Abstract
Radiation therapy (RT) has been described as the most effective single agent in the treatment of lymphoma; however, contemporary lymphoma treatment rarely relies on single agents. In the modern era, the selection of appropriate patients for combined modality therapy has become increasingly complex over the last decade with the transition to immunochemotherapy, the emergence of functional imaging for response evaluation, and the improvement in conformal avoidance of normal tissues when delivering RT. Recent evidence demonstrates that selected patients with DLBCL have significantly better outcomes when RT is added to immunochemotherapy; however, there are important knowledge gaps regarding the use of functional imaging to facilitate treatment selection. This article will review the current evidence regarding the optimal use of combined modality therapy for DLBCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combined modality therapy has been the standard of care in North America and the UK for many patients with diffuse large B-cell lymphoma (DLBCL), particularly those with limited stage disease or bulk. However, the incorporation of rituximab into the standard chemotherapy regimen of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), and the associated increase in progression-free and overall survival compared to CHOP, has led to a reevaluation of the role of radiation therapy (RT) in the management of DLBCL. Moreover, the emergence of fluoro-deoxyglucose positron emission tomography (FDG-PET) as a means of evaluating response and a growing body of work describing genetic signatures that may differentiate different subtypes of DLBCL potentially add further opportunity to individually tailor treatment selection. Despite the complexity of the disease, there can be a temptation to broadly apply a policy of six cycles of R-CHOP with no additional treatment for patients with a “negative” FDG-PET scan for practically all patients with DLBCL. Available evidence, however, suggests that such an approach likely compromises the chance of cure for some patients. The aim of this paper is to review the evidence regarding selection of DLBCL patients for RT and some technical aspects of RT delivery.
Late Effects of RT: Distinct Considerations for DLBCL
Much of the recent clinical concern regarding the use of RT in hematologic malignancies has revolved around the recognized risk of late toxicity among survivors, typically young HL patients, treated with RT. While risk of late toxicity among young patients should always be a consideration, direct extrapolation of HL survivorship studies to patients with DLBCL is misguided.
At least two factors critically distinguish RT-related late toxicity considerations for DLBCL patients from those for HL patients. Firstly, despite the improvement in outcome with R-CHOP, DLBCL remains a much more lethal disease than HL. For example, in a population-based analysis of DLBCL patients treated in the R-CHOP era, 2-year progression-free and overall survival rates were 69 and 78 %, respectively [1], illustrating that there is still considerable scope for improving initial lymphoma cure and this, rather than reduction in treatment intensity, should remain the primary concern for most DBLCL patients.
Secondly, the RT-related risk of second malignancy is age-dependent, and even most studies of HL survivors find the excess risk among patients receiving RT after the age of 45 to be small [2]. This is relevant given that the median age of DLBCL diagnosis is 60–65 years. As a result, DLBCL patients are more likely than HL patients to die from their lymphoma, and moreover, other age-related competing causes of mortality attenuate the risk of late effects compared to HL patients.
These factors likely explain why RT has not been associated with increased risks of second cancer in population-based studies of DLBCL survivors. The British National Lymphoma Investigation study, for example, reported the outcome of 2465 patients treated for NHL before age 60 years, including 1219 patients receiving CHOP (average age at first treatment, 46.5 years) [3]. Relative risks were increased for all second cancers combined (SIR = 1.3; 95 % CI = 1.1 to 1.6) and leukemia (SIR = 8.8; 95 % CI 5.1–14.1). Examining individual second cancers, CHOP chemotherapy was associated with significantly increased risks of lung cancer (SIR = 2.1), colorectal cancer (SIR = 2.4), and leukemia (SIR = 14.2), whereas combined modality therapy was associated with increased risks of cancers of the mouth and pharynx (SIR = 5.7) and leukemia (SIR = 13.0) [3]. Similarly, in a European study of 748 aggressive-histology NHL treated in four consecutive trials (1980–1999), RT was not associated with an increased risk of second cancer even after adjusting for patient age, whereas salvage therapy did increase this risk [4]. Another study of 1280 patients with diffuse large B-cell lymphoma diagnosed 1988–2003 also found that RT was not associated with the risk of second cancer [5].
This is not to say that the potential toxicity of RT in the treatment of DLBCL is not a consideration. However, sophisticated judgments regarding the optimal use of RT should not conflate the late toxicity considerations facing young HL survivors with challenges facing DLBCL patients, which primarily relate to disease control.
The Role of Consolidative RT: Lessons from the Pre-Rituxumab Era
The South West Oncology Group (SWOG) 8736 study reported on 442 patients with stage I/II nonbulky disease randomized between 8 cycles of CHOP chemotherapy or 3 cycles of CHOP plus 40–55 Gy IFRT [6]. Patients receiving combined modality therapy had 5-year progression-free (PFS) of 77 %, compared to 64 % among those treated with chemotherapy alone (P < 0.03), and overall survival (OS) of 82 versus 72 %, respectively (P < 0.02) [6]. An update reported that the differences in PFS and OS had diminished with longer follow-up [7]. Life-threatening toxicity occurred in 40 % of patients treated with chemotherapy alone and 30 % of patients treated with abbreviated chemotherapy and RT (P = 0.06), and evidence of cardiac toxicity was significantly higher among patients treated with 8 cycles of CHOP (P = 0.02) [6].
The ECOG 1484 trial included 399 patients with stages I–II aggressive histology NHL. All patients received 8 cycles of CHOP chemotherapy, and patients achieving complete response (CR) to chemotherapy were randomized to 30 Gy IFRT or no further therapy. Among 172 CR patients, the 6-year disease-free survival (DFS) was 73 % following RT versus 56 % for chemotherapy alone (P = .05), and OS was 82 % for those treated with combined modality therapy and 71 % for those treated with chemotherapy alone (P = 0.24), although the study was only powered to detect a 20 % difference in PFS [8].
Despite other studies finding no benefit to RT in young patients treated with dose-intense chemotherapy, or elderly patients treated with CHOP [3, 5, 9–11], combined modality therapy remained the standard of care for patients with localized DLBCL in many institutions in North America and the UK.
The major limitation of these trials is that they do not account for the superior outcome seen when rituximab is added to CHOP. In both elderly [12••] and young [13••] patients with aggressive histology NHL, rituximab significantly reduces relapse rates and improves overall survival compared to CHOP alone. Consequently, these trials left open the possibility that improved systemic therapy employing R-CHOP negated the benefit of consolidative RT. Only recently are data emerging that can address this issue.
Indications for Consolidative RT After R-CHOP
Disease bulk has been recognized as a poor prognostic factor although it is not part of the International Prognostic Index (IPI). The RICOVER-60 trial compared R-CHOP to CHOP among patients 61–80 years old with aggressive B-cell NHL of any stage or IPI risk group [12••]. Involved-field RT (IFRT) 36 Gy was given to patients with bulk (defined as conglomerate mass ≥7.5 cm in diameter) or extranodal involvement, and approximately 54 % of patients received combined modality therapy. Three-year progression-free and overall survival were significantly improved by the addition of rituximab to CHOP (e.g., PFS 73.4 vs. 56.9 %; P < 0.001) [12••].
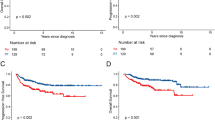
Subsequently, an extended single arm companion study, RICOVER-noRTh, treated elderly patients with the 6 cycles of R-CHOP–14 and two additional cycles of rituximab, but without RT. Notably, 60 % of patients in this study had stage III/IV disease, 63 % had extranodal sites, and 29 % had bulky disease [14•]. One hundred and sixty-four patients were eligible for evaluation and were compared with 306 patients from RICOVER-60 who had received 6× R-CHOP–14-2R plus 36 Gy IFRT [14•]. Among patients with bulky disease, the omission of RT was associated with a significant reduction in 3-year EFS (54 vs. 80 %; P = 0.001), PFS (62 vs. 88 % P = 0.001), and OS (65 vs. 90 % P = 001) (Fig. 1). In multivariable analysis adjusting for relevant prognostic factors, the hazards of progression (HR = 4.4) and death (HR = 4.3) were both significantly increased among patients with bulk who did not receive RT [14•].
a Event-free, b progression-free, and c overall survival of patients with bulky disease in RICOVER-60 (6 vs. 8 cycles of biweekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20 + B-cell lymphomas) and RICOVER-noRTh (no radiotherapy) cohorts. From Held et al. [14]
The MabThera International Trial (MInT) randomized 824 patients aged 18–60 years with 0–1 age-adjusted IPI factors to six cycles of CHOP-like chemotherapy and rituximab (n = 413) or to six cycles of CHOP-like chemotherapy alone (N = 411) [13••]. A subset analysis among 824 patients on this trial concluded that rituximab decreased but did not eliminate the adverse prognostic effect of bulk and that the effect of maximum tumor dimension on prognosis was linear, although the effect of RT could not be effectively evaluated due to selection bias for RT use in the protocol [15].
The impact of consolidative RT was subsequently evaluated in the UNFOLDER trial (DSHNHL 2004-3; ClinicalTrials.gov identifier NCT00278408), which enrolled patients aged 18 to 60 with an age-adjusted IPI score of 1, or an IPI score of 0 with bulky tumor (≥7.5 cm). This trial involved a four-way randomization to compare 14- versus 21-day cycles of R-CHOP and consolidative RT versus observation after chemotherapy. The no-RT arms were closed when interim analysis revealed significantly worse EFS with the omission of RT. Taken together, these results provide the best available evidence demonstrating that even following 6 cycles of R-CHOP, patients with aggregate masses ≥7.5 cm derive significant benefit from 36 Gy IFRT, with a substantial reduction in relapse rate and potentially superior overall survival.
Limited Stage Favorable Risk Disease
Abbreviated Chemotherapy and RT
Given the increased cardiac toxicity in the elderly associated with ≥6 cycles of CHOP, in some circumstances, it is preferable to give 3–4 cycles followed by RT in order to reduce anthracycline exposure. Nonrandomized trials have shown that with appropriate selection of patients, the combination of abbreviated chemotherapy and IFRT is very effective. The Southwest Oncology Group (SWOG) S0014 study reported on 60 patients with limited-stage disease and at least one IPI risk factor. Patients received 3 cycles of R-CHOP and an additional dose of rituximab, followed by 40 to 46 Gy IFRT. Four-year progression-free survival (PFS) was 88 %, and overall survival (OS) was 92 % [16]. Similar findings have been reported by others [17].
Careful selection of patients for abbreviated chemotherapy is important, as poor EFS has been reported for patients with more than two IPI risk factors when only 3–4 cycles of chemotherapy are given [18]. However, for patients with limited volume disease (commonly in the head and neck), especially if older age is the only IPI factor, abbreviated chemotherapy with 30–36 Gy IFRT can produce good PFS while limiting acute side effects and reducing the risk of anthracycline-induced cardiac toxicity.
Chemotherapy Alone
Data are emerging that for early stage, favorable risk patients who can tolerate 6 cycles of R-CHOP, RT may be omitted without significant loss of disease control [19]. The Lysa/Goelams Group reported preliminary results of a randomized trial including 301 evaluable DLBCL patients without bulk, most of whom had modified IPI scores of 0 (n = 170) or 1 (n = 113). Patients with complete response after 4–6 cycles of R-CHOP (defined as PET-negative and >50 % reduction in CT volume) were randomized to 40 Gy IFRT or no further treatment. With a median follow-up of 51 months, the intent to treat analysis revealed 5y-EFS of 87 % in the R-CHOP alone arm versus 91 % in the R-CHOP + RT arm (HR = 0.55; P = 0.13), and 5-year OS is 90 % in the R-CHOP arm versus 95 % in the R-CHOP + RT arm (HR = 0.60, P = 0.32). Results were equally good for the patients receiving 4 cycles of R-CHOP [20].
So, for patients with limited stage and favorable risk disease, reasonable treatment options include three cycles of R-CHOP chemotherapy followed by 30–36 Gy IFRT, or 4–6 cycles of R-CHOP with RT only used if patients do not achieve a CR. Notably, the Lysa/Golems trial had both CT and PET response criteria for achieving CR, not PET alone (see below), and the “as treated” analysis has not yet been published . The relative merits of these different approaches will depend on whether reducing anthracycline exposure seems desirable (e.g., in an elderly patient with heart disease), how patients tolerate the acute toxicity of R-CHOP, and whether IFRT might be expected to produce clinically significant toxicity (e.g., pelvic involvement in a young women wanting to preserve fertility).
Advanced Stage Disease
Given that RT is a local treatment, defining its appropriate use in advanced stage disease is challenging. Marcheselli et al. reported outcomes of 216 patients with DLBCL treated in two Italian Lymphoma Study Group trials with six R-CHOP cycles and involved-field radiotherapy (IFRT) [21]. Most patients (65 %) had stage III/IV disease. The protocols did not specify which patients should receive RT, although patients with stage I/II disease or bulky disease were more likely to receive IFRT. Response to chemotheraphy was evaluated without PET. Among patients achieving a CR, IFRT was associated with significantly better 5-year EFS (88 vs. 59 % without IFRT; P = 0.035) and a trend toward better 5-year OS of (91 vs. 79 %; P = 0.141). In multivariable analysis adjusted for stage, lactate dehydrogenase (LDH) level, age, and performance status, IFRT had a significant favorable effect on EFS among patients in CR or partial response (PR) (HR 0.33; P = 0.044), or CR alone (HR 0.24; P = 0.037) [21].
Shi et al. reported a retrospective study of 110 patients with stage III/IV DLBCL who achieved a CR to R-CHOP [22]. Fourteen patients received consolidative RT (median dose = 30.6 Gy); seven of these to all initial sites and seven to sites of initial bulk only. Among all patients, the 5-year PFS and OS were 50.5 and 72.9 %, respectively. Consolidative RT was associated with significantly improved 5-year PFS (85.1 vs. 44.2 %, P < .0001) and OS (92.3 vs. 68.5 %, P < .0001) compared with patients who received R-CHOP alone. On multivariate analysis, after adjusting other prognostic factors, consolidative RT was predictive of superior PFS (HR 9.64, P = 0.028) and a trend toward better OS (HR = 5.905, P = 0.083) [22]. Although these results suggest a benefit to RT, it is notable that the 5-year PFS among patients treated with chemotherapy alone is quite poor, possibly suggesting an imbalance in risk factors not completely accounted for by the multivariable analysis.
Phan et al. reported a single institution retrospective evaluation of 469 patients with DLBCL treated with R-CHOP chemotherapy [23]. All stages of disease were included although 59 % had stage III/IV and 37 % had bulky disease (>5 cm). Thirty-nine patients (14 %) were treated with consolidation RT based on clinician preference. Among complete responders to R-CHOP, patients getting RT had better OS (89 % with RT added vs. 66 % with R-CHOP alone; P = .0008) and PFS (76 % vs. 55 %; P = .003). Notably, when the analysis was limited to patients with stage III/IV disease, IFRT was still associated with significantly better 5-year PFS (76 vs. 55 %; P = 0.003) and OS (89 vs. 66 %; P = 0.008) [23].
Similarly, a multi-institutional study evaluated the impact of RT on 841 patients with advanced stage disease, 84 % of whom had received 6 to 8 cycles of R-CHOP [24]. An analysis of 76 stage patient pairs with stage III/IV disease matched by age, stage, IPI score, B symptoms, disease bulk, and response to chemotherapy indicated that RT was associated with improved FFS (HR = 0.77, P = 0.34) and OS (HR = 0.53, P = 0.07), although these associations did not meet statistical significance [24].
Skeletal involvement is occasionally a manifestation of advanced stage disease. Held et al. evaluated the outcome of 292 patients with skeletal involvement treated on German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) trials (total sample size 3840). Among 161 patients who achieved a CR, CR unconfirmed, or PR to immunochemotherapy, patients who received RT to sites of skeletal involvement had a better 3-year EFS (75 vs. 36 %; P < 0.001) and OS (86 vs. 71 %; P = 0.064) than those treated with systemic therapy alone (Fig. 2) [25•]. Notably, the benefit of RT for patients with skeletal involvement remained after adjusting for IPI score and disease bulk, and was seen for both patients with advanced stages (HR for EFS = 0.3, P = 0.001) and limited stages (HR for EFS = 0.4; P = 0.146) [25•].
a Event-free and b overall survival of patients with diffuse large B-cell lymphoma with skeletal involvement treated with and without radiotherapy to sites of skeletal involvement. Gold lines represent patients treated with (n = 133), and blue lines represent patients treated without (n = 28) radiotherapy to skeletal sites. From Held et al. [25•]
These studies suggest that some patients with advanced stage disease benefit from adjuvant RT, although they have several limitations relating to the uncertainty about the criteria for selecting patients for RT. The first of these is that nonrandom treatment selection and incomplete accounting for prognostic factors may bias the apparent benefit of RT in these retrospective studies (although it is not obvious in what direction this bias would operate). Moreover, clinical application of the results is challenging because it is not clear which advanced-stage patients benefitted from RT and what the appropriate RT target volume for advanced stage patients should be. The DSHNHL data supports treating sites of initial bulk and skeletal involvement, and many oncologists would also include sites of incomplete anatomic response. An additional source of controversy is whether metabolic response should also be used to select patients for RT.
Use of FDG-PET to Guide Selection of Patients for RT
In recent years, FDG-PET/CT has been shown to be useful for response assessment after treatment. PET/CT became the standard method to assess remission in the 2007 international workshop criteria [26] and subsequently in 2014 Lugano criteria [27, 28]. Additionally, an early PET scan during a course of chemotherapy (commonly known as interim PET, iPET) can show response early and predict prognosis, leading to several studies testing the change of treatment on the basis of early PET response. As a result, there has been an interest in exploring whether PET/CT at the end of chemotherapy (ePET) or interim PET (iPET) can be used to select patients who are likely to benefit from RT and those who may have RT omitted without a detrimental effect on their chance of cure.
Complete Metabolic Response (CMR) Following R-CHOP
In the Emory University study of 110 DLBCL patients discussed previously, 86 % of CRs were confirmed with both PET/CT (14 % were assessed by CT alone) [22]. Despite achieving metabolic CR, RT was still associated with significantly better PFS after adjustment for conventional prognostic factors.
In another study from Duke University [29], 79 patients with stage III/IV DLBCL 65 % of whom were treated with R-CHOP achieved a CR on PET after chemotherapy (13 % had CR on CT alone and 14 % had Gallium-67 scans). RT to all or selected sites of disease was given to 38/79 (48 %) patients. RT was associated with superior EFS and a trend toward improved OS. Among 13 relapses in the no RT group, ten involved previous sites and 3 were isolated distant failures. Multivariable analyses supported the finding that RT improved PFS (HR = 4.3, P = 0.014) [29].
Similarly, in the study of 469 patients from the MDACC [30•], RT was given to patients with CR on PET/CT, whereas PR were treated with salvage chemotherapy. Again, despite good metabolic response, RT improved PFS and OS, and RT was associated with significantly better PFS and OS after adjustment for chemotherapy response and other prognostic factors, including bulk and IPI score [30•].
Despite their retrospective nature and the heterogeneous patient populations, in the absence of prospective trials, these studies provide the best available information about whether CMR at the end of R-CHOP therapy can be used to select patients to be treated without RT, and all suggest that RT after CMR results in significantly better PFS and potentially superior OS. Further evidence from prospective studies is required, and the ongoing German OPTIMAL > 60 study (ClinicalTrials.gov Identifier NCT01478542) is adopting a PET-guided strategy for consolidation RT, where PET-negative patients will not receive RT and PET-positive patients will. The UK-NCRI is planning a randomized study to test the benefit of RT after complete metabolic response to full course chemotherapy.
Consolidation/Salvage RT for Patients with Residual FDG Uptake
Another related controversy is whether RT alone can effectively eradicate disease that remains PET-avid following R-CHOP. Dorth et al. reported on 99 patients who had post-chemotherapy PET (80 %) or Gallium-67 scans (20 %) and received consolidation RT [31]. Although patients with negative metabolic imaging had a better outcome, two thirds of patients with residual activity remained in long-term remission after RT. Among the 21 patients who had residual metabolic activity, 5y-infield control, EFS and OS were 71 %, 65 %, and 73 %, respectively. Another small study reported similar results in 59 patients, of whom 22 patients had a post-chemotherapy-positive PET scan and achieved 90 % 3y-PFS following 36 Gy (range 28.8–50 Gy) [32]. Investigators from BCCCA reported on their policy of PET-guided RT [33]. Patients who had a residual >2-cm mass after R-CHOP (n = 262) underwent PET, and PET-negative patients received no further treatment, while those with a positive PET received RT. The authors reported that the outcome for PET-positive patients who had RT (n = 60) was as favorable as those with negative PET (n = 167) (4-year time to progression and OS 81 and 85 versus 74 and 83 % for PET-positive and negative-patients, respectively) and was better than PET-positive patients who did not receive RT (n = 22, TTP 33 %, OS 30 %) [33].
Although these studies are not randomized, they demonstrate that consolidation radiotherapy may be an adequate treatment for selected cases with localized residual PET positivity after chemotherapy especially in patients who may not be candidates for salvage chemotherapy and autologous stem cell transplant.
iPET for Selection of Patients for RT
Early response to chemotherapy is evident on interim PET/CT, with many patients achieving CMR after 2–4 cycles. Furthermore, patients with early response on iPET seem to have a better prognosis than those who still have residual activity, although results have not been consistent in all studies. This raises two questions in the management of DLBCL; is early CMR enough to omit consolidation RT? And is the lack of early CMR an indication for adding RT at the end even if ePET is negative?
With regard to omitting RT in patients with early CMR, a phase 2 study by BCCCA was reported in abstract and included 155 patients with stages 1–2 with B symptoms or bulky disease (≥10 cm) or advanced stages [34]. After 4 cycles of R-CHOP, patients had an iPET scan, and those with negative iPET (defined according to IHP criteria, equivalent to Deauville score 1–2) received two further R-CHOP and no RT, while iPET-positive patients were switched to RICE chemotherapy for 4 cycles with additional RT if they remained PET-positive. The 4-year PFS was 91 % and 59 % for PET-negative and PET-positive groups, respectively. Corresponding OS was 96 % and 73 %. Despite switching chemotherapy, only 22 % converted to PET-negative. In the iPET-negative group, the omission of RT did not seem to have a negative effect on outcome, although there was no control group for comparison, and the outcome for patients with initial bulk is not described [34].
There are no studies directly examining the outcome of consolidation RT in those patients with positive iPET who convert eventually to a negative ePET. Whether the addition of RT to slow responders could improve outcome is unknown. More data is required to establish whether iPET can be used to guide RT.
RT Dose and Volume
RT doses for DLBCL have typically been in the range of 30–40 Gy, although until recently, the evidence regarding any potential dose–response was limited largely to institutional case series. More recently, however, Lowry et al. reported the results of a randomized study that provides some of the best available data regarding whether doses >30 Gy are required in the adjuvant treatment of DLBCL. The study reported 602 patients with aggressive histology NHL (approximately 82 % with DLBCL) who received either 40–45 Gy in 20–23 fractions or 30 Gy in 15 fractions [35]. Most patients (86 %) received RT as part of their initial treatment, and the majority of these had limited stage disease. There was no difference in overall response rate (91 % in both arms), complete response rate (83 % vs. 82 % in the high-dose and low-dose arms, respectively), or 5-year freedom from local progression (83.5 % vs. 82.2 %) [35]. Among the 469 patients given RT as part of first-line therapy, there was no difference in the crude rate of PFS (67 % vs. 64 % in the high- and low-dose arms, respectively; P = 0.43). Notably, this pragmatic trial has some important limitations: Only 12 % of the first-line combined modality regimens included rituximab, results could not be analyzed according to the presence of initial bulk, and the impact of metabolic response as seen on FDG-PET imaging on RT dose–response could not be assessed. Nevertheless, the results are supported by prior nonrandomized studies that have found local control rates >90 % among patients with nonbulky disease (i.e., <5 cm in diameter) in anatomic CR after 30 Gy, suggesting that higher doses are not needed for these patients [36].
Less is known about the optimal adjuvant RT dose for patients with bulk disease. The UK study of 30 Gy versus 40–45 Gy included patients with bulk disease but did not report these patients separately [35]. The RICOVER-NoRTh study found a significant reduction in the risk of relapse among patients with disease bulk >7.5 cm with the use of 36 Gy in 1.8–2-Gy fractions after R-CHOP chemotherapy, demonstrating this to be an effective dose [14•]. The reported local control rates after lower doses are variable, and most reports precede the use of rituximab, which in one recent study was associated with improved local control compared to CHOP [37]. Local control in some series is excellent with 30 Gy or less even for patients with initial bulk [36, 38]; however, others report loss of disease control with doses <36 Gy [39, 40]. Among patients with initial bulk achieving both anatomic and metabolic CR/CRu, 36 Gy is appropriate, and lower doses are also likely to be effective. In our view, it is unlikely that doses >36 Gy are required unless a CR is not achieved.
The management of a localized mass of DLBCL which has had a suboptimal response to chemotherapy is challenging. For patients achieving only a PR, particularly in the context of initial bulk, doses 36–40 Gy are appropriate, and some may recommend doses up to 45 Gy, although the evidence supporting doses >40 Gy is very limited. For patients with clearly chemo-refractory disease, single arm studies have shown that RT dose of 39.6–40.5 Gy can produce a 2-year local control rate of 70 % [41, 42].
Recent guidelines have described the utilization of CT and FDG/PET imaging to delineate the clinical target volume (CTV) for non-Hodgkin lymphoma. The CTV should encompass the original lymphoma volume, but typically, it is not necessary to treat the axial width of the pre-chemotherapy volume of the nodal mass. An internal target volume (ITV) should be added to the CTV in situations in which internal organ movement is likely, and this volume can be expanded further to create a planning target volume (PTV) that accounts for other setup and geometric uncertainties. These margins are typically 5–15 mm depending on the anatomic location (Fig. 3). More detailed descriptions of the appropriate ITV and PTV expansions and other methods for managing organ motion are described in detail elsewhere [43, 44].
a Staging FDG-PET/CT scan of a 35-year old female with diffuse large B-cell lymphoma presenting as a bulky mediastinal mass. Axial view (left), sagittal view (middle), and coronal view (right). b Radiotherapy plan using butterfly-VMAT (Volumetric Modulated Arc Therapy) technique with two 60° anterior and posterior arcs and a non-co-planar cranio-caudal anterior arc. Axial dose distribution (upper). Coronal dose distribution (lower left). Sagittal dose distribution (lower right). CTV (clinical target volume) (orange line). PTV (planning target volume) (red line). Note sparing of breasts, relative sparing of heart, and avoidance of low-dose bath to lungs. Treatment was delivered with daily IGRT (image-guided radiotherapy) using cone-beam CT
Meticulous attention to the initially involved sites and appropriate CTV contouring can influence toxicity, insofar as intensity modulated RT (IMRT) can often be employed to limit the dose to the salivary glands, heart, or other critical structures to doses associated with minimal risks of toxicity. However, inadequate pre-chemotherapy imaging can necessitate enlargement of the irradiated volume if the initial locations of disease cannot be identified accurately.
Conclusion
The treatment of patients with DLBCL requires multidisciplinary collaboration to ensure optimal outcome. Following R-CHOP, RT is an important contributor to cure for patients with bulk disease and skeletal involvement. Current data do not clearly support the use of FDG-PET response to determine whether patients should receive RT following chemotherapy: One nonrandomized prospective study reported early favorable outcomes using interim PET to assign RT (but did not report on those with bulk disease), whereas three retrospective studies suggest that RT improves EFS and possibly OS even among those with complete metabolic response to 6 cycles of R-CHOP. This issue should be clarified with the results on the ongoing OPTIMAL >60 trial. It is, for example, in circumstances of complete metabolic response in patients with borderline bulk that multidisciplinary evaluation of factors such as the expected RT toxicity and patient eligibility for salvage therapy is particularly valuable.
For early-stage patients with 0–1 IPI risk factors, 3–4 cycles of R-CHOP with 30 Gy RT is effective, and recent randomized data suggest that 4–6 cycles of R-CHOP alone is equivalent provided that patients achieve a significant anatomic and complete metabolic response. Again, the choice between chemotherapy alone or combined modality therapy cannot be determined from the outset in these cases but requires response evaluation, and further, given the potential increase in cardiac toxicity in some patients with a ≥300 mg/m2 of doxorubicin, balancing this with relative toxicities of an individual’s RT volume warrants multidisciplinary care. Factors that influence eligibility for autologous stem cell transplant—most notably age—are also important to consider since some patients will have only one curative chance and initial treatment should be maximized accordingly.
In the RiCOVER study, approximately 50 % of patients were treated with RT based on simple criteria (initial bulk or extranodal involvement), and ongoing studies will further refine disease and patient characteristics that could improve selection for RT. Future work can improve the outcome of patients by evaluating functional imaging and ideally the biologic characterization of the disease to allow more sophisticated judgments regarding the optimal use of RT for DLBCL.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(22):5027–33.
Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, et al. Long-term solid cancer risk among five-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25(12):1489–97.
Mudie NY, Swerdlow AJ, Higgins CD, Smith P, Qiao Z, Hancock BW, et al. Risk of second malignancy after non-Hodgkin’s lymphoma: a British cohort study. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(10):1568–74.
Moser EC, Noordijk EM, van Leeuwen FE, Baars JW, Thomas J, Carde P, et al. Risk of second cancer after treatment of aggressive non-Hodgkin’s lymphoma; an EORTC cohort study. Haematologica. 2006;91(11):1481–8.
Sacchi S, Marcheselli L, Bari A, Marcheselli R, Pozzi S, Gobbi PG, et al. Second malignancies after treatment of diffuse large B-cell non-Hodgkin’s lymphoma: a GISL cohort study. Haematologica. 2008;93(9):1335–42.
Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339(1):21–6.
Miller T, Leblanc M, Spier C, et al. CHOP alone compared to CHOP plus radiotherapy for early stage aggressive non-Hodgkin’s lymphomas: Update of the Southwest Oncology Group (SWOG) randomized trial. Blood 2001;98(724a).
Horning SJ, Weller E, Kim K, Earle JD, O’Connell MJ, Habermann TM, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern cooperative oncology group study 1484. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(15):3032–8.
Reyes F, Lepage E, Ganem G, Molina TJ, Brice P, Coiffier B, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352(12):1197–205.
Bonnet C, Fillet G, Mounier N, Ganem G, Molina TJ, Thieblemont C, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(7):787–92.
Moser EC, Noordijk EM, van Leeuwen FE, le Cessie S, Baars JW, Thomas J, et al. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood. 2006;107(7):2912–9.
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–16. A randomized trial of 1222 elderly patients comparing 6–8 cycles of CHOP with or without rituximab. The addition of rituximab to 6 cycles of CHOP improved 3-year EFS by 19.3 % and 3-year OS by 10.4 %. Patients with bulk (≥7.5 cm) or extranodal disease received RT.
Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–22. Randomized trial including 813 patients aged 18–60 assigned CHOP with or without rituximab. 6-year event-free survival was 55 · 8 % for patients treated with CHOP alone and 74 · 3 % for those recieiving R-CHOP; p < 0 · 0001).
Held G, Murawski N, Ziepert M, Fleckenstein J, Poschel V, Zwick C, et al. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(11):1112–8. A prospective trial of 166 patients evaluating the effect of omitting RT among patients achieving Cr/CRu with R-CHOP chemotherapy. Compared to patients on the RICOVER trial (who received RT), the omission of RT among patients with bulk was associated with significantly worse 3-year EFS (54 % vs. 80 %; P = .001), PFS (62 % vs. 88 %; P < .001) and OS (65 % vs. 90 %; P = .001).
Pfreundschuh M, Ho AD, Cavallin-Stahl E, Wolf M, Pettengell R, Vasova I, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008;9(5):435–44.
Persky DO, Unger JM, Spier CM, Stea B, LeBlanc M, McCarty MJ, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(14):2258–63.
Tondini C, Zanini M, Lombardi F, Bengala C, Rocca A, Giardini R, et al. Combined modality treatment with primary CHOP chemotherapy followed by locoregional irradiation in stage I or II histologically aggressive non-Hodgkin’s lymphomas. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(4):720–5.
Shenkier TN, Voss N, Fairey R, Gascoyne RD, Hoskins P, Klasa R, et al. Brief chemotherapy and involved-region irradiation for limited-stage diffuse large-cell lymphoma: an 18-year experience from the British Columbia Cancer Agency. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(1):197–204.
Tomita N, Takasaki H, Miyashita K, Fujisawa S, Ogusa E, Matsuura S, et al. R-CHOP therapy alone in limited stage diffuse large B-cell lymphoma. Br J Haematol. 2013;161(3):383–8.
Lamy T, Damaj G, Gyan E, Soubeyran P, Bouabdallah K, Cartron G, et al. R-CHOP with or without radiotherapy in non-bulky limited-stage Diffuse Large B Cell Lymphoma (DLBCL): preliminary results of the prospective randomized phase III 02–03 Trial from the Lysa/Goelams Group. https://ash.confex.com/ash/2014/webprogram/Paper69734.html Accessed Jun3 6, 2015. 2014; American Society of Hematology, abstract 393.
Marcheselli L, Marcheselli R, Bari A, Liardo EV, Morabito F, Baldini L, et al. Radiation therapy improves treatment outcome in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2011;52(10):1867–72.
Shi Z, Das S, Okwan-Duodu D, Esiashvili N, Flowers C, Chen Z, et al. Patterns of failure in advanced stage diffuse large B-cell lymphoma patients after complete response to R-CHOP immunochemotherapy and the emerging role of consolidative radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):569–77.
Phan J, Mazloom A, Abboud M, Salehpour M, Reed V, Zreik T, et al. Consolidative radiation therapy for stage III Hodgkin lymphoma in patients who achieve complete response after ABVD chemotherapy. Am J Clin Oncol. 2011;34(5):499–505.
Dabaja BS, Vanderplas AM, Crosby-Thompson AL, Abel GA, Czuczman MS, Friedberg JW, et al. Radiation for diffuse large B-cell lymphoma in the rituximab era: analysis of the national comprehensive cancer network lymphoma outcomes project. Cancer. 2015;121(7):1032–9.
Held G, Zeynalova S, Murawski N, et al. Impact of rituximab and radiotherapy on outcome of patients with aggressive B-cell lymphoma and skeletal involvement. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(32):4115–22. Analysis of 292 patients with skeletal involvement treated on German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) trials (total sample size 3,840). Among 161 patients who achieved a CR, CR unconfirmed, or PR to immunochemotherapy, patients who received RT to sites of skeletal involvement had a better 3-year EFS (75 % v 36 %; P < 0.001), and OS (86 % v 71 %; P = 0.064) than those treated with systemic therapy alone.
Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(5):571–8.
Barrington SF, Mikhaeel NG. When should FDG-PET be used in the modern management of lymphoma? Br J Haematol. 2014;164(3):315–28.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(27):3059–68.
Dorth JA, Prosnitz LR, Broadwater G, Diehl LF, Beaven AW, Coleman RE, et al. Impact of consolidation radiation therapy in stage III–IV diffuse large B-cell lymphoma with negative post-chemotherapy radiologic imaging. Int J Radiat Oncol Biol Phys. 2012;84(3):762–7.
Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(27):4170–6. Analysis of 469 patients with DLBCL treated with or without RT. Matched-pair analyses of patients who received six to eight cycles of R-CHOP with stage I or II disease (44 pairs) and all stages (74 pairs) indicated that RT improved OS (hazard ratio [HR], 0.52 and 0.29, respectively) and PFS (HR, 0.45 and 0.24, respectively) compared with no RT.
Dorth JA, Chino JP, Prosnitz LR, Diehl LF, Beaven AW, Coleman RE, et al. The impact of radiation therapy in patients with diffuse large B-cell lymphoma with positive post-chemotherapy FDG-PET or gallium-67 scans. Ann Oncol. 2011;22(2):405–10.
Halasz LM, Jacene HA, Catalano PJ, Van den Abbeele AD, Lacasce A, Mauch PM, et al. Combined modality treatment for PET-positive non-Hodgkin lymphoma: favorable outcomes of combined modality treatment for patients with non-Hodgkin lymphoma and positive interim or postchemotherapy FDG-PET. Int J Radiat Oncol Biol Phys. 2012;83(5):e647–54.
Sehn L, Klasa R, Shenkier T, et al. Long-term experience with pet-guided consolidative radiation therapy (XRT) in patients with advancedstage diffuse large B-cell lymphoma (DLBCL) treated with R-CHOP. Hematol Oncol. 2013;31(Supp1):137. abstract 123.
Laurie H Sehn, Edward L G Hardy, Karamjit K Gill, Abdulwahab J Al-Tourah, Jesse Shustik, Nicol A Macpherson, et al. Phase 2 trial of interim PET scan-tailored therapy in patients with advanced stage Diffuse Large B-Cell Lymphoma (DLBCL) in British Columbia (BC). https://ash.confex.com/ash/2014/webprogram/Paper76195.html; Accessed June 8, 2015. 2014.
Lowry L, Smith P, Qian W, Falk S, Benstead K, Illidge T, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100(1):86–92.
Dorth JA, Prosnitz LR, Broadwater G, Beaven AW, Kelsey CR. Radiotherapy dose–response analysis for diffuse large B-cell lymphoma with a complete response to chemotherapy. Radiat Oncol. 2012;7:100.
Lao L, Tsang R, Pintilie M, et al. Combined modality therapy for stage I–II diffuse large B-cell lymphoma provides excellent local control and clinical outcome in the rituximab era. Eur J Cancer. 2011;37(Supp 1):S644. abstract 2919.
Krol AD, Berenschot HW, Doekharan D, Henzen-Logmans S, van der Holt B, van’t Veer MB. Cyclophosphamide, doxorubicin, vincristine and prednisone chemotherapy and radiotherapy for stage I intermediate or high grade non-Hodgkin’s lymphomas: results of a strategy that adapts radiotherapy dose to the response after chemotherapy. Radiother Oncol. 2001;58(3):251–5.
Wilder RB, Rodriguez MA, Ha CS, Pro B, Hess MA, Cabanillas F, et al. Bulky disease is an adverse prognostic factor in patients treated with chemotherapy comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy for aggressive lymphoma. Cancer. 2001;91(12):2440–6.
Kamath SS, Marcus Jr RB, Lynch JW, Mendenhall NP. The impact of radiotherapy dose and other treatment-related and clinical factors on in-field control in stage I and II non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 1999;44(3):563–8.
Tseng YD, Chen Y, Catalno PJ, et al. Rates and durability of response to salvage radiation therapy among patients with refractory or relapsed aggressive non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;91(1):223–31.
Martens C, Hodgson DC, Wells WA, Sun A, Bezjak A, Pintilie M, et al. Outcome of hyperfractionated radiotherapy in chemotherapy-resistant non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2006;64(4):1183–7.
Illidge T, Specht L, Yahalom J, Aleman B, Berthelsen AK, Constine L, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2014;89(1):49–58.
Alcorn S, Agbahiwe H, Terezakis S. Non-Hodgkin lymphoma. In: Lee NY, Riaz N, Lu J, editors. Target volume delineation for conformal and intensity modulated radiation therapy. Switzerland: Springer International; 2014.
Compliance with Ethics Guidelines
Conflict of Interest
David C. Hodgson and N. George Mikhaeel declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lymphomas
Rights and permissions
About this article
Cite this article
Hodgson, D.C., Mikhaeel, N.G. Consolidative Radiation in DLBCL: Evidence-Based Recommendations. Curr Oncol Rep 17, 49 (2015). https://doi.org/10.1007/s11912-015-0472-y
Published:
DOI: https://doi.org/10.1007/s11912-015-0472-y