to a dissimilarity with subsequent follow-up imaging [17]. Secondly, the timing of the post-operative MRI can be highly variable and often reveal the temporal development of surgically induced reactive contrast enhancement and blood products, rendering the radiographic assessment difficult [18]. Furthermore, corticosteroid dose can be extremely variable in the immediate post-operative period and not well documented since patients are usually not yet enrolled in clinical trials at this time [17]. The RANO criteria incorporated the evaluation of nonenhancing (T2/FLAIR) abnormality which is subjective and does not accurately predict overall survival [19]. Because of these pitfalls, the RANO criteria were later modified [17] and included a suggestion for consideration of the use of volumetric measurements for response evaluation (a proposal that has not come to pass). The modified RANO (mRANO) criteria also provided a clearer definition of non-measurable disease and advocated for the use of a post-radiation MRI as the baseline for response evaluation in patients with newly diagnosed glioblastoma. Other RANO working groups including the RANO-low grade glioma [20], immunotherapy RANO [21], RANO leptomeningeal metastases [22], RANO brain metastases [23], and RANO-corticosteroids [24] have emerged to provide guidance and assessment of response and end points in other areas of neuro-oncology clinical trials.
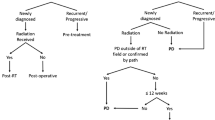
In 2021, the World Health Organization published an updated classification for brain and spinal tumors and for the first time introduced the role of molecular data for central nervous system tumor classification, building on the 2016 updated fourth edition and the work of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy [25]. Accordingly, astrocytic tumors are stratified as those that harbor the isocitrate dehydrogenase 1 gene mutation (IDH) and those without the mutation (wildtype), designated as glioblastoma IDH-wildtype. The WHO observed that IDH-wildtype astrocytomas ascribed to grade 2 or 3 by morphology-based criteria exhibit and an aggressive phenotype much like glioblastoma. As such, molecular aberrations that confer an aggressive behavior of an IDH-wildtype diffuse astrocytoma including EGFR amplification, TERTp mutations, gain of chromosome 7 and loss of chromosome 10 were evaluated [26]. Consequently, an IDH-wildtype diffuse astrocytoma with at least one of these genetic signatures establishes the diagnosis of glioblastoma IDH-wildtype CNS WHO grade 4 even in the absence of the histopathological features of glioblastoma. In response, the RANO working group proposed an updated response criteria (RANO 2.0) for the evaluation of all grades of glial tumors regardless of IDH status or therapies being evaluated [27••, 28••]. Data showing PFS obtained by RANO and modified RANO criteria correlated similarly with OS in patients with newly diagnosed and recurrent glioblastoma was another impetus for RANO 2.0 [29••]. Similar to the mRANO, RANO 2.0 advocates using the post-radiation MRI as the baseline scan for response evaluation in patients with newly diagnosed glioblastoma. Because the incidence of pseudoprogression is high within the first 12 weeks following chemoradiotherapy, to confirm radiographic progression when equivocal lesions are encountered during this interval, the working group proposed a repeat MRI (in 4–8 weeks) to determine true progression in clinically stable patients [28••]. However, confirmation scans are not mandatory after this period or for recurrent tumors since these scans do not appear to improve reliability in determining progression. On the contrary, for agents with a high likelihood to induce pseudoprogression, for instance immunogenic cell death-inducing therapies, a mandatory confirmation of progression with a repeat MRI is an option. RANO 2.0 will employ two-dimensional tumor analysis like the RANO criteria but will allow volumetric acquisition. The steering committee recognized the fact that non-enhancing progression does not improve correlation of PFS with survival in patients with enhancing glioblastomas [30, 31], and proposed the elimination of measuring non-enhancing tumor in agreement with the mRANO criteria. Finally, the group also noted that the application of immunotherapy RANO in patients who received immune checkpoint blockade monotherapy did not increase the correlation between PFS and OS when compared to RANO and mRANO.
Advances in Neuroimaging Approaches in Glioma
The poor specificity of T1-gadolinium enhancement changes is a well-recognized challenge in radiographic monitoring of gliomas [32]. Multipronged efforts are made to address this problem through standardizing routine clinical neuroimaging, improving image postprocessing and analysis methods, as well as developing advanced MRI acquisition techniques and amino acid radiotracers. For instance, multidisciplinary consensus recommendations called the Brain Tumor Imaging Protocol (BTIP) were published in 2016, setting minimum and ideal specifications used for the acquisition of 3D T1-weighted pre-gadolinium, 2D FLAIR, and diffusion-weighted images (DWI), as well as post-gadolinium 2D T2-weighted and 3D T1-weighted images [33]. Standard specifications for the commonly used MRI perfusion method, dynamic susceptibility contrast (DSC) imaging, were subsequently published [34]. These efforts aimed to reduce inter-scanner and inter-institutional variability in MRIs and address the extreme inconsistencies in the diagnostic performance of perfusion MRI.
Advanced image assessment techniques are being developed, such as histogram analysis, primarily applied in apparent diffusivity coefficient (ADC) maps. This approach utilizes changes in a summary characteristic, for instance, the reductions in the diffusivity on the lowest 5th percentile of voxels, which was shown to identifying true progression with 89% accuracy [35]. Although relatively easy to perform in a clinical setting, the main limitation of histogram analyses is the loss of spatial information that may mask focal tumor progression in an otherwise necrotic background [36]. Parametric response maps (PRMs) use imaging at pre- and post-intervention timepoints to map out voxel-wise changes to detect early responses, or identify PsP [37]. PRMs can be applied to virtually any single or a combination of imaging modalities. For example, in PRMs of ADC, an increase in high-diffusivity areas was associated with fivefold longer survival [38], or identified pseudoprogression with an 86% accuracy [39], or predicted a 1-year OS advantage when bevacizumab is used [40]. PRMs of relative cerebral blood volume (rCBV) maps also showed a successful identification of true progression by a reduction in the proportion of voxels with low rCBV, while the difference between PsP and true progression (TP) were obscured when whole-tumor changes were measured [39]. However, significant barriers to the routine clinical implementation of PRMs include not only the need for two scans ideally performed on the same scanner, but also the significant expertise and effort required in image pre-processing (co-registration, normalization, segmentation, etc.) that would require nearly full automation to conform to the clinical workflow.
Advances in automated tumor segmentation as demonstrated annually by the Radiological Society of North America-American Society of Neuroradiology (RSNA-ASNR) Brain Tumor Segmentation (BraTS) challenge [41] may offer the most accurate method for tumor measurement by enabling rapid volumetric monitoring of gliomas, an approach suggested to be especially helpful to detect subtle and often slowly emerging responses to IDH-inhibitor therapy [42, 43]. In terms of special image acquisition, MR spectroscopy (MRS) and chemical exchange saturation transfer (CEST) stand out. MRS detects nuclear magnetic resonance signals produced by variations in the chemical composition of tissues, such as lactate content, which was shown to be a highly sensitive marker of PsP when normalized to the neuronal marker N-acetyl-aspartate (NAA); however, its specificity appears limited [44•]. An emerging role of MRS is to serve as a pharmacodynamic biomarker for metabolically targeted therapies such as dichloroacetate therapy against glioma (hypothesized to decrease lactate) [45], or potentially detecting changes in 2-hydroxyglutarate levels in the context of emerging IDH-inhibitor therapies that tend to be linked to slow and subtle responses on conventional MRI [42, 43]. CEST imaging is a special MRI technique that allows the quantification of endo- or exogenous molecules by using radiofrequency signals to saturate the protons these molecules exchange with surrounding water and thus amplifying their signal to MRI detectable levels. Amide proton transfer-weighted imaging (APTw) is a CEST technique that provides contrast based on tissue protein and peptide content, which is increased in neoplasms such as gliomas [46]. APTw was recently demonstrated to differentiate PsP from TP with a high resolution and accuracy in a histopathologically confirmed cohort [47].
Amino acid PET tracers have also been heavily researched for disease monitoring in gliomas. These are especially advantageous in neuro-oncology due to their low uptake in normal brain and extremely high contrast between normal and tumor tissue. In 2019, joint recommendations from the European Association of Neuro-Oncology and RANO committees extended the standardization efforts to PET acquisition and analysis approaches as well [48, 49]. Among the tracers listed is [18F]Fluoro-ethyl tyrosine (FET), which showed high accuracy for detecting early as well as late progression following radiotherapy [50]. Another amino acid tracer [18F]FDOPA, preferentially studied in North America, performs similarly well in glioma monitoring. In a prospective trial assessing its spatial sensitivity/specificity to identify true progression, FDOPA far exceeded the performance of conventional MRI (76%/100% vs 52%/50%, respectively) [32]. Fluciclovine PET is another amino acid tracer approved for prostate cancer imaging that is currently prospectively studied in radiation response monitoring in glioma (NCT03926507).
Neurologic Assessment in Neuro-Oncology
The most widely used tools to assess performance in activities of daily living in cancer patients are the Karnofsky Performance Status (KPS) and the Eastern Cooperative Oncology Group (ECOG) performance status. These assessment tools have proven helpful for monitoring the clinical trajectories of patients with cancer receiving experimental chemotherapies [51]. However, these scales are not without shortcomings. For instance, scores are subjectively [52] assigned by a health care provider, lack reproducibility [53], and fail to assess deficits in neurologic function objectively. In the early 1990s, recursive partitioning analysis (RPA) of prognostic factors helped the design, stratification, and outcome comparison for multiple glioblastoma clinical trials [54]. The classification was simplified in 2011 and resulted in three distinct prognostic groups defined by age, performance status, the extent of resection, and neurologic function (able to work versus not) for use in glioblastoma clinical trials [55]. Similar to prior assessment tools, the RPA model did not include data from the standard neurologic examination. Although the Macdonald and RANO criteria incorporated the patient’s clinical status in the assessment of progressive disease, a quantifiable measure of the true clinical picture was not clearly defined. To illustrate, multiple assessment tools are readily available for clinicians to report changes in neurological status in patients across several neurologic subspecialties such as neurocritical care (Glasgow Coma Scale [GSC]), stroke (National Institutes of Health Stroke Scale (NIHSS) and the Canadian Neurological Scale (CNS)), multiple sclerosis (Expanded Disability Status Scale (EDSS)), Parkinson disease (Unified Parkinson Disease Rating Scale (UPDRS)), ataxia (Scale for Assessment and Rating of Ataxia), myopathy (Kendall muscle scale), and amyotrophic lateral sclerosis (Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R)) [56,57,58]. Despite this progression in neuroscience, a standardized metric to measure neurologic function in patients with brain tumors was not available and generated a surge in interest in implementing a measurable tool to quantify neurological function in this patient population. To this end, the Neurologic Assessment in Neuro-Oncology (NANO) scale was drafted and provided an objective clinician-reported outcome of neurologic function for patients with brain tumors [56]. The NANO scale integrated components of the standard neurologic examination conducted in routine clinic visits and measured neurologic function across nine relevant neurological domains, including gait, strength, upper extremity ataxia, sensation, visual field, facial strength, language, level of consciousness, and behavior [56]. The scale has been introduced into clinical trials with preliminary confirmation of high interobserver agreement and reliability for assessing disease response to novel therapies [56].
Patient-Reported Outcomes
Glioblastoma portends an unfavorable prognosis and a high symptom burden that adversely impacts the quality of survival. Thus, maintaining or improving the patient’s quality of life (QoL) and palliation are critical considerations during the disease trajectory. For this reason, health-related quality of life (HRQoL) has become a crucial outcome measure in clinical trials, supplementary to traditional outcome measures such as OS, PFS, and radiological response to treatment [59]. HRQoL is a multidimensional concept covering physical, psychological, and social domains and symptoms induced by the disease and its treatment [59]. Generally speaking, HRQoL focuses on the impact of the disease and interventions on the emotional, social, and physical aspects of the patient’s daily life. HRQoL data is garnered through a patient-reported outcome (PRO) measure, which captures reports from patients about their health without the interpretation or influence of the clinician. Several PROs questionnaires have been developed and are available in brain tumor clinical trials and daily clinical practice. The European Organization for Research and Treatment of Cancer (EORTC) developed a generic questionnaire, the EORTC QLQ-C30, to measure HRQoL in patients with cancer [60]. This questionnaire was designed to assessed clinical variables (disease stage, weight loss, performance status, and treatment toxicity) in heterogenous group of patients with cancer, and includes 30 items, organized into five functional scales (physical, role, emotional, cognitive, and social functioning), three symptom scales (fatigue, nausea and vomiting, and pain), one global health status scale, one overall quality of life scale, and six single-items symptom measures (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). The EORTC QLQ-C30 is a reliable and valid measure of the QoL in cancer patients in multicultural clinical research settings [60]. Following this, Osoba and colleagues developed a brain tumor-specific questionnaire, the EORTC QLQ-BN20, designed to complement other core or general QoL questionnaires when studying patients with brain cancer in clinical trials [61]. This module included 20 items organized into four scales (future uncertainty, visual disorders, motor dysfunction, and communication deficit) and seven single items (headache, seizures, drowsiness, hair loss, itchy skin, weakness of the legs, and bladder control). Another well-established tool is the Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire. The FACT-G (version 4) consists of a 27-item questionnaire covering four domains of the HRQOL in cancer patients: physical, social, emotional, and functional well-being [62]. A brain cancer-specific module, the FACT-brain, is available in addition to this generic questionnaire. This disease-specific questionnaire consists of 50 items that cover physical, social/family, emotional, and functional well-being and concerns relevant to patients with brain tumors, including concentration, memory, seizures, eyesight, personality, expression of thoughts, weakness, coordination, and headaches [62,63,64]. The FACT measures cover more psychosocial aspects of the disease and its treatment, while the EORTC measures focus more on functioning and symptoms [59]. Finally, the MD Anderson Symptom Inventory (MDASI) questionnaire was designed to measure the severity of symptoms in cancer patients (13 items) as well as the hindrance of these symptoms on basic activities necessary for independent living (6 items) [65]. A brain tumor-specific module (MDASI-BT) has been developed in addition to the core questionnaire [66].
Neurocognitive Assessments
Patients with glioblastoma are exceedingly vulnerable to neurocognitive decline as they progress through standard therapy. Supportive treatment such as anti-seizure medication and dexamethasone may also contribute to cognitive dysfunction [67]. A report found deterioration in neurocognitive performance in the domains of information processing, psychomotor function, and attentional tasks in patients with glioblastoma eight months after completion of radiotherapy [68]. By the same token, the long-term survival of patients with glioblastoma is associated with substantial impairment in HRQOL and disabling cognitive deficits [69, 70]. Thus, assessing neurocognitive function in HGG patients is of immense value. The Mini-Mental State Examination (MMSE) is a relatively quick and easy-to-perform screening tool for general neurocognitive function; however, the questionnaire lacks sensitivity and fails to detail memory, verbal fluency, visual-motor speed, and executive function, frequent cognitive changes in patients with brain tumors [71, 72]. Additionally, the MMSE does not account for patient-to-patient variation based on age and education [73]. Neurocognitive function can be evaluated more comprehensively with other screening instruments such as the Wechsler Adult Intelligence Scale-Third Edition and the Wechsler Memory Scale-Revised, the Hopkins Verbal Learning Test–Revised, the Trail Making Tests, and the Controlled Oral Word Association, which are highly valuable, objective, and comprehensive measures of cognition and with reported prognostic value [72,73,74,75].
Seizure Control
Tumor-related epilepsy (TRE) is one of the most common comorbidities in patients with brain tumors, and it is well-established that TRE is influenced by oncobiology and tumor growth [76]. Seizures occur in > 80% of low-grade glioma (LGG) patients and 40–60% of patients with classical, histopathologically-defined glioblastoma [76, 77]. Approximately 48–65% of patients with the newly recognized diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade 4 are confronted with seizures at presentation [78,79,80]. Uncontrolled epilepsy and the capriciousness of TRE can impact a patient’s HRQoL and adversely compromise independence in self-care. Emerging data suggest that effective seizure control and radiographic response can be achieved with tumor-directed therapies alone in patients with LGG [81,82,83]. In fact, a change in chemotherapy has been proposed in patients with inadequate seizure control, even in the absence of radiographic progression [84]. Similarly, achieving seizure freedom may herald a radiographic response to therapy [84, 85]. From this frame of reference, the RANO seizure working group proposed seizure control as an adjunctive secondary outcome measure in determining treatment response in clinical trials of patients with LGG [86]. The assessment tool includes an evaluation of seizure classification, frequency, and a rating system for seizure outcome and incorporates a variety of HRQoL and symptom burden scales [86].
Liquid Biomarker Discovery
Liquid biopsy is an attractive noninvasive complement to radiographic assessment for monitoring therapeutic response in patients with glioblastoma. Success with this technique hinges on leveraging the discovery and quantification of diverse classes of tumoral content, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTC), extracellular vesicles (EV), and glioma-specific oncogenic markers that are shed into peripheral blood and cerebrospinal fluid (CSF) during tumor cell turnover. This paradigm shift in oncology has modernized prognostication parameters for patients with solid cancers such as breast cancer [87], head and neck cancer [88], and lung cancer [89]. At present, several assays have been approved by the FDA to detect genetic alterations in plasma cell-free DNA (cfDNA) in patients with advanced-stage systemic cancer, marking a turning point for integration into daily practice [90]. Comparatively, very little progress has been made in validating circulating biomarkers for primary CNS malignancies. Still, the detection and quantification of tumoral content released by glioblastoma may be helpful for diagnosis, monitoring tumor evolution, and unveiling the molecular landscape of primary and recurrent disease.
To date, liquid biopsy studies in glioblastoma have shown a detection rate of CTCs ranging from 20–77% [91,92,93]. This variability is primarily due to the heterogeneity of analytical methods, the lack of standardized tumor-specific cell surface markers, and the absence of methodological uniformity to permit meaningful comparison among studies [94, 95•]. Nonetheless, there is growing evidence that the enumeration of CTCs could reflect tumor burden, which has potential value when monitoring tumor progression and therapeutic response [93, 96•]. Researchers in a recent pilot study isolated CTCs from whole blood in 20 newly diagnosed patients with glioblastoma before and after surgery and reported that patients with a CTC count of zero after surgery had a significantly longer PFS, suggesting that postoperative CTC quantification may have potential utility as a prognostic biomarker [97]. Moreover, CTC detection may be vital in distinguishing tumor recurrence from radiation necrosis in patients with glioma; larger studies are needed to clarify this potential benefit [93].
Circulating tumor DNA analysis is a novel approach for interrogating the genomic landscape of primary brain tumors without invasive tissue acquisition. Studies performing comprehensive ctDNA analysis in patients with primary brain tumors report a detection rate of 10–50% in plasma, with higher detection rates associated with glioblastoma [98,99,100,101]. However, all studies unanimously report much lower ctDNA concentrations when compared to other advanced-stage tumors. The postulated explanation for this lower yield in ctDNA concentrations relates to the physical hurdle of the blood–brain barrier, which limits the passage of tumoral content into the peripheral circulatory system. In contrast, several studies have identified CSF as a rich and reliable source of ctDNA in patients with primary brain tumors [102, 103]. Molecular characterization of ctDNA in CSF of patients with primary brain tumors has confirmed the feasibility of capturing a broad spectrum of mutations and copy number alterations, including TERT promoter, TP53, and IDH1 mutations as well as CDKN2A/B deletions [104, 105]. Tracking CSF ctDNA longitudinally may have applications in determining therapeutic efficacy and potentially transform how we evaluate responses to glioma therapies. Longitudinal data have illustrated the ability of CSF ctDNA analysis to capture the genomic response to treatment and tumor evolution [102, 104]. For instance, in one study, high H3F3A K27M copy number in CSF ctDNA correlated with the presence of contrast-enhancing and total tumor cross-sectional area on MRI in pediatric diffuse intrinsic pontine gliomas [106].
Liquid biopsy based analytes also hold promise as prognostic biomarkers in glioma [107]. In an analysis of CSF ctDNA in 85 adults with glioma, tumor-derived DNA positivity was a statistically significant prognostic factor, even after adjustment for percent extent of resection at original diagnosis, tumor burden at CSF collection, and IDH status [104]. Moreover, a prospective study of 42 patients with newly diagnosed glioblastoma showed that a high plasma cfDNA concentration prior to initial surgical resection is independently associated with poor outcomes in patients undergoing standard of care therapy [108]. Equally important, the emergence of plasma cfDNA methylomics is gaining momentum in liquid biopsy diagnostics for the detection and discrimination of glioma from other primary intracranial tumors [109]. For instance, the implementation of a cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq) [110] to blood samples from glioma patients, patients with extracranial cancers, and healthy controls showed high sensitivity and discriminative capacity for distinguishing patients with gliomas from patients with systemic cancers and healthy controls [111]. Additionally, a more recent study profiled the methylome of circulating cfDNA in the serum from a cohort of patients with gliomas and other tumors and non-neoplastic conditions and identified a DNA methylation-based signature that recapitulated the epigenetic features of glioma tissue [112]. The methylation-based signature discriminated patients with glioma from non-glioma patients with 100% sensitivity and 98% specificity [112]. Interestingly, the researchers tested the signature in an independent discovery and validation cohorts that enabled the development and verification of a score metric (the “glioma-epigenetic liquid biopsy score” or GeLB) that reflected clinicopathological changes during surveillance (i.e., progression, pseudoprogression, and response to treatment to standard and or experimental therapies). Still, the practicality of using ctDNA as a therapy-monitoring biomarkers remains unresolved. More large-scale prospective cohorts are needed to define its clinical utility.
Extracellular vesicles (EVs) are membrane-bilayered vesicular particles that carry molecules such as oncoproteins and oncopeptides, RNA species (microRNAs, mRNAs, and long non-coding RNAs), lipids, and DNA fragments from donor to recipient cells [113]. EVs hold several advantages over other liquid biopsy analytes. As a first point, EVs shed from glioblastoma and the tumor microenvironment are more diffusible than CTCs and may recapitulate the whole tumor when compared to trace CTCs that represent only a fraction of the multiclonal tumor heterogeneity [114]. Additionally, EVs are biologically stable, which permits storage at a variety of temperatures without degradation of their contents [115]. Finally, genomic analysis of plasma exosomal nucleic acids has a higher sensitivity for detecting common mutations than mutational analysis of plasma ctDNA [116]. Among the potential biomarkers with clinical utility in diagnosis, monitoring, and predicting treatment response in patients with glioblastoma, miRNAs are the most promising. For instance, there is evidence that changes in the mRNA level of alkylating repair enzymes within glioblastoma-derived exosomes from blood may potentially predict the emergence of temozolomide resistance during therapy [117, 118]. Another study reported a dramatic decrease in circulating exosomal miR-21, miR-222, and miR-124-3p in patients with glioblastoma, supporting the rationale of using microRNA-based biomarkers when monitoring for post-surgical progression [119]. On the contrary, extracellular vesicle enumeration from plasma, rather than detecting exosomal tumor-associated proteins and RNA levels may be helpful in detecting tumor presence, tracking responses to therapy, and confirming tumor progression [120]. Ongoing prospective trials are expected to provide longitudinal analyses of liquid biopsies in primary brain tumors to validate findings and enable entry into clinical practice (NCT05383872), (NCT05099068), (NCT04931732), and (NCT04940507).
Artificial Intelligence Methods
Progress in artificial intelligence (AI) methods applied to a vast amount of imaging, clinical, and molecular data will revolutionize treatment response monitoring in neuro-oncology. Machine learning (ML) and deep learning (DL) algorithms are being rapidly adopted in radiomics research to relate large-scale extracted imaging data to clinical and biological endpoints, making personalized precision cancer care possible [121]. ML can process unlabeled data without specified outcome variables; most ML used in medicine utilizes labeled data provided as “ground truth” and is thus supervised [121]. ML algorithms are being rapidly adopted in the task of automated tumor segmentation, a key task for rapid and reliable volumetric studies [41], as well as in radiomics research where large-scale multimodality imaging data is matched with clinical and biological endpoints, promising truly personalized cancer care [121]. Recent machine learning approaches have successfully predicted pseudoprogression [122, 123], tumor recurrence [124,125,126], and radiation necrosis [127] in glioblastoma. Furthermore, a machine learning algorithm has emerged as a putative imaging biomarker for identifying patients who may benefit most from antiangiogenic therapy [128]. Likewise, a recent deep learning model discriminated true progression from pseudoprogression in glioblastoma patients with a moderate accuracy comparable to advanced imaging methods [129]. As discussed earlier, there are several pitfalls in the RANO criteria for evaluating treatment response which may be remedied by AI. For example, Kickingereder et al. established an infrastructure to allow fully automated quantitative analysis of MRI and examined its effectiveness for tumor response assessment [130]. Based on their neural network, the researchers’ evaluation of tumor response yielded a better surrogate endpoint than the RANO assessment for predicting overall survival in an EORTC dataset. Additionally, the automatic evaluation of tumor response enabled a higher agreement to radiologist assessment than using RANO criteria [130]. Although artificial intelligence–based techniques may outperform standard response evaluation in neuro-oncology, the translation of radiomics and artificial intelligence algorithms into everyday multidisciplinary care plans is far from ready for daily use, and numerous translational challenges persist. These include the limited quality and clinical value of ground truth data used for AI training, and the lagging regulatory framework for interinstitutional data sharing and publication standards. Federated learning offers a promising solution to some of these issues, by enabling model-training to take place locally and multiple parallel sites, where raw data is stored and only models are shared aggregated between the sites maintaining a high level of data privacy and security and thus enabling extremely large-scale projects [131], and projects. Thus, enthusiasm towards developing platforms to permit more rapid integration of new AI algorithms into the clinical-neuroradiological workflow is highly justified [132, 133]. Several clinical research studies are underway to clarify the feasibility and clinical utility of artificial intelligence in the management of patients with glioblastoma (NCT05624736), (NCT05735171), (NCT04359745), and (NCT03452774).
Conclusions
Glioblastoma remains the most aggressive and recalcitrant of all the primary brain tumors in adults and is associated with a dismal prognosis despite multimodal therapy and decades of interventional studies. Evaluating the direct impact of standard therapies and novel investigational agents for the treatment of glioblastoma remains a formidable challenge in neuro-oncology. Several brain tumor assessment criteria have been developed and revised in the past decade to address these challenges. Currently, the RANO criteria are generally used in clinical trials to evaluate the effectiveness of investigational agents; nonetheless, efforts are underway to refine and standardize these guidelines. Several advanced imaging modalities have emerged with the potential to complement visual interpretation of glioblastoma response to therapies. In the era of precision medicine, liquid biopsy and artificial intelligence methodologies are poised to modernize our methods for measuring disease response. While the field of neuro-oncology continues to evolve, accelerating the pace and breath of these preliminary achievements into clinical practice will require large prospective randomized controlled studies. Furthermore, the implementation of these technological innovations on a large scale will also require industry to overcome issues with infrastructure, knowledge gaps, and disparities in access to care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Aum DJ, Kim DH, Beaumont TL, Leuthardt EC, Dunn GP, Kim AH. Molecular and cellular heterogeneity: the hallmark of glioblastoma. Neurosurg Focus. 2014;37(6):E11.
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–80.
Sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5(11):634–44.
Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008;29(3):419–24.
van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s Criteria. J Clin Oncol. 2009;27(18):2905–8.
Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72.
Leao DJ, Craig PG, Godoy LF, Leite CC, Policeni B. Response assessment in neuro-oncology criteria for gliomas: practical approach using conventional and advanced techniques. AJNR Am J Neuroradiol. 2020;41(1):10–20.
Abbasi AW, Westerlaan HE, Holtman GA, Aden KM, van Laar PJ, van der Hoorn A. Incidence of tumour progression and pseudoprogression in high-grade gliomas: a systematic review and meta-analysis. Clin Neuroradiol. 2018;28(3):401–11.
Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–7.
Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–49.
Han K, Ren M, Wick W, et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol. 2014;16(5):696–706.
Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5.
Prados M, Cloughesy T, Samant M, et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(1):143–51.
Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13(1):50–6.
Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–70.
Dempsey MF, Condon BR, Hadley DM. Measurement of tumor“size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR Am J Neuroradiol. 2005;26(4):770–6.
Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–20.
Rykkje AM, Larsen VA, Skjøth-Rasmussen J, Nielsen MB, Carlsen JF, Hansen AE. Timing of early postoperative MRI following primary glioblastoma surgery-a retrospective study of contrast enhancements in 311 patients. Diagnostics (Basel, Switzerland). 2023;13(4).
Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82(19):1684–92.
van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–93.
Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–42.
Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–92.
Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-278.
Arvold ND, Armstrong TS, Warren KE, et al. Corticosteroid use endpoints in neuro-oncology: response assessment in neuro-oncology working group. Neuro Oncol. 2018;20(7):897–906.
Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51.
Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” Acta Neuropathol. 2018;136(5):805–10.
Wen PY. RANO 2.0 glioma. Society for neuro-oncology 27th Annual Meeting & Education Day. Tampa Bay, Fl. 2022. https://www.eventscribeapp.com/live/videoPlayer.asp?lsfp=YlBEUzdlNFgyK2dMQS9tWStJU21RSk9JY0tWQ215L2ozTGtyU0NmbEJTaz0. This presentation provides a summary of the proposed updated response criteria (RANO 2.0) by the RANO working group.
Bent MJVD, Youssef G, Cloughesy TF, et al. RANO 2.0: proposal for an update to the response assessment in neuro-oncology (RANO) criteria for high- and low-grade gliomas in adults. J Clin Oncol. 2023;41(16_Suppl):2017–2017. This abstract provides a summary of the proposed updated response criteria (RANO 2.0) by the RANO working group.
Youssef G, Rahman R, Bay C, et al. Evaluation of standard response assessment in neuro-oncology, modified response assessment in neuro-oncology, and immunotherapy response assessment in neuro-oncology in newly diagnosed and recurrent glioblastoma. J Clin Oncol. 2023;41(17):3160–71. In this retrospective study, the authors compared the RANO criteria with updated mRANO and iRANO criteria in patients with newly diagnosed and recurrent glioblastoma to evaluate the performance of each set of criteria and inform the development of the planned RANO 2.0 update. The authors concluded that their data suggest that PFS obtained by RANO and modified RANO criteria correlates similarly with OS in patients with newly diagnosed and recurrent glioblastoma. Among patients who received immunotherapy, Spearman correlations were similar among RANO, modified RANO, and immunotherapy RANO.
Huang RY, Rahman R, Ballman KV, et al. The impact of T2/FLAIR evaluation per rano criteria on response assessment of recurrent glioblastoma patients treated with bevacizumab. Clin Cancer Res. 2016;22(3):575–81.
Boxerman JL, Zhang Z, Safriel Y, et al. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 2013;15(7):945–54.
Youland RS, Pafundi DH, Brinkmann DH, et al. Prospective trial evaluating the sensitivity and specificity of 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (18F-DOPA) PET and MRI in patients with recurrent gliomas. J Neurooncol. 2018;137(3):583–91.
Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–98.
Boxerman JL, Quarles CC, Hu LS, et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro Oncol. 2020;22(9):1262–75.
Chu HH, Choi SH, Ryoo I, et al. Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology. 2013;269(3):831–40.
Chenevert TL, Malyarenko DI, Galbán CJ, et al. Comparison of voxel-wise and histogram analyses of glioma ADC maps for prediction of early therapeutic change. Tomography (Ann Arbor, Mich). 2019;5(1):7–14.
Aime S, Travagin F, Terreno E, Giovenzana GB. Chapter 24 -chemistry of molecular imaging: an overview. In: Ross BD, Gambhir SS, editors. Molecular Imaging (second edn): Academic Press. 2021. p. 423–43.
Hamstra DA, Galbán CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26(20):3387–94.
Tsien C, Galbán CJ, Chenevert TL, et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol. 2010;28(13):2293–9.
Ellingson BM, Malkin MG, Rand SD, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31(3):538–48.
Baid U, Ghodasara S, Mohan S, et al. The RSNA-ASNR-MICCAI BraTS 2021 benchmark on brain tumor segmentation and radiogenomic classification. ArXiv. 2021;abs/2107.02314
Ellingson BM, Kim GHJ, Brown M, et al. Volumetric measurements are preferred in the evaluation of mutant IDH inhibition in non-enhancing diffuse gliomas: evidence from a phase I trial of ivosidenib. Neuro Oncol. 2022;24(5):770–8.
Kamson DO, Puri S, Sang Y, et al. Impact of frontline ivosidenib on volumetric growth patterns in isocitrate dehydrogenase (IDH) mutant astrocytic and oligodendroglial tumors. Clinical Cancer Res. 2023;CCR-23–0585.
Sidibe I, Tensaouti F, Gilhodes J, et al. Pseudoprogression in GBM versus true progression in patients with glioblastoma: a multiapproach analysis. Radiother Oncol. 2023;181:109486. This study investigated the feasibility of using a multiapproach analysis combining clinical data, diffusion- and perfusion-weighted imaging, and 3D magnetic resonance spectroscopic imaging to distinguish true tumor progression from pseudoprogression in patients with glioblastoma.
Kamson DO, Chinnasamy V, Grossman SA, et al. In-vivo magnetic resonance spectroscopy of lactate as a non-invasive biomarker of dichloroacetate activity in cancer and non-cancer central nervous system disorders. Front Oncol. 2023;13:1077461.
Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50(6):1120–6.
Jiang S, Eberhart CG, Lim M, et al. Identifying recurrent malignant glioma after treatment using amide proton transfer-weighted MR imaging: a validation study with image-guided stereotactic biopsy. Clin Cancer Res. 2019;25(2):552–61.
Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–57.
Juhász C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging. 2014;13. https://doi.org/10.2310/7290.2014.00015.
Galldiks N, Dunkl V, Stoffels G, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–95.
Timmermann C. ‘Just give me the best quality of life questionnaire’: the Karnofsky scale and the history of quality of life measurements in cancer trials. Chronic Illn. 2013;9(3):179–90.
Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky performance status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak. 2013;13:72.
Hutchinson TA, Boyd NF, Feinstein AR, Gonda A, Hollomby D, Rowat B. Scientific problems in clinical scales, as demonstrated in the Karnofsky index of performance status. J Chronic Dis. 1979;32(9–10):661–6.
Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–10.
Li J, Wang M, Won M, et al. Validation and simplification of the radiation therapy oncology group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–30.
Nayak L, DeAngelis LM, Brandes AA, et al. The neurologic assessment in neuro-oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625–35.
Iacono LA, Wells C, Mann-Finnerty K. Standardizing neurological assessments. J Neurosci Nurs. 2014;46(2):125–32.
Nilanont Y, Komoltri C, Saposnik G, et al. The Canadian neurological scale and the NIHSS: development and validation of a simple conversion model. Cerebrovasc Dis (Basel, Switzerland). 2010;30(2):120–6.
Dirven L, Aaronson NK, Heimans JJ, Taphoorn MJ. Health-related quality of life in high-grade glioma patients. Chin J Cancer. 2014;33(1):40–5.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5(1):139–50.
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9.
Chow R, Lao N, Popovic M, et al. Comparison of the EORTC QLQ-BN20 and the FACT-Br quality of life questionnaires for patients with primary brain cancers: a literature review. Support Care Cancer. 2014;22(9):2593–8.
Chiu N, Chiu L, Zeng L, et al. Quality of life in patients with primary and metastatic brain tumors in the literature as assessed by the FACT-Br. World J Oncol. 2012;3(6):280–5.
Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46.
Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neuro-Oncol. 2006;80(1):27–35.
Phillips KA, Fadul CE, Schiff D. Neurologic and medical management of brain tumors. Neurol Clin. 2018;36(3):449–66.
Bosma I, Vos MJ, Heimans JJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9(1):53–62.
Gately L, McLachlan SA, Dowling A, Philip J. Life beyond a diagnosis of glioblastoma: a systematic review of the literature. J Cancer Survivorship. 2017;11(4):447–52.
Solanki C, Sadana D, Arimappamagan A, et al. Impairments in quality of life and cognitive functions in long-term survivors of glioblastoma. J Neurosci Rural Pract. 2017;8(2):228–35.
Dick JP, Guiloff RJ, Stewart A, et al. Mini-mental state examination in neurological patients. J Neurol Neurosurg Psychiatry. 1984;47(5):496–9.
Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–9.
Johnson DR, Sawyer AM, Meyers CA, O’Neill BP, Wefel JS. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2012;14(6):808–16.
Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro Oncol. 2003;5(2):89–95.
Klein M, Postma TJ, Taphoorn MJ, et al. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology. 2003;61(12):1796–8.
Berendsen S, Varkila M, Kroonen J, et al. Prognostic relevance of epilepsy at presentation in glioblastoma patients. Neuro Oncol. 2016;18(5):700–6.
van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. The Lancet Neurol. 2007;6(5):421–30.
Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol. 2020;22(4):515–23.
Grogan D, Bray DP, Cosgrove M, et al. Clinical and radiographic characteristics of diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma: a single institution review. J Neurooncol. 2022;157(1):187–95.
Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018;20(1):103–12.
Soffietti R, Rudà R, Bradac GB, Schiffer D. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43(5):1066–73.
Pace A, Vidiri A, Galiè E, et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Annals Oncol. 2003;14(12):1722–6.
Haggiagi A, Avila EK. Seizure response to temozolomide chemotherapy in patients with WHO grade II oligodendroglioma: a single-institution descriptive study. Neuro-oncology Pract. 2019;6(3):203–8.
You G, Sha ZY, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro Oncol. 2012;14(2):230–41.
Koekkoek JA, Dirven L, Heimans JJ, et al. Seizure reduction is a prognostic marker in low-grade glioma patients treated with temozolomide. J Neurooncol. 2016;126(2):347–54.
Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol. 2017;19(1):12–21.
Zhang L, Riethdorf S, Wu G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18(20):5701–10.
Kulasinghe A, Schmidt H, Perry C, et al. A collective route to head and neck cancer metastasis. Sci Rep. 2018;8(1):746.
Kulasinghe A, Kapeleris J, Cooper C, Warkiani ME, O’Byrne K, Punyadeera C. Phenotypic characterization of circulating lung cancer cells for clinically actionable targets. Cancers. 2019;11(3):380.
Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18(5):297–312.
Müller C, Holtschmidt J, Auer M, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014;6(247):247ra101.
Macarthur KM, Kao GD, Chandrasekaran S, et al. Detection of brain tumor cells in the peripheral blood by a telomerase promoter-based assay. Can Res. 2014;74(8):2152–9.
Gao F, Cui Y, Jiang H, et al. Circulating tumor cell is a common property of brain glioma and promotes the monitoring system. Oncotarget. 2016;7(44):71330–40.
Gatto L, Franceschi E, Di Nunno V, Tosoni A, Lodi R, Brandes AA. Liquid biopsy in glioblastoma management: from current research to future perspectives. Oncologist. 2021;26(10):865–78.
Soffietti R, Bettegowda C, Mellinghoff IK, et al. Liquid biopsy in gliomas: A RANO review and proposals for clinical applications. Neuro-oncology. 2022;24(6):855–71. This review article summarizes the potential clinical applications of liquid biopsy in the management of glioma and provides suggestions for integrating liquid biopsies into clinical trials.
Kolostova K, Pospisilova E, Pavlickova V, et al. Next generation sequencing of glioblastoma circulating tumor cells: non-invasive solution for disease monitoring. Am J Transl Res. 2021;13(5):4489–99. This study confirms the feasibility of using CTCs in peripheral blood in patients with glioblastoma as a source of DNA to performing next-generation anaysis in to confirm diagnosis, identifying mutations present, monitoring tumor evolution, and response to therapy.
Müller Bark J, Kulasinghe A, Hartel G, et al. Isolation of circulating tumour cells in patients with glioblastoma using spiral microfluidic technology - a pilot study. Front Oncol. 2021;11:681130.
Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224.
Zill OA, Banks KC, Fairclough SR, et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin Cancer Res. 2018;24(15):3528–38.
Piccioni DE, Achrol AS, Kiedrowski LA, et al. Analysis of cell-free circulating tumor DNA in 419 patients with glioblastoma and other primary brain tumors. CNS Oncol. 2019;8(2):Cns34.
Schwaederle M, Husain H, Fanta PT, et al. Detection rate of actionable mutations in diverse cancers using a biopsy-free (blood) circulating tumor cell DNA assay. Oncotarget. 2016;7(9):9707–17.
Pentsova EI, Shah RH, Tang J, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34(20):2404–15.
De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839.
Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565(7741):654–8.
Mouliere F, Mair R, Chandrananda D, et al. Detection of cell-free DNA fragmentation andcopy number alterations in cerebrospinal fluid from glioma patients. EMBO Mol Med. 2018;10(12):e9323.
Stallard S, Savelieff MG, Wierzbicki K, et al. CSF H3F3A K27M circulating tumor DNA copy number quantifies tumor growth and in vitro treatment response. Acta Neuropathol Commun. 2018;6(1):80.
Friedman JS, Hertz CAJ, Karajannis MA, Miller AM. Tapping into the genome: the role of CSF ctDNA liquid biopsy in glioma. Neuro-oncology Adv. 2022;4(Suppl 2):ii33–40.
Bagley SJ, Nabavizadeh SA, Mays JJ, et al. Clinical utility of plasma cell-free DNA in adult patients with newly diagnosed glioblastoma: a pilot prospective study. Clin Cancer Res. 2020;26(2):397–407.
Carpenter EL, Bagley SJ. Clinical utility of plasma cell-free DNA in gliomas. Neuro-oncology Adv. 2022;4(Suppl 2):ii41–4.
Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563(7732):579–83.
Nassiri F, Chakravarthy A, Feng S, et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med. 2020;26(7):1044–7.
Sabedot TS, Malta TM, Snyder J, et al. A serum-based DNA methylation assay provides accurate detection of glioma. Neuro Oncol. 2021;23(9):1494–508.
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–38.
Westphal M, Pantel K, Ricklefs FL, et al. Circulating tumor cells and extracellular vesicles as liquid biopsy markers in neuro-oncology: prospects and limitations. Neuro-oncology Adv. 2022;4(Suppl 2):ii45–52.
Zhou B, Xu K, Zheng X, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5(1):144.
Möhrmann L, Huang HJ, Hong DS, et al. Liquid biopsies using plasma exosomal nucleic acids and plasma cell-free DNA compared with clinical outcomes of patients with advanced cancers. Clin Cancer Res. 2018;24(1):181–8.
Shao H, Chung J, Lee K, et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun. 2015;6:6999.
Zeng A, Wei Z, Yan W, et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018;436:10–21.
Santangelo A, Imbrucè P, Gardenghi B, et al. A microRNA signature from serum exosomes of patients with glioma as complementary diagnostic biomarker. J Neurooncol. 2018;136(1):51–62.
Osti D, Del Bene M, Rappa G, et al. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019;25(1):266–76.
Avanzo M, Wei L, Stancanello J, et al. Machine and deep learning methods for radiomics. Med Phys. 2020;47(5):e185–202.
Cha J, Kim ST, Kim HJ, et al. Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol. 2014;35(7):1309–17.
Patel M, Zhan J, Natarajan K, et al. Machine learning-based radiomic evaluation of treatment response prediction in glioblastoma. Clin Radiol. 2021;76(8):628.e617-628.e627.
Chang PD, Chow DS, Yang PH, Filippi CG, Lignelli A. Predicting glioblastoma recurrence by early changes in the apparent diffusion coefficient value and signal intensity on FLAIR images. AJR Am J Roentgenol. 2017;208(1):57–65.
Chang PD, Malone HR, Bowden SG, et al. A multiparametric model for mapping cellularity in glioblastoma using radiographically localized biopsies. AJNR Am J Neuroradiol. 2017;38(5):890–8.
Akbari H, Macyszyn L, Da X, et al. Imaging surrogates of infiltration obtained via multiparametric imaging pattern analysis predict subsequent location of recurrence of glioblastoma. Neurosurgery. 2016;78(4):572–80.
Hu X, Wong KK, Young GS, Guo L, Wong ST. Support vector machine multiparametric MRI identification of pseudoprogression from tumor recurrence in patients with resected glioblastoma. J Magn Reson Imaging. 2011;33(2):296–305.
Kickingereder P, Götz M, Muschelli J, et al. Large-scale radiomic profiling of recurrent glioblastoma identifies an imaging predictor for stratifying anti-angiogenic treatment response. Clin Cancer Res. 2016;22(23):5765–71.
Moassefi M, Faghani S, Conte GM, et al. A deep learning model for discriminating true progression from pseudoprogression in glioblastoma patients. J Neurooncol. 2022;159(2):447–55.
Kickingereder P, Isensee F, Tursunova I, et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 2019;20(5):728–40.
Rieke N, Hancox J, Li W, et al. The future of digital health with federated learning. NPJ Digit Med. 2020;3:119.
Wagner MW, Namdar K, Biswas A, Monah S, Khalvati F, Ertl-Wagner BB. Radiomics, machine learning, and artificial intelligence-what the neuroradiologist needs to know. Neuroradiology. 2021;63(12):1957–67.
Ciecierski-Holmes T, Singh R, Axt M, Brenner S, Barteit S. Artificial intelligence for strengthening healthcare systems in low- and middle-income countries: a systematic scoping review. NPJ Digit Med. 2022;5(1):162.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kester A. Phillips, David O. Kamson, and David Schiff declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Phillips, K.A., Kamson, D.O. & Schiff, D. Disease Assessments in Patients with Glioblastoma. Curr Oncol Rep 25, 1057–1069 (2023). https://doi.org/10.1007/s11912-023-01440-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01440-2