Abstract
Purpose of Review
Transcranial magnetic stimulation (TMS) is a method of Non-Invasive Brain Stimulation that is based on electro-physical principles discovered by Michael Faraday. A TMS device is made of one or two copper coils, positioned superficially to a site of interest in the brain, to non-invasively produce a brief magnetic pulse to an estimated depth from the surface of the scalp with the following axonal depolarization. This axonal depolarization activates cortical and subcortical networks with multiple effects. There are different methods of TMS used, all with different mechanisms of action. TMS is well tolerated with very few side effects.
Recent Findings
TMS is now approved for major depression disorder and obsessive-compulsive disorder. There is significant data to consider approval of TMS for many neurological disorders. This is a review of the uses of TMS in diverse neurological conditions, including stroke and spasticity, migraine, and dementia.
Summary
TMS is a device that utilizes non-invasive brain stimulation, and it has shown promising results with objective clinical and basic science data. Its ability to trigger neuronal plasticity and potentiating synaptic transmission gives it incredible therapeutic potential. There are diverse mechanisms of action, and this could be troublesome in elaborating clinical trials and standardization of therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introduced in the early 1980s but originating from the bioelectrical movement once instituted by Galvani and Volta in the late seventeenth and early eighteenth century, respectively, transcranial magnetic stimulation (TMS) has found its way into the mainstream in the areas of non-invasive therapy for multiple neurological and psychiatric disorders.
Michael Faraday discovered electromagnetism in 1831, when he found that changing magnetic fields could produce electricity. TMS is based on the Maxwell-Faraday equation and principles. When “active,” the coil of wire creates a plane with a flow of current, and this current creates a magnetic field which itself produces a perpendicular electric field, stronger in the periphery than the coil, and absent and weaker in the center. This magnetic current depolarizes transmembrane potentials and activates neurons located directly under the coil [1••]. The coils presented in different models that determine the focality of the stimulation, the circular (the original), the figure-of-eight, or butterfly coil and the H-coil.
The transcranial method followed the peripheral stimulation of nerve trunks. AT Barker and IL Freston from Sheffield, England, described the repetitive method in 1985. They described the use of a capacitor discharge system connected to a coil placed on top of the scalp, able to elicit an action potential from a muscle in the arm, expanding the idea from single pulses that was achieved by A. Merton and H. B. Morton at the National Hospital in London [2, 3]. They used a brief, high-voltage electric shock to activate the motor cortex and produce a relatively synchronous muscle response, the motor-evoked potential (MEP). Unfortunately, the first attempts to the technique were unsuccessful, but in 1985 TMS was done for the first time without pain, or little pain. In order for TMS to work, pulses must be created repetitively at different frequencies that stimulate or inhibit cerebral function.
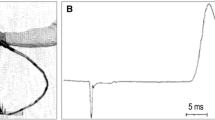
A TMS device is made of one or two copper coils, positioned superficially to a site of interest in the brain, to non-invasively produce a brief (100 to 400 μs) magnetic pulse (generating a 1.5 to 2 T magnetic field) to an estimated depth of ~2–2.5 cm from the surface of the scalp with the following axonal depolarization of a patch of approximately two square centimeters, see Fig. 1. Low-frequency repetitive stimulation has been hypothesized to result in prolonged synaptic depression when each incoming pulse arrives during the late inhibitory phase produced by the previous pulse.
High-current pulse is produced in a coil of wire, the magnetic coil, which is placed above the scalp. A magnetic field is produced with lines of flux passing perpendicularly to the plane of the coil. An electric field is induced perpendicularly to the magnetic field. In a homogeneous medium, the electric field will cause current to flow in loops parallel to the plane of the coil. The loops with the strongest current will be near the circumference of the coil itself. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature, Transcranial magnetic stimulation and the human brain, Mark Hallet, 2000
Alvaro Pascual Leone, whom I met and worked with briefly while doing research in the laboratory of Alexander Rotenberg (who introduced me to this field of neurostimulation and modulation) at Children’s Hospital Boston, was a pioneer in demonstrating how rTMS can reveal how the brain works. In his studies with blind individuals, he and his colleagues stimulated magnetically the visual cortex and noticed an increase in speed in Braille reading, demonstrating neuronal plasticity. His work has expanded into multiple areas including epilepsy, stroke, Parkinson’s disease, pain, autism, depression, and dementia [4].
TMS is part of the non-invasive electrical brain stimulation (NIBS) methods where inducing a virtual transient neurological deficit is intended. Investigation of mechanism of action has been an area of increasing and intense interest. Depending on the frequency of stimulation, low frequency (< 1 Hz) or high frequency (> 5–20 Hz), we will obtain a specific result, long-term depression or potentiation, respectively. Low-frequency rTMS (< 1 Hz) reduces/inhibits cortical excitability increases cortical silent period duration and reduces motor-evoked potential amplitudes. Higher frequencies (> 5 Hz) enhance cortical excitability and induce stimulation. LTD and LTP are forms of neuronal plasticity in the neuronal circuitry [5]. A good example of TMS functionality is the study of visual perception. Professor Amassian at the State University of New York in Brooklyn were able to elicit an inhibitory postsynaptic potential (IPSP) in calcarine cortical neurons with topographical relationship to the stimulus. In similar fashion, Kammer and collaborators at the University of Ulm with the use of the magnetic coil elicited phosphenes and scotomas. Stereotactic positioned TMS over the occipital pole is able to induce contralateral phosphenes by suppressing visual perception on the stimulated side. Using this technique, they were able to map the retinotopic organization of individual cortices providing valuable information of the structures that could suppress vision (see Fig. 2).
Artistic illustration showing an area (red) of coil-induced stimulation on the left posterior-occipital region inducing changes on the contra-lateral visual field, representing inhibition of vision, as well as scotomas and phosphenes. This TMS-induced phenomena has been documented in several key experiments that were important to elucidate utility and possible mechanisms of actions of TMS [1••, 6, 7] TMS Illustration courtesy of Gabriel Sabbagh, 2019.
TMS became a safe and non-invasive method to study the brain, a process that could be done inexpensively and repeatedly. Initially it was used mainly for cortical mapping, but it now is used for treatment or potential treatment in multiple conditions. There are multiple types of TMS stimulation techniques that include cortical and deep stimulation. Here the focus will be cortical stimulation, surface or standard TMS, and its different forms since most of the developments of our interest have been noted on this area. Deep brain TMS is an expanding area of psychiatric and neurological treatment development, but most of the subject is beyond the focus of this review. Of note, the magnetic force may dissipate with depth as it is harder to induce specific therapy in deeper structures of the brain, and attempting may induce damage to delicate structures such as optic nerve. Deeper stimulation would require higher magnitude with an increased risk in considerable side effects. However, we will see the use of deep brain stimulation with the use of the H-coil as well briefly explained in stroke.
TMS has been used the most widely in the field of psychiatry. In 2008 the FDA cleared TMS use in the treatment of major depression. A main target of stimulation is the dorsolateral prefrontal cortex (DLPFC) (see Fig. 3). Many areas have been targeted including the orbitofrontal cortex or pre-supplementary motor area (pre-SMA). It is like electroconvulsive therapy but much less likely to have seizures as a side effect. In 2018 a 3-minute high-frequency stimulation called the Express TMS was also recently approved for depression treatment via intermittent theta burst stimulation (iTBS) that was non-inferior to 10 Hz rTMS for the treatment of depression). iTBS is a newer form of TMS that can deliver powerful stimulation in a very short period [8•], exciting for the world of psychiatry. In a systematic review, a meta-analysis completed with literature from randomized control trials though 2016 showed that low-frequency stimulation over the SMA offered the most effective responses in the treatment of obsessive-compulsive disorder (OCD) [9]. In August 2018, the FDA cleared the way for TMS in the treatment of OCD.
The Neurology Experience
The neurological perspective has been very “exciting,” but it is still a work in progress. Now we will look at diverse areas of neurology in which TMS use is rapidly expanding.
Epilepsy
RTMS has been proposed as a potential non-invasive treatment for the management of refractory epilepsy, as an alternative to epilepsy surgery. The rationale for using rTMS to suppress seizures in real time relates to its potential to interrupt synaptic potential and focal cortical excitability. The main concern for TMS is that in epileptic populations, it may actually induce seizures, although in the series of EPC this was not the case [10]. The prolonged inhibitory effects of TMS are thought to reduce cortical hyperexcitability associated with various epilepsies. TMS has been used to probe cortical excitability in various epilepsy syndromes, to assess the effects of antiepileptic drugs on the brain, and to help identify areas of the brain more prone to seizure for surgical removal [5]. In 2016, A Cochrane review done on seven pilot studies completed in different countries around the world showed interesting results. All recruited participants had drug-resistant epilepsy of varying definitions across studies, generally defined as at least one complex partial or secondarily generalized seizure per month (but most required three or more seizures per week) and an unchanging drug regimen of at least two antiepileptic medications. All used standard figure 8 coils to deliver rTMS, although sham methods differed. The conclusion from this review was that the quality of evidence was low overall. While TMS was safe with no clear significant adverse events, the efficacy of rTMS for seizure reduction was lacking, despite rTMS reducing epileptiform discharges. There was too much variability in the studies regarding TMS techniques and outcome reporting [11••].
Stroke, Spasticity, and Rehabilitation
In the area of post-stroke rehabilitation, responses have been if anything controversial. Numerous studies have failed replication. In Europe, the CE, Conformité Européenne, has approved the use of the H-coil deep TMS stimulation for the therapy of patients with aphasia due to stroke either ischemic or hemorrhagic. The stimulation is guided to the ipsilateral side of the stroke. Significant improvement of non-fluent aphasias were noted when excitatory10 Hz rTMS targeting the inferior frontal gyrus (IFG) right homolog language region. No significance was noted with low frequency or inhibitory stimulation. The H-coil attempts to stimulate targets that are deeper and larger compared to the most commonly used figure 8 coil Fig. 3b. The area of stimulation was localized 5 cm anterior and 1.5 cm lateral to the right-hand motor area with an intensity set at 100% if resting motor threshold, meaning the minimal intensity that would evoke a twitch of the left-hand muscles. The idea to stimulate the contralateral hemisphere of the stroke is to induce cerebral plasticity and reorganization of the cerebral circuitry and networks. Of interest, on this small study, the only patient who did not improve on the treatment leg was the patient with a subcortical stroke. Functional MRI studies have shown that the pars opercularis and pars orbitalis on the right IFG reactivated on individuals who are affected by an inferior frontal cortex stroke [12].
The same group, Chieffo et al., were able to demonstrate that the result of deep stimulation with the H-coil in right-handed patients (n = 10) who had suffered (> 6 months to 3 years from the initial insult) a right or left middle cerebral artery territory subcortical stroke was improved walking speed even shortly after stimulation and at follow-up visits (at least 4 weeks), when compared to sham stimulation patients. The residual neurological deficit (National Institutes of Health Stroke Scale – NIHSS) and the degree of disability (Barthel Index and modified Rankin Scale) were established upon enrollment. The stimulation was again at high frequency at 20 Hz over the lower limb motor cortex bilaterally.
This is now being used and approved in certain areas of Europe. Of note, previous rTMS with patients who suffered a stroke involving the cortical motor area have failed to show improvement [13, 14]. Interestingly, Galvão et al. showed significantly that inhibitory stimulation of the unaffected primary motor area by doing 1500 pulses of 1 Hz and at 90% of resting motor threshold for the first dorsal interosseous muscle resulted in 90% of the patients at post-intervention and 55.5% at follow-up with a decrease of ≥ 1 in the Modified Ashworth scale (MAS score) [15].
In a recent systematic review of 168 individual articles and 70 studies, filtered from nearly 700 records that focused on motor function rehabilitation, published between 2005 and 2016 and involving 3744 adult patients, many learning points surfaced. A protocol needs to be established and larger randomized control trials need to be made. There was difference in methodology in these studies, but they conclude that rTMS can help improve mobility and spasticity, especially if it is combined with physical therapy and rehabilitation. Studies are needed to standardize timing and length of stimulation and site of inhibition and excitation. It is not clear what is more important, to inhibit the contralateral unaffected hemisphere or to excite the stroke side. One of the important take-home messages from the use of rTMS was the little and tolerable side effect profile [16•]. This is different from what was experienced with direct contact electrodes on the scalp when Merton and Morton al were doing their own experiments with unfortunately very painful and uncomfortable adverse reactions. [17].
Spasticity is a major cause of disability, and it is seen on multiple neurological conditions that affect the corticospinal tract. Gunduz and Pascual-Leone did a literature search on the effect of non-invasive brain stimulation, including rTMS, on spasticity in stroke, MS, spinal cord injury (SCI), and cerebral palsy (CP). In the area of MS, the study completed by Centonze et al. in 2007 was highlighted. On this study 19 patients were treated with a figure 8 coil and stimulated over the primary motor cortex. There was improvement of the modified Ashworth scale (MAS) after stimulating daily for a 2-week period with 15 rTMS at 5 Hz. In a similar study, Morin and colleagues 3 years later were able to induce improvement of MAS with suppression of H reflex (also noted by Centonze with 5 Hz stimulation), in this case with TBS (10 bursts, composed of three stimuli at 50 Hz, repeated at a theta frequency of 5 Hz) applied over primary motor cortex of lower extremity for 2 weeks. The improvement remained on both studies at least 1 week after the last day of stimulation. In SCI, Kumru showed on two studies that daily high-frequency rTMS applied over the motor cortex area of the legs from 5 to 15 days of therapy could decrease the level of spasticity reflected by lowering scores of MAS, spinal cord assessment tool for spasticity (SCAT), modified Penn spasm frequency scale (MPSFS), and spinal cord injury spasticity evaluation tool (SCI-SET). The latter scaling tool failed to show improvement on the Kumru 2013 study. Unfortunately in cerebral palsy rTMS at 5 Hz did not induce significant improvement of the spasticity measure with MAS, but a partial improvement in range of motion was noted. No change was seen when completed with 1 Hz [18].
Multiple Sclerosis
The H-coil rTMS was studied for safety and efficacy for deep brain stimulation in patients with fatigue in the setting of multiple sclerosis (MS) [19]. Thirty-seven patients were randomized for stimulation with high frequency of the left prefrontal cortex (PFC), motor cortex (MC), and sham control stimulation. This pilot study showed class III evidence that stimulation of deep structures via the H-coil rTMS may possibly alter the lateral, dorsolateral, and ventrolateral prefrontal cortical network and their projections to subcortical networks. It is believed that fatigue is closely related to damage in connectivity between different cortical and subcortical networks in white and gray matter. Connections between PFC and posterior cingulated cortex and cortical motor areas may be involved, similar to what was seen in major depressive disorder (MDD). However, improvement was more pronounced on the MC group. It is possible to believe that deep rTMS could improve connectivity at least momentarily in MC with supplementary motor areas. The study appeared to be safe, and preliminary efficacy was assessed based on changes in Fatigue Severity Scale (FSS) and Beck Depression Inventory scores. Due to the small number of participants, a larger study needs to be made.
Migraine
Nearly 1 billion people worldwide suffer from migraine, which is one of the top five the most common neurological disorders in the world. In this field, single-pulse TMS (sTMS) showed promising results in its ability to block mechanically and chemically induced cortical spreading in animal models. Given that migraine entitles cortical spreading depression on its physiopathology, it is reasonable to immediately think that inhibiting this spread could suppress the hypersensitivity and pain. Several human trials ensued the animal studies. A significant reduction in pain severity and other migraine-associated symptoms when compared to sham TMS stimulation were seen in these trials when sTMS was used a rescue therapy in studies with a non-dismissible number of participants. Responses were recorded by participants at different time points, and no significant side effects were reported. This is an important situation as many of the current abortive therapies have serious side effects and are contraindicated in many individuals, especially in the more advanced age population or with vascular or gastrointestinal disease. The findings also showed that sTMS showed non-inferiority for migraine-associated symptoms such as photophobia, phonophobia, and nausea after 2 h [20••, 21].
Take for example, an open-label study of sTMS of 190 selected patients (n = 449) with migraine with aura in 20 headache-specialized clinic set in the UK. In the study, they used a “small and lightweight” machine which the CE approved for clinical practice at home for the treatment of breakthrough moderate to severe migraine attacks and withholding rescue pharmaceuticals. The machine was placed below the occipital bone and was based on escalating amount pulses sTMS until symptoms had resolved, as much as one pulse every 15 min until pain and symptoms had resolved. Sixty-two percent of these patients reported a reduction in pain, 59% a decrease in attack duration, and 59% reported reduction in headache days. Twenty-five percent reported no change. A total of 3,802 attacks of migraine with aura and nearly 6,000 attacks without aura were treated on this study. The machine and the stimulation were well tolerated and overall patients felt “clearer” the following day [22].
The situation seemed similar in regard to preventative chronic migraine TMS therapy. There were a lot of mixed results in this setting with “dramatic” placebo, noted in a study that combined chronic migraine and medication overuse headache. Studies ran into the problem that there were too many variables, with some conflictive results. Meta-analysis of the data suggests a respectable level of efficacy, but the parameters including the area of stimulation needed to be standardized. [23]. In 2018 the FDA-approved TMS for migraine prevention based on an open-label study completed in prestigious treatment centers in the USA and UK. This study, called the eNeura SpringTMS Post-Market Observational US Study of Migraine (ESPOUSE), observed 263 patients between 2014 and 2016 over 15 months. It suggested efficacy and tolerability of sTMS as an option for migraine prevention. The criteria for headache were 4 to 25 headache days per month (confirmed by 1-month baseline diary, minimum of 5 completely headache-free days/month). Reponses using the same target as in the depression trial, the dorsolateral pre-frontal cortex had been negative. The same area that was used for the rescue therapy was used, the occiput. Patients pressed the button to deliver the single pulse twice daily, and additional pulses could be given for acute treatment, once at the onset, and then repeated after 15 min. Subjects were allowed to use medication if they experienced no relief 30 min after the pulses were delivered. The intensity of the pulse was 0.9 T, rise time of 180 μsec, and total pulse length less than 1 ms [24•].
The mechanism believed to be involved in sTMS is the blockade of the before-mentioned cortical spreading depression. Andreou and collaborators elucidated this very finely. STMS delivered through a coil with a rise time of 170 μsec was able to block mechanically induced cortical depression via a needle prick. They saw a similar phenomenon when inducing CSD via potassium chloride measuring cerebral blood flow and intracortical DC-shift changes. A significant inhibition of the thalamocortical signaling via the ventroposteromedial thalamic nucleus neuronal activity and dural C-fiber-mediated trigeminovascular activity was observed. The role of the thalamus as (10 Hz) a potential target for the treatment of migraine was noted. No clear effect was seen on the trigeminovascular activity recorded on the second order neurons. Finally, sTMS may also involve the endogenous opioid system [25••].
Pain
TMS and other types of non-invasive brain stimulation have not shown significantly clear or statistically strong data to support its use in the area of chronic pain. Fibromyalgia showed the most encouraging pool of results. The Cochrane library data has been meta-analyzed for all the different methods and did not show strong enough evidence in the change in pain intensity, severity or enough to have TMS considered as a possible tool in the treatment of chronic pain. The pooled mean decrease in pain scores with rTMS therapy from a scale ranging from “0” (no pain) to “10” (worst pain) was 0.4 or 7% where an accepted improvement should be > 30% to be considered clinically important. The quality of the data was considered low and highly variable. However, some efficacy was projected if it is used in concert with conventional medical treatment. Transient reduction in pain has been shown on small trials by applying high-frequency (20 min of 10 Hz) rTMS over the motor cortex corresponding to the painful area. The area was mapped and confirmed by recording motor-evoked potentials the muscles of the affected areas, the first dorsal interosseous muscle in patients with thalamic stroke and in the masseter of the painful hemiface in patients with trigeminal neuralgia [26,27,28].
Amyotrophic Lateral Sclerosis
In the area of neurodegenerative disease, TMS has been in mapping areas of the brain that are involved in amyotrophic lateral sclerosis (ALS) in addition to symptomatic treatment of cramps and spasticity. TMS may help to differentiate Alzheimer’s disease from other types of dementia, and it could help in speech impairment and disease progression in primary progressive aphasia. In combination with aerobic exercise, TMS can help improve motor symptoms. ALS is a disease associated with degeneration of upper and lower motor neuron, known in the USA as Lou Gehrig’s disease and in other areas of the world as Charcot’s disease. TMS has helped the understanding of the pathogenic process that area active or inactive in the cortex in patients with motor neuron disease, offering a possible biomarker to hopefully assess preclinical progression. In a Russian study, Chervyakov [29] and colleagues were able to elucidate cortical areas affected in patients with ALS in a study comparing to healthy controls. Using a technique called navigational TMS (nTMS), they studied the areas affected by the degeneration, to investigate whether these areas could be the neurodegenerative focus. They also studied which motor neuron is triggered in the onset of the disease, examining if upper motor neurons are involved prior to or independently from the lower motor neurons. This is an important aspect in understanding the neuropathology and disease activity in ALS.
By evaluating the motor threshold (MT), defined here as the lowest possible stimulation intensity able to produce motor responses of 50 μV in five of ten trials with the patient at rest, and by looking at motor evoked potentials via EMG of a target muscle, the abductor pollicis brevis, in order to “map,” TMS was performed using a 70 mm figure 8 coil, maximal magnetic field strength of 199 V/m, and magnetic impulse duration of 280 μsec. In addition, functional MRI (fMRI) were used to find the corresponding area on the motor cortex representing the abductor pollicis brevis, basically on the lower anterior central gyrus, area Brodmann 4,1 and 6. The group found that as the disease progressed, the higher the MT, and there were broader or absent MEPs. At the same time, there were worse functional rating scales, as measured by the ALSFRS-R, the revised ALS Functional Rating Scale. In ALS the patients exhibited a decreased motor cortex volume in both hemispheres, reduced map size. In addition, a phenomenon called “sparseness” was found: the shape of the motor area functional zone changed and became “patchy.” This could be interpreted as progression and severity or is part of a neuroplasticity occurring while neurons degenerate. This patchy enigma could indicate that disease activity lacks a focus of onset and that neurodegeneration could follow a multifocal pattern as well. These arising concepts need further investigation but raise significant and exciting questions. Besides a subtle headache and increased fasciculations during the stimulation, no other side effect or adverse events were recorded during the study.
There is still a lot of understanding ahead of us, but findings like this make TMS a potential tool for disease mechanism and therapy.
In a study published in the Journal Neurology in 2017, Benussi et al. from Brescia, Italy showed class III evidence. By using the different paradigms of TMS (see Fig. 4), they were able to distinguish differences in intracortical circuits from confirmed patients suffering from Alzheimer’s dementia (AD) or frontotemporal dementia (FTD) and healthy controls. The paradigms used were short-interval intracortical inhibition (SICI) and facilitation (ICF), long-interval intracortical inhibition, and short-latency afferent inhibition (SAI) [30••, 31]. The differences were noted with high accuracy even in mild disease or early stages. TMS has become a tool to assess this circuitry. Particular differences were seen in each condition, noted earlier in smaller studies and now confirmed in this large study. Impairment of the different neurotransmitters GABAergic, glutamatergic, and cholinergic circuits can be assessed by TMS paradigms SAI, SICI, and ICF. SAI circuit was impaired in AD because it relies on cholinergic circuits known to be affected in this disease. Therefore SAI can be used as a marker and as an important diagnostic finding and tool. SICI seems to be spared in AD, known to be dependent of GABAergic inhibitive activity. On the other hand, patients with FTD showed a significant impairment of SICI/ICF regulated by glutamatergic NMDA receptors. Conclusion AD and FTD differ in SICI/ICF and SAI but not in LICI circuits even in early stages of the diseases and with high sensitivity and specificity, 91.8% and 88.6%, respectively, and accuracy of > 85%. TMS is a useful, inexpensive, non-invasive, quick, and reliable tool to help diagnose and differentiate and prognosticate dementias.
Transcranial magnetic stimulation (TMS) paradigms. (A) A single pulse of TMS over M1 evokes an MEP recorded over the contralateral first dorsal interosseous. (B) Two pulses separated by 2 ms evoke an MEP of smaller amplitude, revealing short interval intracortical inhibition (SICI). (C) Two pulses separated by 12 ms evoke an MEP of larger amplitude, revealing intracortical facilitation (ICF). De Beaumont, Louis; Lassonde, Maryse, Long-term and Cumulative Effects of Sports Concussion on Motor Cortex Inhibition, Neurosurgery, 2007, 61, 2, 331, by permission of Oxford University Press
Conclusions
Transcranial magnetic stimulation seems to have finally found a clear position in the treatment of neuropsychiatric disorders. After an extensive review of literature, one can have many conclusions. Above all, it is a device that is non-invasive. It has shown very promising results with objective clinical and basic science data, including triggering neuronal plasticity and potentiating synaptic transmission. There are diverse mechanisms of action; it can work as a stimulant or an inhibitor of cerebral activity and both towards the same goal. We saw that in post-stroke spasticity, there are stimulation protocols as well as inhibition. Different coil may induce different stimulus, resulting in a different experience; also taking into account the individual owns “plastic homeostasis.” One-size-coil and magnetic impulse that does not fit all philosophy is in place. There are many variables that could be studied and arranged to improve functionality and network connections.
No frequent or significant complications or mayor adverse effects are usually the rule. Local pain at the site of stimulation or a mild and transient headache or neck pain is usually triggered following a session of TMS. TMS is tolerable, inexpensive, safe, and evolving. In my opinion, the field of epilepsy is where it may have the most resistance, since one of the significant side effects are seizures, about 0.6%. Epilepsy is a contraindication of TMS for the treatment of MDD and OCD. RTMS does not seem to have higher risk for triggering seizures than single or paired pulses. Certainly, in a patient with known epilepsy, the risk of having a seizure following TMS can rise to nearly 30%. Similar increased risk can be seen in patients who suffered a cortical stroke, arteriovenous or other vascular malformations, tumor, or patients with known psychiatric disorders who are treated with medications known to decrease seizure threshold. In a survey study by Lerner and colleagues, stimulation of the primary motor cause seizures was the most sensitive area to trigger seizures, probably because it is one of the most frequent areas to be stimulated. In this survey, 2% of patients treated for depression reported hypomania or mania, although statistically low. More than 60% of seizures occurred after or during the first TMS treatment. Syncope and pre-syncope were also reported at a higher rate than seizures. Cochlear implant or any metallic objects in close proximity to the TMS (< 30 cm) are an absolute contraindication. Cardiac pacemakers are usually contraindicated but are unlikely to be damaged by TMS [28, 32, 33].
Now that the FDA has approved TMS for medication resistant MDD and OCD where remission has been clear with improved disability, skyrocketing practices are seen and other fields are starting to notice. There are currently 1,641 studies currently listed as ongoing, recruiting, completed, or withdrawn on the clinicaltrials.gov website, more than 60 in dementia alone. RTMS for the treatment of primary progressive aphasia (PPA) is one of many ongoing trials based on small studies with significant results that showed improvement of patient suffering from PPA.
TMS warrants further exploration. Study designs need to have standardization of techniques, focusing on primary end points, stimulation location, and depth of stimulation among many “electrical” variables. Epilepsy may be one of the fields with more variable results and controversy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. 2017;18(11):685–93. https://doi.org/10.1038/nrn.2017.113 This articles provides an updated, eloquent information regarding the fundamentals and mechanism of action of TMS.
Tang A, Thickbroom G, Rodger J. Repetitive transcranial magnetic stimulation of the brain: mechanisms from animal and experimental models. Neuroscientist. 23(1):82–94.
Barker AT, Freston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;110601107.
Pascual-Leone Á. A pioneer of non-invasive brain stimulation. Marcos Z. Lancet Neurol. 2013;12(9):853. https://doi.org/10.1016/S1474-4422(13)70129-7.
Vasilios K. Kimiskidis, Transcranial magnetic stimulation for drug-resistant epilepsies: rationale and clinical experience. Eur Neurol. 2010;63:205–10.
Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74(6):458–62. https://doi.org/10.1016/0168-5597(89)90036-1.
Kammer T, Puls K, Erb M, et al. Transcranial magnetic stimulation in the visual system. II. Characterization of induced phosphenes and scotomas. Exp Brain Res. 2005;160:129. https://doi.org/10.1007/s00221-004-1992-0.
• Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–92. https://doi.org/10.1016/S0140-6736(18)30295-2 Large multicenter trial showing efficacy of the different form of TMS in psychiatric disorders proving the multiple methods can be used with good efficacy and with shorter treatments.
A Meta-Analysis of the Effectiveness of Different Cortical Targets Used in Repetitive Transcranial Magnetic Stimulation (rTMS) for the Treatment of Obsessive-Compulsive Disorder (OCD), Rehn, S., Eslick, G.D. & Brakoulias, V. Psychiatr Q (2018) 89: 645. https://doi.org/10.1007/s11126-018-9566-7
Rotenberg A, et al. Epilepsy Behav. 2009;14:253–7.
•• Chen R, Spencer DC, Weston J, Nolan SJ. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database Syst Rev. 2016; (8):CD011025. https://doi.org/10.1002/14651858.CD011025.pub2. Review. Very detailed comparative studies of TMS in its attempt to treat seizures.
Chieffo R, Ferrari F, Battista P, Houdayer E, Nuara A, Alemanno F, et al. Excitatory deep transcranial magnetic stimulation with H-coil over the right homologous Broca's region improves naming in chronic post-stroke aphasia. Neurorehabil Neural Repair. 2014;28(3):291–8. https://doi.org/10.1177/1545968313508471.
Chieffo R, De Prezzo S, Houdayer E, Nuara A, Di Maggio G, Coppi E, et al. Deep repetitive transcranial magnetic stimulation with H-coil on lower limb motor function in chronic stroke: a pilot study. Arch Phys Med Rehabil. 2014;95(6):1141–7. https://doi.org/10.1016/j.apmr.2014.02.019.
Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66(3):298–309. https://doi.org/10.1002/ana.21725.
Barros Galvão SC, Borba Costa dos Santos R, Borba dos Santos P, Cabral ME, Monte-Silva K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2014;95(2):222–9. https://doi.org/10.1016/j.apmr.2013.10.023.
• Dionísio A, Duarte IC, Patrício M, Castelo-Branco M. The Use of Repetitive Transcranial Magnetic Stimulation for Stroke Rehabilitation: A Systematic Review. J Stroke Cerebrovasc Dis. 2018;27(1):1–31. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.09.008 Provides a state of the art systematic review of TMS and its use in stroke. The study found a wide variability in the factors that need to be considered when comparing outcomes of rTMS studies and emphasizes in understanding the mechanism of action better in order to find a more guided treament.
Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human cortex through the scalp. Review Article. Exp Physiol. 1991;76:159–200.
Kumru H, Pascual-Leone A, Gunduz A. Outcomes in spasticity after repetitive transcranial magnetic and transcranial direct current stimulations. Neural Regen Res. 2014;9(7):712–8. https://doi.org/10.4103/1673-5374.131574.
Gaede G, Tiede M, Lorenz I, Brandt AU, Pfueller C, Dörr J, et al. Safety and preliminary efficacy of deep transcranial magnetic stimulation in MS-related fatigue. Neurol Neuroimmunol Neuroinflamm. 2017;5(1):e423. https://doi.org/10.1212/NXI.0000000000000423 eCollection 2018 Jan.
•• Lan L, Zhang X, Li X, Rong X, Peng Y. The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails Review. J Headache Pain. 2017;18(1):86. https://doi.org/10.1186/s10194-017-0792-4 Provides a well-rounded understanding of TMS in the treatment of migraine, its level of efficacy and different parameters that could be used.
Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomized, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9(4):373–80. https://doi.org/10.1016/s1474-4422(10)70054-5.
Bhola R, Kinsella E, Giffin N, Lipscombe S, Ahmed F, Weatherall M, et al. Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: evaluation of outcome data for the UK post market pilot program. J Headache Pain. 2015;16:535. https://doi.org/10.1186/s10194-015-0535-3.
Granato A, Fantini J, Monti F, Furlanis G, Musho Ilbeh S, Semenic M, et al. Dramatic placebo effect of high frequency repetitive TMS in treatment of chronic migraine and medication overuse headache. J Clin Neurosci. 2019;60:96–100. https://doi.org/10.1016/j.jocn.2018.09.021.
• Starling AJ, Tepper SJ, Marmura MJ, Shamim EA, Robbins MS, Hindiyeh N, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia. 2018;38(6):1038–48. https://doi.org/10.1177/0333102418762525 Important findings of TMS as a powerful therapy to prevent migraines, not only working as rescue therapy but also in prophylaxis.
•• Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain. 2016;139(7):2002–14. https://doi.org/10.1093/brain/aww118 Significant findings in the mechanisms of action of TMS in mouse models and its effect in cortical spreading depression and trigeminothalamic modulation.
O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018;(4):CD008208. https://doi.org/10.1002/14651858.CD008208.pub5.
Lefaucheur JP, Drouot X, Nguyen JP. Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin. 2001;31(4):247–52.
Transcranial magnetic stimulation. Jean-Pascal Lefaucheur. Chapter 37Clinical Neurophysiology: Basis and Technical Aspects Edited by Kerry H. Levin, Patrick Chauvel. Volume 160.
Chervyakov AV, Bakulin IS, Savitskaya NG, Arkhipov IV, Gavrilov AV, Zakharova MN, et al. Navigated transcranial magnetic stimulation in amyotrophic lateral sclerosis. Muscle Nerve. 2015;51(1):125–31. https://doi.org/10.1002/mus.24345.
•• Benussi A, Di Lorenzo F, Dell’Era V, Cosseddu M, Alberici A, Caratozzolo S, et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89(7):665–72. https://doi.org/10.1212/WNL.0000000000004232 Cutting edge functionality of TMS and how it can be effective in diagnosing different types of dementia by detecting specific underlying pathologic process and associated neurotransmitter deficit.
De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery. 2007;61(2):329–36 discussion 336–7.
Lerner AJ, Wassermann EM, Tamir DI. Seizures from Transcranial magnetic stimulation 2012-2016: results of a survey of active laboratories and clinics. Clin Neurophysiol. 2019. https://doi.org/10.1016/j.clinph.2019.03.016.
Taylor R, Galvez V, Loo C. Transcranial magnetic stimulation (TMS) safety: a practical guide for psychiatrists. Australas Psychiatry. 2018;26(2):189–92. https://doi.org/10.1177/1039856217748249.
Acknowledgments
I thank Rachel Iglesias, Juan J. Del Risco, and Gabriel Sabbagh for their advice and support and for providing graphic illustrations. Dr. Mark Hallett and Dr. Louis De Beaumont for giving me permission to use their figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Iglesias has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of Topical Collection on Neurology of Systemic Diseases
Rights and permissions
About this article
Cite this article
Iglesias, A.H. Transcranial Magnetic Stimulation as Treatment in Multiple Neurologic Conditions. Curr Neurol Neurosci Rep 20, 1 (2020). https://doi.org/10.1007/s11910-020-1021-0
Published:
DOI: https://doi.org/10.1007/s11910-020-1021-0