Abstract
Purposeof Review
Head and neck cancer (HNC) comprises a group of malignancies, amongst which squamous cell carcinoma accounts for more than 90% of the cases. HNC has been related to tobacco use, alcohol consumption, human papillomavirus, Epstein-Barr virus, air pollution, and previous local radiotherapy. HNC has been associated with substantial morbidity and mortality. This review aims to summarize the recent findings regarding immunotherapy in HNC.
Recent Findings
The recent introduction of immunotherapy, with the use of programmed death 1 (PD-1) inhibitors pembrolizumab and nivolumab, which have been FDA approved for the treatment of metastatic or recurrent head and neck squamous cell carcinoma, has changed the field in metastatic or recurrent disease. There are many ongoing trials regarding the use of novel immunotherapeutic agents, such as durvalumab, atezolizumab, avelumab, tremelimumab, and monalizumab.
Summary
In this review, we focus on the therapeutic potential of novel immunotherapy treatment modalities, such as combinations of newer immune-checkpoint inhibitors; the use of tumor vaccines such as human papillomavirus-targeted vaccines; the potential use of oncolytic viruses; as well as the latest advances regarding adoptive cellular immunotherapy. As novel treatment options are still emerging, a more personalized approach to metastatic or recurrent HNC therapy should be followed. Moreover, the role of the microbiome in immunotherapy, the limitations of immunotherapy, and the various diagnostic, prognostic, and predictive biomarkers based on genetics and the tumor microenvironment are synopsized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HNC is the seventh-most common cancer worldwide [1]. It has been estimated that it accounts for more than 600,000 new cases and approximately 325,000 deaths annually [2, 3]. In addition, its incidence will increase by 30% in 2030 [2,3,4]. This increasing trend is observed particularly in oropharyngeal carcinoma [4].
Head and neck cancer comprises a variety of malignancies, of which more than 90% involve head and neck squamous cell carcinoma (HNSCC) [5]. Although HNC is typically diagnosed among older patients with a history of heavy tobacco and alcohol use, this trend has declined in the Western world due to the decrease in tobacco consumption. On the contrary, human papillomavirus-associated (HPV-associated) HNC has increasingly been recognized among younger patients in northern Europe and the USA [6, 7]. Of the 120 types of HPV, the oncogenic types 16 and 18 account for more than 90% of HPV-associated HNSCC [8]. There is an increased likelihood of developing HPV-associated HNSCC after 10 to 30 years of oral sex. Oral sex has been implicated in the development of HPV-associated HNSCC in many studies [9, 10]. Another virus, Epstein-Barr virus has also been suggested to be involved in the pathogenesis of nasopharyngeal carcinoma, while air pollutants and previous local radiotherapy have also been implicated in the development of HNC [12, 13••]. Besides, in southern Asia, betel chewing has also been documented as an established risk factor [4]. Moreover, genetic predisposition related to specific loci, diet, and the microbiome have also been involved in the pathogenesis of HNSCC [4].
Despite advances in the treatment of local HNSCC with the use of transoral robotic surgery (TORS), metastatic or recurrent disease occurs in approximately 50 to 60% of patients with stages III or IV of the disease [14]. Notably, the majority of recurrences are not eligible for surgery or/and local radiotherapy. It is estimated that approximately 60% of patients with HNSCC in the UK present with stages III or IV of the disease. Interestingly, most patients with oral or oropharyngeal cancer present with stage IV at diagnosis, whereas most patients with laryngeal cancer present with stage I of the disease [18]. Metastatic disease had a poor prognosis, with a median overall survival (OS) of 6 months in the past [15]. However, the advent of immunotherapy has revolutionized our understanding as well as the treatment modalities in recurrent or metastatic (R/M) HNSCC [16, 17•, 18]
In this review, we aim to discuss current treatment choices regarding immunotherapy in HNSCC as well as explore novel immunotherapeutic agents that are candidates for future treatment protocols. Special emphasis is given to combinations of newer immune checkpoint inhibitors; the use of tumor vaccines such as human papillomavirus-targeted vaccines; the potential use of oncolytic viruses; the latest advances in the use of adoptive cellular immunotherapy; the role of the microbiome in immunotherapy; and the limitations of immunotherapy. Finally, we will synopsize various diagnostic, prognostic, and predictive biomarkers based on genetics and the tumor microenvironment (TME).
The Concept of Immunotherapy in Cancer
Immunotherapy has emerged as a novel treatment modality in cancer during the last 2 decades [19]. However, the concept of the participation of the immune system in cancer prognosis dates back to 1893, when Dr. William Coley noted that patients with cancer and post-surgical infection had better outcomes [20]. In particular, he reported ten cases of cancer patients who were administered heat-killed bacteria-causing erysipelas, the so-called “Coleys’ toxins,” and who exhibited a better prognosis than patients without infection [20]. For this conception, which was really outstanding, Dr. William Coley has been recognized as the father of cancer immunotherapy [21]. Nowadays, it is widely known that the immune system plays a key role in cancer cell regulation. More specifically, T cells and antigen-presenting cells (APCs) are the cornerstone of immune system responses in cancer. Immunotherapy focuses on the development of therapeutic agents that may mitigate T cell and APC responses in the context of the TME. T cell receptor was discovered in 1982 by Allison, who has extensively studied T cell responses in cancer. Allison and Honjo were awarded the Nobel Prize in 2018 in Physiology for their research on immune checkpoint inhibitors (ICIs). ICIs are suggested to limit the inflammatory responses taking place after the activation of T cells [18]. The first ICI that was developed was the CTLA-4, which was discovered by Brunet et al. in the 1980s [22]. Ipilimumab, a CTLA-4 monoclonal antibody, was the first immunotherapeutic agent approved by the Food and Drug Administration (FDA) in 2011 for the treatment of metastatic melanoma [19]. Programmed cell death 1 (PD-1) gene was first discovered in 1992 by Honjo et al. and programmed cell death ligand 1 (PD-L1) ensued within a few years [23]. In 2016, the FDA granted the anti-PD-1 antibodies pembrolizumab and nivolumab accelerated approval for treating non-small cell lung carcinoma due to their durable objective responses. Nowadays, anti-PD-1/PD-L1 agents are rapidly emerging as treatment options in various types of cancer, such as metastatic melanoma, non-small cell lung carcinoma, small cell lung carcinoma, triple-negative breast cancer, pancreatic cancer, platinum-resistant ovarian cancer, cervical cancer, renal cell carcinoma, gastric and gastroesophangeal junction adenocarcinoma, colorectal cancer, hepatocellular carcinoma, and prostate cancer [18,19,20,21,22,23].
Immunotherapy in Recurrent or Metastatic HNSSC
PD-1 and PD-L1 as Immunotherapeutic Agents
PD-1 monoclonal antibody pembrolizumab has been the first immunotherapeutic agent used for R/M HNSSC [17•]. The KEYNOTE studies have assessed the efficacy and adverse effects of pembrolizumab in R/M HNSCC. The KEYNOTE studies comprise three studies: KEYNOTE-012, which was a phase I study that ended in 2016, the KEYNOTE-055, which was a phase II study that ended in 2017, and the KEYNOTE-048, which was a phase III study that ended in 2019 [24,25,26]. In the KEYNOTE-012 study, 192 patients with R/M HNSCC were enrolled. Among them, 60 patients were administered pembrolizumab 10 mg/kg every 2 weeks, and 132 patients were administered pembrolizumab 200 mg every 3 weeks. A complete response (CR) was noted in 4% of treated patients and a partial response (PR) in 14%. Seventy-one percent of responses lasted more than 12 months, which is indicative of the durability of responses to this monoclonal antibody [24]. In addition, pembrolizumab has also been administered in comparison with standard therapy in the KEYNOTE-055 and KEYNOTE-048 studies, where it showed similar results to the KEYNOTE-012 study [25, 26]. In particular, in the KEYNOTE-055 study, a phase II single-arm trial, 171 patients with HNSCC received 200 mg of pembrolizumab every 3 weeks. The overall response rate (RR) was 16%, with a median duration of 8 months [25]. In the KEYNOTE-048 study, an open-label phase III trial, 247 patients with HNSCC received 200 mg of pembrolizumab every 3 weeks, and 248 patients received methotrexate, docetaxel, or cetuximab as a standard of care therapy. Median overall survival (OS) was 8.4 months in the pembrolizumab group and 6.9 months in the standard-of-care group [26]. Adverse effects were fatigue, diarrhea, decreased appetite, hypothyroidism, adrenal insufficiency, pneumonitis, fever, rash, and pruritus [25, 26]. Based on KEYNOTE-012, KEYNOTE-055, and KEYNOTE-048 studies, pembrolizumab has shown a significant prolongation in the OS and a favorable safety profile when compared to chemotherapy [24, 26].
Apart from pembrolizumab, nivolumab, another PD-1 monoclonal antibody, has been employed in the CheckMate 141 trial among 361 patients with R/M HNSCC who progressed after platinum chemotherapy [27]. The CheckMate 141 trial was a randomized phase III study that evaluated the efficacy of the administration of nivolumab at a dose of 3 mg/kg every 2 weeks in 240 patients, while 121 patients received standard-of-care therapy. Among these patients, there was an estimated survival rate at 1 year of 36% with nivolumab versus 16.6% with standard treatment (methotrexate, docetaxel, or cetuximab). The response rate was 13.3% in the nivolumab group versus 5.8% in the standard single-agent therapy group. Regarding adverse effects, fatigue, nausea, decreased appetite, rash, pruritus, and hypothyroidism were reported [27]. Nivolumab was granted FDA approval on November 10, 2016, for the treatment of R/M HNSCC as a result of the promising outcomes of the CheckMate 141 trial.
Based on the KEYNOTE-012 and the CheckMate 141 trials, respectively, pembrolizumab and nivolumab were the first immunotherapeutic agents approved for R/M HNSSC. Pembrolizumab and nivolumab were documented to result in improved OS as well as increased PFS compared to standard treatment [28].
Nevertheless, as there is ongoing research in this field, other agents have also been investigated in this regard. PD-L1 blockade by the monoclonal antibody durvalumab has been evaluated in the HAWK study among 112 patients with R/M HNSSC who exhibited PD-L1 tumor expression ≥ 25% [29]. Median OS and PFS were 7.1 months (95% CI, 4.9–9.9) and 2.1 months (95% CI, 1.9–3.7), respectively. OS and PFS at 12 months were 33.6% (95% CI, 24.8–42.7) and 14.6% (95% CI, 8.5–22.1). Adverse effects included fatigue, nausea, decreased appetite, hypothyroidism, diarrhea, pruritus, and rash [30].
Two other immunotherapeutic anti-PD-L1 agents, atezolizumab and avelumab, have also been used. Atezolizumab was administered in 32 patients with advanced HNSSC and showed a median OS of 6 months and a median PFS of 2.6 months without major adverse events. Interestingly, the questionnaire evaluating the quality of life yielded positive outcomes regarding its administration [31]. On the other hand, avelumab was administered in the JAVELIN study among 153 patients with R/M HNSSC and demonstrated a median OS of 8 months. Adverse effects were documented in 83 of the 153 patients, the most common being fatigue, fever, and pruritus [32]. Atezolizumab and avelumab have been reported to result in objective response rates of 22 and 13.1%, respectively, which are equal to or slightly better compared to pembrolizumab, nivolumab, and durvalumab [17•].
Overall, PD-1/PD-L1 blockade is capable of restoring anti-tumor immune responses, mainly mediated by CD8 + lymphocytes in cases of R/M HNSSC [16]. It is noteworthy that Chen et al. have demonstrated that p16 protein expression, which translates into HPV-positivity, is highly associated with PD-L1 expression in HNSSC [33]. This association may account for the better response rates with PD-1/PD-L1 blockade among HPV-positive HNSSC patients when compared to HPV-negative HNSSC ones [16].
Other Immuno-Based Treatment Modalities Beyond PD-1/PD-L1
Tregs are a sub-group of CD4 + lymphocytes that express the transcription factor 3, Foxp-3 (forkhead box protein 3), and CD25 [34••, 35]. This subgroup exists in the blood as well as in the stroma of HNSSC, where it exerts tumor-promoting effects [36]. More specifically, Tregs are capable of secreting inhibitory cytokines, such as IL-10, IL-35, and TGF-β, upregulating inhibitory receptors, as well as depriving the local TME of IL-2 through the increase of CD25 expression [37, 38]. Cytotoxic T lymphocyte antigen 4 (CTLA-4), is highly expressed in intratumoral Tregs. This expression is further enhanced after treatment with cetuximab [39]. Cetuximab is a monoclonal antibody targeting epidermal growth factor receptor (EGFR), which has gained FDA approval for HNSCC treatment in 2006 [39, 40]. CTLA-4 Tregs exert inhibitory effects on natural killer (NK) cell functionality after treatment with cetuximab. Ipilimumab and tremelimumab, which are monoclonal antibodies against CTLA-4, seem to restore the functionality of NK cells via the depletion of Tregs, thus exhibiting immunotherapeutic potential [39, 40]. As Tregs are suggested to promote an immunosuppressive TME in HNSSC, their inhibition in the TME of HNSSC may restore immune responses [39, 40]. Moreover, as resistance to cetuximab may rapidly develop among patients with R/M HNSSC, the use of anti-CTLA-4 therapy could be of special interest. Due to the fact that Tregs may mitigate the efficacy of treatment with anti-PD-1/PD-L1, the administration of an anti-CTLA-4 agent may play a crucial role in ameliorating sensitivity to anti-PD-1/PD-L1 drugs [15]. In this context, CONDOR and EAGLE studies were performed to further assess the co-administration of the PD-L1 monoclonal antibody durvalumab with the anti-CTLA-4 agent tremelimumab [40, 41]. In the CONDOR study, 256 patients with R/M/HNSSC were enrolled, and the median PFS in the combination group was 2 months, while in the monotherapy groups, it was 1.9 months for each of the two drugs administered, i.e., durvalumab and tremelimumab. Notably, adverse effects, such as fatigue and diarrhea were similar in the group receiving durvalumab monotherapy, tremelimumab monotherapy, and the group receiving their combination [40]. In the EAGLE Study, 736 patients with R/M HNSSC were enrolled, and the median PFS and OS were similar in the three different groups, i.e., the group receiving only durvalumab, the group receiving durvalumab plus tremelimumab, and the last group receiving standard of care chemotherapy (cetuximab, taxane, methotrexate, or fluoropyrimidine). The most common adverse effect for durvalumab and durvalumab plus tremelimumab was hypothyroidism, whereas anemia was the most frequent adverse effect in the standard of care group [41]. Despite the fact that the outcomes from the CONDOR and EAGLE studies were not very encouraging, there is ongoing investigation regarding anti-CTLA-4 drugs as monotherapy or in combination with different category agents in R/M HNSSC.
Apart from the anti-CTLA-4 drugs, other agents with therapeutic potential against Tregs include anti-TIM-3 and anti-LAG-3 targeting drugs. Anti-TIM-3 agents are heading towards T cell immunoglobulin and mucin domain-containing protein 3 [16]. In addition, anti-LAG-3 agents are being developed targetινγ lymphocyte activation gene 3. More specifically, eftilagimod alpha is a soluble LAG-3 protein that binds to MHC II, thereby activating APCs as well as CD8 + T cells. Eftilagimod alpha is expected to increase the anti-tumor responses of PD-1/PD-L1 when used in combination [16]. A trial expected to enroll 189 participants with R/M HNSSC to receive eftilagimod alpha together with pembrolizumab is active but not recruiting yet [NCT03625323]. Regarding anti-TIM-3 therapy, there is an ongoing trial administering INCAGN02385 and INCAGNO2390 together with the anti-PD-L1 retifanlimab among 162 patients with R/M HNSSC [NCT05287113]. The results of this trial are eagerly anticipated.
Other treatment modalities include monoclonal antibodies, which prevent the binding of NK group 2 member A (NKG2A) to HLA-E in NK cells [42]. HLA-E is a member of the non-classical HLA (human leukocyte antigen) histocompatibility complex, which is overexpressed in HNSSC [42]. NKG2A is a receptor of the NK cells as well as of a sub-group of CD8 + T cells. Monalizumab is the first monoclonal antibody blockading the NKG2A receptor that has been evaluated in the UPSTREAM study. The immunotherapy 1 cohort of the UPSTREAM study has enrolled 26 patients with R/M HNSSC and has shown a median PFS of 1.7 months (95% CI, 1.5–1.8) and a median OS of 6.7 months (95% CI, 3.0–9.6). In this cohort, monalizumab presented limited effectiveness in patients with R/M HNSSC. However, an immunotherapy cohort 2 with the addition of durvalumab to monalizumab is under investigation within the UPSTREAM study [43]. Moreover, Andre et al. have examined the efficacy of monalizumab when added to cetuximab in patients with HNSSC [44]. The results have shown a 31% objective response rate, which was attributed to the dual activity enhancement of NK cells as well as T cells. The most common adverse effects included fatigue, fever, and headache [44].
Another possible target is the inducible T cell co-stimulator (ICOS) together with its ligand, the inducible T cell co-stimulator ligand (ICOSL). The INDUCE-3 trial has embarked on investigating the effects of the ICOS receptor agonist antibody GSK3359609, feladilimab, as an add-on therapy to pembrolizumab among 315 patients with R/M HNSSC [NCT04128696]. The INDUCE-4 trial is an active trial of the effects of GSK3359609, feladilimab, together with pembrolizumab and 5FU-platinum chemotherapy among 118 patients with R/M HNSSC [NCT04428333]. The results of both INDUCE-3 and INDUCE-4 trials have not been published yet.
Overall, there is ongoing research regarding the development of various immunotherapeutic agents beyond the PD-1/PD-L1 axis. Currently, these agents are being studied either alone or in combination with the anti-PD-1/PD-L1 drugs. Notably, the latter are considered the mainstay of immunotherapy among patients with R/M HNSSC.
Tumor Vaccines
Tumor vaccines may be used for the activation of the immune system against the development of cancer. They are categorized into prophylactic and therapeutic tumor vaccines. Paradigms of prophylactic tumor vaccines are vaccines against hepatitis B virus, which protect against the development of hepatocellular carcinoma, and against HPV, which protects against HPV-associated cervical carcinoma. Bacillus Calmette-Guerin (BCG) vaccine is an FDA-approved vaccine for the treatment of early-stage bladder cancer, while Sipuleucel-T is FDA-approved for the treatment of prostate cancer. Sipuleucel-T comprises APCs, that have been activated ex vivo with a recombinant fusion protein, PA2024. This PA2024 fusion protein consists of a prostate antigen, such as prostatic acid phosphatase, which is fused to an immune-cell activator, the granulocyte-macrophage colony-stimulating factor [45••].
The expression of E6 and E7 proteins by HPV results in the degradation of p53 gene; hence, leading to uncontrolled cellular proliferation. HPV vaccines are suggested to prevent more than 90% of HPV-associated head and neck pre-cancerous lesions [18]. However, the majority of tumor antigens are being recognized as self-antigens by the immune system. HPV vaccines targeting E6/E7 oncogenes of HPV16 are not able to induce complete remission of HNSCC by themselves [45••]. Therefore, they are increasingly being tested in conjunction with ICIs in R/M HNSCC, promoting T cell responses [45••]. Indeed, there are various ongoing trials that study a variety of combinations of HPV16 E6/E7 targeted oncogenes in therapeutic vaccines with different novel ICIs. Table 1 depicts ongoing trials employing HPV16-targeted vaccines in combination with various immunotherapeutic agents.
Adoptive Cellular Immunotherapy
Adoptive cellular immunotherapy refers to the transfer of immune cells, which possess anti-tumor properties to patients with cancer [46, 47]. Chimeric antigen receptor T cell (CAR-T cell) therapy is the most widely known paradigm of adoptive cellular immunotherapy, which has been increasingly used in hematologic malignancies [46, 47]. CAR-T cell therapy works by recognizing and eradicating specific targets on the surface of cancer cells. In HNSCC, potential targets for CAR-T cells are the following: CD27, EGFR, MICA, MICB, MAGE-A4, FAP, EPCAM, CD70, and B4GALNT1 [48••]. Besides, one of the potential targets belongs to the ErbB family, consisting of ErbB1 (EGFR), ErbB2 (HER2/neu), ErbB 3, and ErbB4. CAR-T cell therapy targeting ErbB2 has resulted in a 56% reduction in tumor size [49]. CAR-T cell therapy targeting the CD70-positive HNSCC cells has shown promising results. CD70/CAR-T cell therapy may be a future candidate for CD70-positive HNSCC, but not for the treatment of HNSCC in general [50]. Furthermore, approximately 15% of HNSCC carry NOTCH 1 mutations, making synNOTCH CAR-T cell therapy suitable for this group of patients with HNSCC [50].
CAR-T cell therapy is classified as HPV-associated HNSCC and non-HPV-associated HNSCC. In the first case, E6 and E7 viral proteins are targeted by T cells. Initial results have shown complete tumor regression after the administration of tumor-infiltrating lymphocytes (TIL). An ongoing trial (NCT03083873) is underway, evaluating the efficacy of TIL (LN-145) administration in R/M HNSCC [51]. Moreover, HPV16E6 peptide T cell receptor gene therapy has shown an objective tumor response in 17% of patients, while HPV16E7 T cell receptor gene therapy (clinical phase I/II trial) has reported an objective tumor response in approximately 50% of patients [52]. For non-HPV-associated HNSCC patients, there are two procedures: Epstein-Barr virus T cells and cancer germline antigens. Melanoma-associated antigen 4 and Kita-Kyushu lung cancer antigen 1 are currently under investigation as possible antigen targets for the treatment of HNSCC in the context of cancer germline therapy [53]. Although there is much progress in the administration of adoptive cellular immunotherapy, this technique is still being performed very rarely among patients with HNSCC. Nevertheless, this is a technique that is evolving. Its substantial toxicity until now, mainly the cytokine release syndrome, which may result even in multi-organ dysfunction, limits its widespread use for the time being [54, 55].
Oncolytic Viruses (OVs)
Oncolytic viruses (OVs) have the ability to differentially target tumor cells and destroy them, whereas they do not affect normal cells in the host. OVs may directly kill tumor cells or indirectly augment anti-tumor immune responses by releasing pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), or tumor-associated antigens (TAAs). They are categorized into naturally occurring OVs and genetically modified OVs, both of which can lead to tumor cell lysis [54, 55]. T-VEC (talimogene laherparepvec) is a doubly mutated HSV-1 that possesses the ability to infect tumor cells and replicate within them. It accomplishes this infection and replication within tumor cells through the utilization of various cell receptors, such as glycoproteins, nectins, and herpesvirus entry mediators [56, 57]. Notably, its replication has been related to the interruption of some other oncogenic signaling pathways, namely protein kinase R and interferon (IFN) type I pathways [57]. Its anti-tumor activity may be enhanced by a granulocyte-macrophage colony-stimulating factor (GM-CSF), which has been documented to recruit dendritic cells to the sites of inflammation, thus further stimulating antigen-presenting cell (APC) functionality and T cell responses [57]. HF10 is another virus that is naturally mutated in the UL56 gene, possessing the ability to replicate and kill tumor cells together with suppressing tumor growth among patients with HNSCC [58]. This novel, evolving technique using OVs could also be combined with chemotherapy or CAR-T cell therapy [54, 55]. Nevertheless, the combination of T-VEC with pembrolizumab has not resulted in an improved ORR when compared to pembrolizumab alone thus far [54, 55].
Diagnostic and Prognostic Biomarkers in HNSCC
Programmed Death Ligand-1 (PD-L1) Expression: Is it Reliable?
Although ICIs have revolutionized the treatment of R/M HNSCC, not all patients are eligible to respond to ICIs. Currently, responders have been authorized to receive ICIs based solely on the expression of PD-L1 in tumor or stroma cells [59]. However, the level of PD-L1 expression is still questionable. In the KEYNOTE-012 study, even expression of PD-L1 in at least 1% of cancer cells or stroma cells by immunochemistry staining has been associated with improved overall survival when compared with patients with a PD-L1 expression of less than 1% [60•]. Therefore, this more than 1% PD-L1 expression has been designated as a marker for a potential response to ICIs. Nonetheless, this 1% index has been a matter of debate, as later studies have shown that even patients with HNSSC with less than 1% expression of PD-L1 could benefit from ICIs [61, 62].
Thus, as PD-L1 expression is not considered a reliable tool to assess the therapeutic potential with ICIs, other biomarkers, such as microsatellite instability (MSI) and tumor mutational burden (TMB), have already been developed [63]. MSI is defined as the DNA mismatch repair defect system in microsatellites, i.e., in repetitive DNA motifs close to important genes. MSI has been generally classified as high-level and low-level MSI [59, 60•, 61,62,63]. High-level MSI has been associated with a better prognosis and response to immunotherapy and may serve as a marker for individualized cancer treatment. However, HNSCC has not been related to a significant MSI, as is the case of adenocarcinomas. This fact precludes its utility in HNSCC [59, 60•, 61,62,63].
Besides, TMB, which is defined as the number of non-inherited mutations per million bases of the investigated genome, is being assessed due to the advent of next-generation sequencing (NGS) [64•, 65, 66]. As TMB has been suggested to correlate with a high burden of neoantigens, the increased presence of neoantigens could be associated with a more evident activation of cytotoxic T cells and a better response to immunotherapy [59, 67•]. Indeed, a better OS has been documented with the use of ICIs among patients with high TMB values [67•, 68, 69]. Nevertheless, even patients with lower than 20 mutations/per million bases may benefit from immunotherapy. In addition, standardization of the techniques used to estimate TMB is mandatory, as differences in NGS platforms as well as bioinformatics analyses may lead to non-comparable results [67•, 68, 69]. Therefore, there is a need for further investigation in this field to establish the effectiveness or not of immunotherapy in HNSCC.
Gene Panels Predicting Response to Immunotherapy
Huang et al. have recently proposed a 25-gene mutation signature, which serves as a better predictor of response to the administration of ICIs than TMB [59]. In particular, they have suggested that the implementation of a 25-gene panel may better predict the patients who will benefit from ICIs than the high-TMB score, i.e., a TMB score ≥ 10. This TMB score ≥ 10 has been designated by the FDA for the approval of pembrolizumab treatment among patients with advanced solid tumors. It is noteworthy that Huang et al. have demonstrated that this 25-gene panel may include patients with a low TMB score, i.e., less than 10, but who will still benefit from the administration of ICIs [59]. More specifically, this 25-gene mutation signature includes genes that were predictive of response to ICIs. For example, EP300 has already been documented as a predictive biomarker of response to ICIs in various cancers [59, 70, 71]. It works by altering the differentiation of CD4+ lymphocytes into T-regulatory cells (Tregs) [70, 71]. Tregs have a pivotal role in suppressing autoimmunity. Their immune-suppressive ability is considered to be a drawback for the response to ICIs in the context of tumor microenvironment. Therefore, this drawback could be overcome by reducing their accumulation in tumor microenvironment, thus allowing for a more robust immune response to ICIs [72, 73]. Apart from EP300, 4 genes of this 25-gene panel belong to the NOTCH family, which has been related to responses to immunotherapy [72, 73]. Indeed, the NOTCH family of genes has been implicated in tumorigenesis and is a good predictor of response to cancer immunotherapy [72, 73]. In addition, the specific squamous cell carcinoma transcription factor TP63 has also been proven to be involved in the proliferation of squamous cell carcinoma cells as well as in responses to immunotherapy in these carcinomas [74]. Besides, mutations in ARID1A gene, which has been included in this 25-gene panel, have been associated with different responses to treatment with PD-1/PD-L1 [70]. In particular, Okamura et al. have demonstrated that alterations in the ARID1A gene may serve as a biomarker of longer progression-free survival (PFS) after treatment with ICIs, regardless of high MSI and TMB. Overall, this 25-gene panel seems to be a very promising biomarker regarding response to immunotherapy. It is noteworthy that this specific 25-gene mutation signature may be further improved by the more extensive use of NGS [74].
Zheng et al. have recently reported the usefulness of a 7-gene panel of transforming growth factor β (TGF-β) in predicting response to immunotherapy. TGF-β acts by reducing CD8 + T cell proliferation as well as by increasing the proliferation and activation of Tregs [75•]. HNSCC is characterized by dense infiltration with Tregs. As TGF-β suppresses CD8+ cells while promoting Tregs activation, the neutralization of TGF-β could result in increased anti-tumor activity. Therefore, the inhibition of the TGF-β receptor I, by diminishing the immunosuppressive effects of TGF-β in the TME, may lead to improved responses to immunotherapy [76]. Redman et al. have documented that the dual anti-PD-L1 and anti-TGF-β treatment enhances anti-tumor activity among patients with non-HPV-associated HNSCC [77]. This beneficial dual inhibition may confer a significant utility of this 7-gene panel regarding TGF-β in predicting responses to immunotherapy [75•, 76, 77].
Starger et al. have advocated the use of a DNA methylation profile to predict response of not to ICIs [78]. In particular, they have checked more than 850,000 CpG sites in patients with metastatic HNSCC who had previously received platinum-based chemotherapy and have found differences in methylation profiles, such as hypo-methylation or hyper-methylation gene patterns, by using microarray assay in well-known cancer-involved pathways. They have tested genes implicated in MAPK, Hippo, and Axon signaling as well as other pathways in cancer and have documented a differential methylation profile, which could distinguish patients who would benefit from PD-1 treatment compared to nonresponders. However, further large-scale studies are needed to confirm their findings [78].
Furthermore, another 18-gene panel has been suggested as a surrogative biomarker of response to immunotherapy. Haddad et al. have documented that this 18-gene T cell inflamed gene expression profile (Tcellinfl-GEP), which refers to infiltrating T cells, interferon-γ, and chemokines, has also been shown as a good biomarker of response to immunotherapy, especially when combined with TMB and PD-L1 expression [79]. This combination may provide distinct features and more information about response to pembrolizumab in HNSCC patients [79].
Major Components of the TME and Their Metabolic Reprogramming as a Potential Biomarker
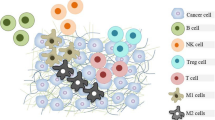
TME comprises the immune cells, the stromal cells, the blood vessel cells, and the extracellular matrix (Fig. 1). It is considered a dynamic entity that plays a crucial role in the development, local invasion, as well as the metastatic spread of cancer [80]. Among the stromal cells, cancer-associated fibroblasts (CAFs) play a vital importance in the progression of cancer [80]. As a major component of TME, CAFs have been documented to interfere with cancer cells to promote differentiation and transformation of normal fibroblasts into CAFs, while they have been implicated in the enhancement of angiogenesis as well as the immunosuppression of T cells. In particular, when a tumor reaches a specific volume, its oxygen supplementation and nutritional needs increase, leading to insufficient oxygen and nutritional defects. In this hypoxic and acidic TME, hypoxia-inducible factors (HIFs) together with angiogenesis-promoting factors, such as the vascular endothelial growth factor (VEGF), are activated in order to maintain sufficient blood supply with enough oxygen and nutritional supplementation to the cancer cells [80]. Therefore, TME with this dynamic process, which is largely attributed to CAFs, is able to maintain and promote cancer progression. Indeed, Luo et al. have only recently proven the plasticity of CAFs and their significance in immune responses, as well as their prognostic and therapeutic potential [81]. With the advent of single-cell RNA sequencing, they have demonstrated the diversity of CAFs and their key role in the development and spread of various types of cancer [81]. Besides, their interactions with tumor-associated macrophages (TAMs) as well as the endothelial-to-mesenchymal transition may result in differential responses to immunotherapy [81].
The tumor microenvironment (TME) plays a crucial role in the immunotherapy of HNSCC. A variety of cells, such as CAFs, TAMs, Tregs, T cells, B-cells, NKCs, and DCs, interact with each other to promote cancer proliferation and spread through the vasculature (vessels in red color). The extracellular matrix (ECM) provides the TME with structural support and biochemical properties, which may be protective against cancer progression or, in the contrary, may serve as a background where the activity of cancer cells is amplified
Du et al. have developed a metabolism-related gene prognostic index (MRGPI) based on 7 genes: HPRT1, AGPAT4, AMY2B, ACADL, CKM, PLA2G2D, and ADA. Patients with a high MRGPI may have a better response to immunotherapy, while patients with a low MRGPI have been designated as nonresponders to immunotherapy [82]. More specifically, a higher MRGPI has been associated with an increased metabolic function, lower anti-tumor immune capacity, and an immunosuppressive TME, which limits response to immunotherapy [82]. This MRGPI seems to be an appealing approach for the prediction of response to immunotherapy [82].
Moreover, Qiang et al. have recently suggested another MRGPI for HNSCC, which could be a useful tool in assessing patients who would have a better response to immunotherapy [83]. This tool is based on 12 genes that have been implicated in the metabolic reprogramming of the TME and could serve as a molecular signature predicting the response to immunotherapy [83]. The expression of P4HA1, ALG3, CYP2D6, POLE2, DNMT1, MTHFD2, and PYGL have already been related to the prognosis of HNSCC, whereas the role of the remaining five genes has not yet been defined.
Wang et al. have proposed that among a 34-gene panel associated with immunogenic cell death, 15 genes were associated with response to immunotherapy: CALR, CXCR3, PDIA3, HSP90AA1, NT5E, ATG5, PRF1, FOXP3, IL17A, CD8A, IL10, IL6, CD8B, CD4, and ENTPD1. The abovementioned panel may be a good predictor of response to immunotherapy, reflecting modifications of the TME in HNSCC [84].
Only recently, a novel predictor of a poor response to immunotherapy has been suggested by Chen et al. They have demonstrated that the expression of the NT5E gene has been related to CAFs in HNSCC patients [85]. More specifically, the increased expression of NT5E on CAFs, i.e., a high NT5E index, has been associated with a poor OS as well as a poor PFS among patients with HNSCC. A higher NT5E expression has been associated with an immunosuppressive TME, which may be translated to a low neo-antigen load, a low TMB, and a reduced response to immunotherapy in HNSCC patients [85].
The Role of Microbiome in Immunotherapy for HNSCC
The microbiome refers to the entire genome of the sum of microorganisms inhabiting the human body, i.e., bacteria, viruses, fungi, and archaea [86, 87]. Recent studies have demonstrated that the microbiome plays a crucial role in the development of various types of cancer. In particular, regarding HNC, Mukheerjie et al. have documented a multi-hit process of microbiome and mycobiome alterations in the pathogenesis of oral cavity cancers [88]. As already mentioned above, alcohol consumption has been linked to the etiopathogenesis of oral cavity carcinoma. Chronic alcohol consumption results in changes in the levels of acetaldehyde, a well-known product of alcohol metabolism. In addition, bacteria may also metabolize alcohol, thus interfering with acetaldehyde production. Acetaldehyde is a highly toxic substance with a negative impact on DNA synthesis and repair mechanisms [89]. It has been suggested that dysbiosis, with the abundance of bacteria synthesizing acetaldehyde such as Rothia, Streptococcus, and Prevotella, has been implicated in oral cavity tumorigenesis [90]. Furthermore, Porphyromonas gingivalis has involved in the pathogenesis of oral cavity cancer. Indeed, higher serum levels of IgG antibodies against Porphyromonas gingivalis have been found among patients with HNC. Moreover, patients with Porphyromonas gingivalis in their oral cavity tend to exhibit a higher mortality. It has been documented that this bacterium decreases the expression of the p53 tumor suppression gene, thereby resulting an increased cell proliferation and tumorigenesis [89]. It is noteworthy that even changes in the oral mycobiome have been associated with the etiopathogenesis of oral cavity cancers. More specifically, among patients with HNSCC, specific strains of Candida albicans were over-presented or under-presented in mouth oral wash, when compared to healthy individuals. Notably, the fungi Schizophyllum commune was also found in abundance among healthy controls [90]. Schizophyllum commune is known to produce the polysaccharide schizophylan, which has been a subject of research as a potential anti-cancerous compound in Japan in the 1980s among patients with HNSCC. It should also be noted that the inter-kingdom and intra-kingdom interplay between the oral bacteriome and the oral mycobiome seems to play a crucial role in the development of the tumor milieu in HNSCC. Whether the modification of the oral microbiome with the administration of probiotics, prebiotics, or synbiotics may prevent the occurrence of HNSCC remains to be elucidated in further large-scale studies [90]. However, Routy et al. have already reported a better outcome among patients receiving immunotherapy who have not been administered antibiotics prior to immunotherapy [91]. The exact role of the oral microbiome in the pathogenesis of oral cavity cancer and the preventive or even therapeutic potential of probiotics or prebiotics in this context will be a subject of investigation in the near future.
Limitations of Immunotherapy in R/M HNSSC
Immunotherapy allows for better management of patients with R/M HNSSC, as it has been associated with an improved OS and PFS [18, 92, 93]. Apart from the improved outcomes, immunotherapy has also been related to a better safety profile when compared to chemotherapy [18, 92, 93]. Regarding adverse effects of ICIs, the most common appear to be fatigue, diarrhea, decreased appetite, rash, fever, pruritus, and pneumonitis; autoimmune endocrinopathies, mainly hypothyroidism; and less frequently, hyperthyroidism and adrenal insufficiency or hypophysitis [18, 92, 93]. In particular, almost 50% of the patients administered ICIs experienced skin rashes. Rarely, neurological complications such as Guillain-Barre syndrome, aseptic meningitis, myasthenia gravis, and optic neuritis occurred. Despite the fact that immunotherapy has been associated with longer durability of its effects, a non-neglected proportion of patients do not respond to immunotherapy, while resistance to immunotherapeutic agents may develop during treatment [94••, 95]. Resistance may be due to the adaptation of cancer cells, T cell proliferation, and alterations in the TME. Changes in the TME have also been in the spotlight of research lately, on account of the complexity of TME and its associated interactions with cancer cells [18, 95, 96].
Regarding the adverse effects of tumor vaccines, their immunogenic properties are limited due to the fact that usually, tumor antigens are recognized as self-antigens, thereby not stimulating the immune system responses. In addition, cancer cells may modulate the TME, resulting in an immunosuppressive TME. Therefore, personalized tumor vaccines are mandated, which are much time consuming and also very expensive [97]. Nowadays, personalized tumor vaccines require approximately 3–4 months to be developed [97]. In the case of CAR-T cell therapy, its major limitation is the development of tachyphylaxis, known as an antigen escape phenomenon in immunology. However, the most dangerous adverse effect is the cytokine release syndrome, which is characterized by the cascade of cytokine release leading to life-threatening complications such as capillary leak syndrome and shock [18]. Regarding OVs, their use is still in its very beginning; thus, our knowledge of adverse effects is limited in the clinical setting.
Conclusion
Nowadays, immunotherapy plays a crucial role in the management of patients with R/M HNSSC. Table 2 summarizes FDA-approved and investigational treatment modalities for HNSCC regarding immunotherapy. Immunotherapy has been linked to more sustained results and fewer and less severe, or at least more easily manageable adverse effects. The advent of anti-PD-1/PD-L1 agents has been the first step in the field of immunotherapy. However, anti-PD-1/PD-L1 drugs are not enough for combating R/M HNSCC. As upregulation of the PD-L1 has been documented in cancers treated with chemotherapy, the strategy of the administration of combinations of immunotherapeutic agents is required. Moreover, the combination of immunotherapeutic agents with vaccines and chemotherapy is still of particular interest. However, there is much to be performed in the field of immunotherapy. Future research on the TME, especially regarding subsets of CAFs and TAMs and their role in immune responses in HNSCC, may be very appealing. Apart from research on TME, the discovery of newer and more precise biomarkers could shed light on when and why immunotherapy could be initiated among patients with various types of R/M HNSSC. Despite the fact that until today, the selection of patients as candidates for immunotherapy is based upon PD-L1 expression, there are many more reasons to extend our choices. Currently, as our knowledge regarding novel biomarkers is rapidly expanding, it seems that a more personalized approach to patients with R/M HNSSC may be feasible. Combined efforts from genetic engineering, molecular biology, with the advent of multi-omics, bioinformatics, immunology, and pharmacology have made possible this personalized approach.
Abbreviations
- APCs:
-
Antigen presenting cells
- CAFs:
-
Cancer associated fibroblasts
- CAR-T cell therapy:
-
Chimeric antigen receptor T cell therapy
- CTLA-4:
-
Cytotoxic T lymphocyte associated protein 4
- DAMPs:
-
Damage associated molecular patterns
- EGFR:
-
Epidermal growth factor receptor
- FDA:
-
Food and Drug Administration
- Foxp-3:
-
Forkhead box protein 3
- HIF:
-
Hypoxia induced factor
- HNC:
-
Head and neck cancer
- HNSSC:
-
Head and neck squamous cell carcinoma
- HPV:
-
Human papillomavirus
- ICIs:
-
Immune checkpoint inhibitors
- ICOS:
-
Inducible T cell co-stimulators
- INF:
-
Interferon
- MRGPI:
-
Metabolism-related gene prognostic index
- MSI:
-
Microsatellite instability
- NGS:
-
Next generation sequencing
- NK cells:
-
Natural killer cells
- NKG2A:
-
Natural killer group 2 A
- OS:
-
Overall survival
- OVs:
-
Oncolytic viruses
- PAMPs:
-
Pathogen associated molecular patterns
- PFS:
-
Progression free survival
- PD-1:
-
Programmed death protein 1
- PD-L1:
-
Programmed death protein ligand 1
- PR:
-
Partial response
- R/M HNSCC:
-
Recurrent/metastatic head and neck squamous cell carcinoma
- TAAs:
-
Tumor associated antigens
- TAMs:
-
Tumor associated macrophages
- TGF-β :
-
Transforming growth factor β
- TILs:
-
Tumor infiltrating lymphocytes
- TMB:
-
Tumor mutational burden
- TME:
-
Tumor microenvironment
- TORS:
-
Transoral robotic surgery
- Tregs:
-
T regulatory cells
- VEGF:
-
Vascular endothelial growth factor
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vos T, Abajobir AA, Abate KH; GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390:1211–59. https://doi.org/10.1016/S0140-6736(17)32154-2.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. https://doi.org/10.1038/s41572-020-00224-3.
Gormley M, Creaney G, Schasche A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dental J. 2022;233(9):780–6. https://doi.org/10.1038/s41415-022-5166-x.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. https://doi.org/10.3322/caac.21442.
Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. https://doi.org/10.1001/jamaoncol.2016.5688.
Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–42. https://doi.org/10.1200/JCO.2015.61.6995.
Yete S, D’Souza W, Saranath D. High-risk human papillomavirus in oral cancer: clinical implications. Oncology. 2018;94:133–41. https://doi.org/10.1159/000485322.
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. https://doi.org/10.1200/JCO.2011.36.4596.
Shah A, Malik A, Garg A, Mair M, Nair S, Chaturvedi P. Oral sex and human papilloma virus-related head and neck squamous cell cancer: a review of the literature. Postgrad Med J. 2017;93:704–9. https://doi.org/10.1136/postgradmedj-2016-134603.
Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen C, Yang CS. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med. 2001;345:1877–82. https://doi.org/10.1056/nejm011610.
Sturgis EM, Pytynia KB. After the smoke clears: environmental and occupational risks for carcinoma of the upper aerodigestive tract. Cancer J. 2005;11:96–103. https://doi.org/10.1097/00130404-200503000-00002.
•• Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60–72. https://doi.org/10.1056/NEJMra171571. An expert’s opinion on the pathogenesis and treatment options regarding head and neck cancer.
Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. https://doi.org/10.1056/NEJM199301213280306.
Peyrade F, Cupissol D, Geoffrois L, Rolland F, Borel C, Ciais C, Faivre S, Guigay J. Systemic treatment and medical management of metastatic squamous cell carcinoma of the head and neck: review of the literature and proposal for management changes. Oral Oncol. 2013;49:482–91. https://doi.org/10.1016/j.oraloncology.2013.01.005.
Fasano M, Corte CMD, Liello RD, Viscardi G, Sparano F, Iacovino ML, Paragliola F, Piccolo A, Napolitano S, Martini G, Morgillo F, Cappabianca S, Ciardiello F. Immunotherapy for head and neck cancer: present and future. Crit Rev Oncol Hematol. 2022;174:103679. https://doi.org/10.1016/j.critrevonc.2022.103679.
• Pereiera D, Martins D, Mendes F. Immunotherapy in head and neck cancer: when, how and why? Biomedicines. 2022;10:2151. https://doi.org/10.3390/biomedicines10092151. A review article elaborating ον immunotherapy in R/MHNSCC.
Chakraborty R, Darido C, Liu F, Maselko M, Ranganathan S. Head and neck cancer immunotherapy: molecular biological aspectsof preclinical and clinical research. Cancers. 2023;15:852. https://doi.org/10.3390/cancers150300852.
Spyrou N, Vallianou N, Kadillari J, Dalamaga M. The interplay of obesity, gut microbiome and diet in the immune checkpoint inhibitors therapy era. Semin Cancer Biol. 2021;73:356–76. https://doi.org/10.1016/j.semcancer.2021.05.008.
Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;10:487–511.
Carlson RD, Flickinger JC, Snook AE. Talkin’ Toxin’s From Coley’s to modern cancer immunotherapy. Toxins. 2020;12(4):241. https://doi.org/10.3390/toxins12040241.
Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin family-CTLA-4. Nature. 1988;328:267–70.
Lipson EJ, Drake CG. Ipilimimab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–62. https://doi.org/10.1158/1078-0432.CCR-11-1595.
Ishida Y. PD-1: its discovery, involvement in cancer immunotherapy and beyond. Cells. 2020;9(6):1376. https://doi.org/10.3390/cells9061376.
Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, Burtness B, Tahara M, Keam B, Kang H, Muro K, Geva R, Chung HC, Lin CC, Aurora-Garg D, Ray A, Pathiraja K, Cheng J, Chow LQM, Haddad R. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119:153–9. https://doi.org/10.1038/s41416-018-0131-9.
Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, Kang H, Gibson MK, Massarelli E, Powell S, Meister A, Shu X, Cheng JD, Haddad R. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase ii study. J Clin Oncol. 2017;35:1542–9. https://doi.org/10.1200/JCO.2016.70.1524.
Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ. KEYNOTE-048 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67. https://doi.org/10.1016/S0140-6736(18)31999-8.
Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. https://doi.org/10.1056/NEJMoa1602252.
Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL, Licitra L, Mehanna H, Mell LK, Raben A, Sikora AG, Uppaluri R, Whitworth F, Zandberg DP, Ferris RL. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7:184. https://doi.org/10.1186/s40425-019-0662-5.
Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, Ready NE, Clement PM, Even C, Jang RW, Wong S, Keilholz U, Gilbert J, Fenton M, Braña I, Henry S, Remenar E, Papai Z, Siu LL, Jarkowski A, Armstrong JM, Asubonteng K, Fan J, Melillo G, Mesía R. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019;107:142–52. https://doi.org/10.1016/j.ejca.2018.11.015.
Colevas AD, Bahleda R, Braiteh F, Balmanoukian A, Brana I, Chau NG, Sarkar I, Molinero L, Grossman W, Kabbinavar F, Fasso M, O’Hear C, Powderly J. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. 2018;29:2247–53. https://doi.org/10.1093/annonc/mdy411.
Guigay J, Lee KW, Patel MR, Daste A, Wong DJ, Goel S, Gordon MS, Gutierrez M, Balmanoukian A, Le Tourneau C, Mita A, Vansteene D, Keilholz U, Schöffski P, Grote HJ, Zhou D, Bajars M, Penel N. Avelumab for platinum-ineligible/refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck: phase Ib results from the JAVELIN solid tumor trial. J Immunother Cancer. 2021;9:e002998. https://doi.org/10.1136/jitc-2021-002998.
Chen SW, Li SH, Shi DB, Jiang WM, Song M, Yang AK, Li YD, Bei JX, Chen WK, Zhang Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int J Biol Markers. 2019;34(4):398–405. https://doi.org/10.1177/1724600819884722.
•• Ruffin AT, Li H, Vujanovic A, Zandberg DP, Ferris RL, Bruno TC. Improving head and neck cancer therapies by immunomodulation of the tumor microenvironment. Nat Rev Cancer. 2023;23(3):173–88. https://doi.org/10.1038/s41568-022-00531-9. An up to date manuscript on the significance of modulating the tumor microenvironment to combat HNSCC.
Dadey RE, Workman CJ, Vignali DA. A regulatory T cells in the tumor microenvironment. Adv Exp Med Biol. 2020;1273:105–34. https://doi.org/10.1007/978-3-030-49270-0-6.
Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, Pennathur A, Corry DB, Luketich JD, Lafyatis R, Chen W, Poholek AC, Bruno TC, Workman CJ, Vignali DAA. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20:724–35. https://doi.org/10.1038/s41590-019-0346-9.
Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, Ferris RL. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–35. https://doi.org/10.1038/bjc.2013.645.
Santuray RT, Johnson DE, Grandis JR. New therapies in head and neck cancer. Trends Cancer. 2018;4:385–96. https://doi.org/10.1016/j.trecan.2018.03.006.
Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep. 2018;20:22. https://doi.org/10.1007/s11912-018-0654-5.
Siu LL, Even C, Mesía R, Remenar E, Daste A, Delord JP, Krauss J, Saba NF, Nabell L, Ready NE, Braña I, Kotecki N, Zandberg DP, Gilbert J, Mehanna H, Bonomi M, Jarkowski A, Melillo G, Armstrong JM, Wildsmith S, Fayette J. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1–low/negative recurrent or metastatic HNSCC. JAMA Oncol. 2019;5:195. https://doi.org/10.1001/jamaoncol.2018.4628.
Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, Clement PM, Mesia R, Kutukova S, Zholudeva L, Daste A, Caballero-Daroqui J, Keam B, Vynnychenko I, Lafond C, Shetty J, Mann H, Fan J, Wildsmith S, Morsli N, Fayette J, Licitra L. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942–50. https://doi.org/10.1016/j.annonc.2020.04.001.
Van Hall T, André P, Horowitz A, Ruan DF, Borst L, Zerbib R, Narni-Mancinelli E, van der Burg SH, Vivier E. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J Immunother Cancer. 2019;7:263. https://doi.org/10.1186/s40425-019-0761-3.
Galot R, Tourneau CL, Saada-Bouzid E, Daste A, Even C, Debruyne PR, Henry S, Zanetta S, Rutten A, Licitra LF. A phase II study of monalizumab in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN): results of the I1 cohort of the EORTC-HNCG-1559 trial (UPSTREAM). Ann Oncol. 2019;30:v449–50. https://doi.org/10.1093/annonc/mdy452.
André P, Denis Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Bléry M, Bonnafous C, Gauthier L, Morel A. Anti-NKG2A MAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731-43.e13. https://doi.org/10.1016/j.cell.2018.10.014.
•• Ghosh S, Shah PA, Johnson FM. Novel systemic treatment modalities including immunotherapy and molecular targeted therapy for recurrent and metastatic head and neck squamous cell carcinoma. Int J Mol Sci. 2022;23:7889. https://doi.org/10.3390/ijms23147889. A very interesting update on immuno therapy in HNSCC.
Ying Z, Huang XF, Xiang X. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25:947–53. https://doi.org/10.1038/s41591-019-0421-7.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30.
•• Damasio MPS, Nascimento CS, Andrade LM, de Oliveira VL, Calzavara-Silva CE. The role of T cells in head and neck squamous cell carcinoma: from immunity to immunotherapy. Front Oncol. 2022;12:1021609. https://doi.org/10.3389/fonc.2022.1021609.eCollection2022. An overview on the role of immunotherapy, with special interest in CAR-T cells therapy in HNSCC.
Warren EA, Liu H-C, Porter CE, Liao KS, Hegde M, Yu W, et al. Abstract 574: overexpression of HER2 in head and neck cancer represents a potential target for T cell immunotherapy. Cancer Res. 2019;79:574–84. https://doi.org/10.1158/1538-7445.am2019-574.
Park YP, Jin L, Bennett KB, Wang D, Fredenburg KM, Tseng JE, Chang LJ, Huang J, Chan EKL. CD70 as a target for chimeric antigen receptor T cells in head and neck squamous cell carcinoma. Oral Oncol. 2018;78:145–50. https://doi.org/10.1016/j.oraloncology.2018.01.024.
Norberg SM, Hinrichs CS. Advances in adoptive cell therapy for head and neck cancer. Otolaryngol Clin N Am. 2021;54:761–8. https://doi.org/10.1016/j.ccell.2022.10.016.
Doran SL, Stevanovic´ S, Adhikary S, Gartner JJ, Jia L, Kwong MLM, Faquin WC, Hewitt SM, Sherry RM, Yang JC, Rosenbetg SA, Hinrichs CS. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol. 2019;37:2759–68. https://doi.org/10.1200/JCO.18.02424.
Marcinkowski B, Stevanovic´ S, Helman SR, Norberg SM, Serna C, Jin B, Gkitsas N, Kadakia T, Warner A, Davis JL, Rooper L, Hinrichs CS. Cancer targeting by TCR gene-engineered T cells directed against Kita-Kyushu lung cancer antigen-1. J Immunother Cancer. 2019;7:229. https://doi.org/10.1186/s40425-019-0678-x.
Bai Y, Hui P, Du X, Su X. Updates to the antitumor mechanism of oncolytic virus. Thorac cancer. 2019;10:1031–5. https://doi.org/10.1111/1759-7714.13043.
Cheng G, Dong H, Yang C, Liu Y, Wu Y, Zhu L, Tong X, Wang S. A review on the advances and challenges of immunotherapy for head and neck cancer. Cancer Cell Int. 2021;21(1):406. https://doi.org/10.1186/s12935-021-02024-5.
Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–9. https://doi.org/10.1111/cas.13027.
Kohlhapp FJ, Kaufman HL. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22:1048–54. https://doi.org/10.1158/1078-0432.CCR-15-2667.
Cheng G, Dong H, Yang C, Liu Y, Wu Y, Zhu L, Tong X, Wang S. A review on the advances and challenges of immunotherapy for head and neck cancer. Cancer Cell Int. 2021;21:406. https://doi.org/10.1186/s12935-021-02024-5.
Huang Y, Liao J, Liang F, Lin P, Wu S, Ye Y, Gao M, Chen R, Zeng H, Yin X, Jiang Y, Ouyang N, Han P, Huang X. A 25-gene panel predicting the benefits of immunotherapy in head and neck squamous cell carcinoma. Int Immunopharmacol. 2022;110:108846. https://doi.org/10.1016/j.intimp.2022.108846.
• Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, Cheng JD, Chow LQ. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–65. https://doi.org/10.1016/S1470-2045(16)30066-3. An original-and why not pioneering- research regarding pembrolizumab for the treatment of R/M HNSCC.
Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the nead and neck cancer. N Engl J Med. 2016;375(19):1856–67. https://doi.org/10.1056/NEJMoa1602252.
Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, Kang H, Gibson MK, Massarelli E, Powell S, Meister A, Shu X, Cheng JD, Haddad R. Pembrolizumab for platinum and cetuximab-refractory head and neck cancer: results from a single-arm phase II study. J Clin Oncol. 2017;35(14):1542–9. https://doi.org/10.1200/JCO.2016.70.1524.
Burtness B, Rischin D, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Brana I, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Ge J, Swaby RF, Gumuscu B, Harrington K. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE 048 Study: subgroup analysis by programmed death ligand -1 combined positive score. J Clin Oncol. 2022;40(21):2321–32. https://doi.org/10.1200/JCO.21.02198.
• Yamamoto H, Watanabe Y, Maehata T, Imai K, Itoh F. Microsatellite instability in cancer: a novel landscape for diagnostic and therapeutic approach. Arch Toxicol. 2020;94(10):3349–57. https://doi.org/10.1007/s00204-020-02833-z. A very interesting manuscript elaborating upon the micro satellite instability in cancer.
Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behavior of tumors. J Cancer Res Clin Oncol. 2019;145(12):2891–9. https://doi.org/10.1007/s00432-019-02942-y.
Merino DM, McShane LM, Fabrizio D, Funari V, Chen S, White JR. Establishing guidelines to harmonize tumor mutational burden: in silico assessment of variation in TMB quantification across diagnostic platforms:phase 1 of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8(1):e100147. https://doi.org/10.1136/jitc-2019-000147.
• Addeo A, Banna GL, Weiss GJ. Tumor mutation burden: from hopes to doubts. JAMA Oncol. 2019;5(7):934–5. https://doi.org/10.1002/cncy22174. A recent manuscript focusing upon tumor mutation burden and analyzing the prons and cons of this parameter.
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–1. https://doi.org/10.1056/NEJMc1713444.
Marcus L, Fashoyin-Aje LA, Donoghue M, Yuan M, Rodriguez L, Gallagher PS, Philip R, Ghosh S, Theoret MR, Beaver JA, Pazdur R, Lemery SJ. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden high solid tumors. Clin Cancer Res. 2021;27(17):4685–9. https://doi.org/10.1158/1078-0432.CCR-21-0327.
Sakaguchi S, Yamaguchi T, Nomura T, Omo M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. https://doi.org/10.1016/j.cell.2008.05.009.
Castillo J, Wu E, Lowe C, Srinivasan S, McCord R, Wagle MC, Jayakar S, Edick MG, Eastham-Anderson J, Liu B, Hutchinson KE, Jones W, Stokes MP, Tarighat SS, Holcomb T, Glibicky A, Romero FA, Magnuson S, Huang SA, Plaks V, Giltnane JM, Lackner MR, Mounir Z. CBP/p300 drives the differentiation of regulatory T cells through transcriptional and non-transcriptional mechanisms. Cancer Res. 2019;79(15):3916–27. https://doi.org/10.1158/0008-5472.CAN-18-3622.
Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. 2020;17(4):204–32. https://doi.org/10.1038/s41571-019-0293-2.
Zhang K, Hong X, Song Z, Xu Y, Li C, Wang G, Zhang Y, Zhao X, Zhao Z, Zhao J, Huang M, Huang D, Qi C, Gao C, Cai S, Gu F, Hu Y, Xu C, Wang W, Lou Z, Zhang Y, Liu L. Identification of deleterious NOTCH mutation as novel predictor to efficacious immunotherapy in NSCLC. Clin Cancer Res. 2020;26(14):3649–61. https://doi.org/10.1158/1078-0432.CCR-19-3976.
Jiang Y, Jiang YY, Xie JJ, Mayakonda A, Hazawa M, Chen L, Xiao JF, Li CQ, Huang ML, Ding LW, Sun QY, Xu L, Kanojia D, Jeitany M, Deng JW, Liao LD, Soukiasian HJ, Berman BP, Hao JJ, Xu LY, Li EM, Wang MR, Bi XG, Lin DC, Koeffler HP. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat Commun. 2018;9(1):3619. https://doi.org/10.1038/s41467-018-06081-9.
• Zheng L, Guan Z, Xue M. TGF-β signaling pathway-based model to predict the subtype and prognosis of head and neck squamous cell carcinoma. Front Genetics. 2022;13:862860. https://doi.org/10.3389/fgene.2022.862860. A very recent manuscript focusing on the significance of TGF-β in the pathogenesis and prognosis of HNSCC.
Bell RB, Gough M, Crittenden R, Young K. Moving beyond the T cell synapse for combination neoadjuvant immunotherapy in head and neck cancer. J Clin Invest. 2022;132(18):e162733. https://doi.org/10.1172/JCI162733.
Redman JM, Friedman J, Robbins Y, Sievers C, Yang X, Lassoued W, Sinkoe A, Papanicolau-Sengos A, Lee CC, Marte JL, Turkbey E, Mydlarz W, Joshi A, London NR, Pierce M, Taylor R, Hong S, Nguyen A, Soon-Shiong P, Schlom J, Gulley JL, Allen CT. Enhanced neoepitope specific immunity following neoadjuvant PD-L1 and TGF-β blockade in HPV-unrelated head and neck cancer. J Clin Invest. 2022;132(18):e161400. https://doi.org/10.1172/JCI161400.
Starzer AM, Heller G, Tomasich E, Melchardt T, Feldmann K, Hatziioannou T, Traint S, Minichsdorfer C, Schwarz-Nemec U, Nackenhorst M, Müllauer L, Preusser M, Berghoff AS, Fuereder T. DNA methylation profiles differ in responders versus nonresponders to anti-PD-1 immune checkpoint inhibitors in patients with advanced and metastatic head and neck squamous cell carcinoma. J Immunother Cancer. 2022;10(3):e003420. https://doi.org/10.1136/jitc-2021-003420.
Haddad RI, Seiwert TY, Chow LQM, Gupta S, Weiss J, Gluck I, Eder JP, Burtness B, Tahara M, Keam B, Kang H, Muro K, Albright A, Mogg R, Ayers M, Huang L, Lunceford J, Cristescu R, Cheng J, Mehra R. Influence of tumor mutational burden, inflammatory gene expression profile and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer. 2022;10(2):e003026. https://doi.org/10.1136/jitc-2021-003026.
Anderson NM, Simon MC. Tumor microenvironment. Curr Biol. 2020;30(16):R921–5. https://doi.org/10.1016/j.cub.2020.06.081.
Luo H, Xia X, Huang LB, An H, Cao M, Kim GD, Chen HN, Zhang WH, Shu Y, Kong X, Ren Z, Li PH, Liu Y, Tang H, Sun R, Li C, Bai B, Jia W, Liu Y, Zhang W, Yang L, Peng Y, Dai L, Hu H, Jiang Y, Hu Y, Zhu J, Jiang H, Li Z, Caulin C, Park J, Xu H. Pan cancer single cell analysis reveals the heterogeneity and plasticity of cancer associated fibroblasts in the tumor microenvironment. Nat Commun. 2022;13(1):6619. https://doi.org/10.1038/s41467-022-34395-2.
Du K, Zou J, Wang B, Liu C, Khan M, Xie T, Huang X, Shen P, Tian Y, Yuan Y. A Metabolism-related gene prognostic index bridging metabolic signatures and antitumor immune cycling in head and neck squamous cell carcinoma. Front Immunol. 2022;13:857934. https://doi.org/10.3389/fimmu.2022.857934.
Qiang W, Dai Y, Xing X, Sun X. Identification of a metabolic reprogramming-related signature associated with prognosis and immune microenvironment of head and neck squamous cell carcinoma by in silico analysis. Cancer Med. 2022;11(16):3168–81. https://doi.org/10.1002/cam4.4670.
Wang X, Wu S, Liu F, Ke D, Wang X, Pan D, Xu W, Zhou L, He W. An immunogenic cell death-related classification predicts prognosis and response to immunotherapy in head and neck squamous cell carcinoma. Front Immunol. 2021;12:781466. https://doi.org/10.3389/fimmu.2021.781466.
Chen X, Liu Y, Tao B, Xue X, Zhang X, Wang L, Zhong H, Zhang J, Yang S, Jiang Q. NT5E upregulation in head and neck squamous cell carcinoma: a novel biomarker on cancer-associated fibroblasts for predicting immunosuppressive tumor microenvironment. Front Immunol. 2022;13:975847. https://doi.org/10.3389/fimmu.2022.975847.
Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, Kounatidis D, Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: current evidence and perspectives. Biomolecules. 2021;12(1):56. https://doi.org/10.3390/biom12010056.
Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. 2020;9(3):179–92. https://doi.org/10.1007/s13679-020-00379-w.
Mukherjee PK, Wang H, Retuerto M, Zhang H, Burkey B, Ghannoum MA, Eng C. Bacteriome and mycobiome associations in oral tongue cancer. Oncotarget. 2017;8(57):97273–89. https://doi.org/10.18632/oncotarget.21921.
Vallianou N, Kounatidis D, Christodoulatos GS, Panagopoulos F, Karampela I, Dalamaga M. Mycobiome and cancer: what is the evidence? Cancers. 2021;13(13):3149. https://doi.org/10.3390/cancers13133149.
Dorobisz K, Dorobisz T, Zdonski T. The microbiome’s influence on head and neck cancer. Curr Oncology Rep. 2023;25(3):163–71. https://doi.org/10.1007/s11912-022-01352-7.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryfel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome infuences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7. https://doi.org/10.1126/science.aan3706.
Meliante PG, Barbato C, Zoccali F, Ralli M, Greco A, de Vincentiis M, Colizza A, Petrella C, Ferraguti G, Minni A. Programmed cell death-ligand 1 in head and neck squamous cell carcinoma: molecular insights, preclinical and clinical data, and therapies. Int J Mol Sci. 2022;23:15384. https://doi.org/10.3390/ijms232315384.
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Basté N, Neupane P, Bratland A, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, González Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D. KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. https://doi.org/10.1016/S0140-6736(19)32591-7.
•• Mody MD, Rocco JW, Yam SS, Haddad RI, Saba NF. Head and neck cancer. Lancet. 2021;398:2289–99. https://doi.org/10.1016/S0140-6736(21)01550-6. An excellent review on the incidence, aetiologies and treatment modalities regarding head and neck cancer.
Emfietzoglou R, Spyrou N, Mantzoros CS, Dalamaga M. Could the endocrine disruptor bisphenol-A be implicated in the pathogenesis of oral and oropharyngeal cancer? Metabolic Consider Future Direct Metabol. 2019;91:61–9. https://doi.org/10.1016/j.metabol.2018.11.007.
Botticelli A, Cirillo A, Strigari L, Valentini F, Cerbelli B, Scagnoli S, Cerbelli E, Zizzari IG, Della Rocca C, D’Amati G, Polimeni A, Nuti M, Merlano MC, Mezi S, Marchetti P. Anti–PD-1 and anti–PD-L1 in head and neck cancer: a network meta-analysis. Front Immunol. 2012;12:705096. https://doi.org/10.3389/fimmu.2021.705096.
Shibata H, Zhou L, Xu N, Egloff AM, Uppaluri R. Personalized cancer vaccination in head and neck cancer. Cancer Sci. 2021;112:978–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal and Human Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vallianou, N.G., Evangelopoulos, A., Kounatidis, D. et al. Immunotherapy in Head and Neck Cancer: Where Do We Stand?. Curr Oncol Rep 25, 897–912 (2023). https://doi.org/10.1007/s11912-023-01425-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01425-1