Abstract
Transcranial magnetic stimulation (TMS) induces phosphenes and disrupts visual perception when applied over the occipital pole. Both the underlying mechanisms and the brain structures involved are still unclear. In the first part of this study we show that the masking effect of TMS differs to masking by light in terms of the psychometric function. Here we investigate the emergence of phosphenes in relation to perimetric measurements. The coil positions were measured with a stereotactic positioning device, and stimulation sites were characterized in four subjects on the basis of individual retinotopic maps measured by with functional magnetic resonance imaging. Phosphene thresholds were found to lie a factor of 0.59 below the stimulation intensities required to induce visual masking. They covered the segments in the visual field where visual suppression occurred with higher stimulation intensity. Both phosphenes and transient scotomas were found in the lower visual field in the quadrant contralateral to the stimulated hemisphere. They could be evoked from a large area over the occipital pole. Phosphene contours and texture remained quite stable with different coil positions over one hemisphere and did not change with the retinotopy of the different visual areas on which the coil was focused. They cannot be related exclusively to a certain functionally defined visual area. It is most likely that both the optic radiation close to its termination in the dorsal parts of V1 and back-projecting fibers from V2 and V3 back to V1 generate phosphenes and scotomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcranial magnetic stimulation (TMS) over the occipital pole is able to induce phosphenes and suppress visual perception. Both effects have been investigated several times, but the relationship between phosphenes and suppressive effects remains unclear. Furthermore, the stimulation site in the brain is still a matter of debate. Phosphenes have been identified in the visual field contralateral to the stimulated hemisphere (Cowey and Walsh 2000; Gothe et al. 2002; Kammer 1999; Kastner et al. 1998; Marg and Rudiak 1994; Meyer et al. 1991; Ray et al. 1998). The same topographic relationship has been found for visual suppression induced by occipital TMS (Amassian et al. 1994; Epstein and Zangaladze 1996; Kamitani and Shimojo 1999; Kammer 1999; Kastner et al. 1998; Potts et al. 1998). Most investigators have suggested V1 as target region for both effects (Amassian et al. 1994; Beckers and Zeki 1995; Corthout et al. 1999; Cowey and Walsh 2000; Kastner et al. 1998; Kosslyn et al. 1999; Meyer et al. 1991; Pascual-Leone and Walsh 2001; Sparing et al. 2002), although arguments have also been made for extrastriate areas (Epstein and Zangaladze 1996; Kastner et al. 1998; Potts et al. 1998) and for the optic radiation (Amassian et al. 1994; Kammer et al. 2001a; Marg and Rudiak 1994) as generator.
In a previous study combining phosphene documentation and perimetric measurement of visual contrast elevation we found a strong correspondence between phosphenes and transient scotomas in the visual field (Kammer 1999). This finding supports the simple view that the target structures in the occipital brain responsible for the generation of phosphenes and scotomas are the same. In the present work we readdress this issue. Since we have developed a stereotactic positioning device that continuously monitors the position of the coil with respect to the position of the head (Kammer et al. 2001b), we are now able to reproduce and maintain a specified coil position for the entirety of a session (Ray et al. 1998). In the first part of this study (Kammer et al. 2004) we characterize the masking effect of occipital TMS in four subjects in detail using sophisticated psychophysical methods. Our results suggest an inhibitory process induced by TMS to be causative for the visual masking effect. The second part of the study focuses on the topography of phosphenes induced over the occipital lobes as a function of coil position in relation to the functional anatomy of the visual system and the relationship between induced phosphenes and visual masking measured by a perimetric approach. To that end, we map the retinotopic areas using functional magnetic resonance imaging (fMRI; Goebel et al. 1998; Sereno et al. 1995). By combining the position data of the coil with retinotopic mapping we are able to analyze the stimulation sites according to the underlying retinotopic organization of the individual cortices. On the basis of this analysis we discuss the putative structures generating phosphenes and visual suppression. Parts of the present study have been published in preliminary form (Kammer et al. 2000).
Materials and methods
Subjects and experimental setup
The four healthy subjects (aged 21–37 years; two men, two women) participating in the study after giving their written informed consent were the same as in the companion contribution (Kammer et al. 2004). The Medtronic Dantec Magpro Stimulator (Skovlunde, Denmark) was used in the biphasic mode (maximal rate 0.33 Hz, restricted by the experiment software). The figure-of-eight coil MC-B70 (outer diameters 96 mm) was fixed on a tripod and the handle was oriented horizontally to the left. The position of the coil was continuously measured by a custom-made positioning system relative to a head-centered coordinate system (see Kammer et al. 2001b). More details on the setup are given in the companion contribution (Kammer et al. 2004).
Phosphene documentation and perimetry
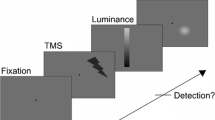
Subjects looked at a fixation point in the middle of the screen at a background intensity of 0.3 cd/m2. They observed the phosphenes induced by a single TMS pulse. For a given site of the coil the subjects themselves released single pulses by pressing a button (maximal rate 0.33 Hz). They drew the contours of the observed phosphene directly on the screen using a mechanical digitizing arm programmed as a drawing device. Traces of the drawing appeared as white lines on the screen. Subjects could freely release stimulation pulses to compare the drawings with the perceived phosphene and if necessary correct the drawing. Finally, the drawing was saved on the computer together with the exact stimulation site of the coil as measured by the positioning system.
Phosphene perception thresholds were measured as described previously (Kammer et al. 2001a). Briefly, 100 magnetic stimuli were delivered at ten different stimulator output intensities in steps of 2%, all randomly intermixed (method of constant stimuli). The subject observed the blank screen with a background intensity of 0.3 cd/m2 and reported the presence or the absence of a phosphene after each stimulus (‘yes/no’). A sigmoidal function was fitted to the measured responses, and the stimulator output intensity of 50% ‘yes’ responses was taken as the phosphene threshold. Perimetric threshold measurements were performed as described in detail in the companion contribution (Kammer et al. 2004). Briefly, detection thresholds for 32 small squares flashed for one frame (for distribution within the visual field see Fig. 1) were determined using a simple up-down staircase. Changes in detection thresholds due to a predetermined TMS stimulus onset asynchrony (SOA) were plotted using a gray scale. Contours of phosphenes were superimposed on the perimetric results (Figs. 1, 2).
Functional imaging of retinotopic maps
Retinotopic maps were delineated using fMRI on a 1.5-T scanner (Siemens Magnetom Vision, Erlangen, Germany). Eccentricity and polar angle were mapped using an expanding checkerboard ring and a rotating checkerboard disk segment, respectively, flickering at 4 Hz (stimulation software courtesy of R. Goebel, Maastricht, The Netherlands). Functional images were sampled in 16 slices oriented parallel to the calcarine fissure (TR=2000 ms, TE=45 ms, flip angle=90°, imaging matrix=64×64, voxel size=3×3×3 mm). Eight cycles with a duration of 9 min were completed for each parameter (eccentricity and polar angle). In addition a high quality T1-weighted anatomical three-dimensional scan was measured (TR=9.7 ms, TE=4 ms, TI=300 ms, flip angle=8°, 192 sagittal slices, matrix 256×256, voxel size=1×1×1 mm). Data were analyzed with BrainVoyager (version 4.6, Brain Innovation, R. Goebel, Maastricht, The Netherlands). After motion correction and temporal high-pass filtering the functional data were carefully coregistered to the anatomical dataset and transformed into three-dimensional space. Eccentricity and polar maps were calculated using cross correlation to a hemodynamically corrected reference function (Goebel et al. 1998). The phase lag indicating eccentricity or polar angle was color coded. A three-dimensional mesh of the cortical surface of each individual subject’s hemisphere was reconstructed based on a segmentation of the white matter in the T1-weighted anatomical scan. This mesh was inflated and functional retinotopic maps were projected onto the mesh. The borders between the retinotopic areas V1, V2, and V3 were delineated manually on the basis of the polar maps (field-sign map; Sereno et al. 1995). The surface meshes were refolded to inspect the individual topography of retinotopic areas in situ (see Fig. 3).
Visualization of magnetic stimulation sites
The coordinates of a set of surface points measured with the positioning system were used to establish a coordinate transformation matrix to the three-dimensional skull and cortex mesh with a surface matching procedure (BrainVoyager). Using this transformation matrix the position and orientation of the TMS coil was visualized on the reconstructed cortical surface of the individual subject (see Figs. 3, 4, 5). Each pin symbol represents the position of the midpoint of the inner coil surface during one threshold measurement (Ray et al. 1998). At this site the maximum electric field is induced by the coil. The direction of the induced current (first upstroke of the biphasic pulse) was coded using an arrow-headed pin symbol.
Results
Phosphenes and perimetry
All four subjects observed phosphenes when they were stimulated. The phosphenes always appeared to be gray or white. They resembled transient clouds or bubbles, usually with a distinct, reproducible contour that could be drawn by the subject. They were found almost exclusively in the lower part of the visual field below the horizontal meridian. With increasing stimulus intensity the impression became stronger and more vivid. It was less vivid or even completely suppressed during measurements of contrast thresholds where subjects concentrated on the detection of visual stimuli but could always be observed immediately on request.
The relationship between phosphenes and elevation in visual contrast thresholds as a function of TMS intensity is shown for subject FS in Fig. 1. With the coil position over the stimulation site used for the extensive contrast threshold measurements reported in the companion contribution (see Figs. 2 and 4 in Kammer et al. 2004) the phosphene threshold was found to be 42.7% of stimulator output intensity. At an intensity of 50% the subject perceived two small phosphenes one in the right visual field just at and below the horizontal meridian with an extension of about 8°×4°, and a second in the left hemifield parafoveally in form of a comma (cross-hatched fields in the upper left panel of Fig. 1). With increasing stimulator output intensity in steps of 10% the phosphenes in both visual fields expanded, merged to a butterflylike area at 70% of output intensity, and finally covered most of the lower visual field at intensities of 90% and 100% (left and middle panels of Fig. 1). They were not restricted to the central ±12° of the visual field as depicted but were documented by the subject using whole screen (width ±24°). A similar enlargement of the phosphene area in the visual field with increasing stimulator output intensities was observed in the three remaining subjects (data not shown).
Phosphenes and scotomas as a factor of TMS intensity. Left, middle columns A series of stimulations in one subject (FS). The appearance of phosphenes within the visual field (±12° of visual angle) with 50% and 60% stimulator output intensity is depicted as cross-hatched areas according to the drawings of the subject. For output intensities of 70–100% the phosphene area is superimposed on the result of a static perimetry with the respective TMS intensity. The mosaic of gray fields indicates the amount of threshold elevation with a TMS pulse at a fixed SOA of 115 ms in comparison to a control measurement in the absence of TMS (transient relative scotoma). Each mosaic area represents the threshold value measured with small light spots. The array of spots is depicted in the lower right panel. In the two outer circles the density of spots is higher in the lower quadrants since threshold elevation was expected mainly in the lower visual field. The amount of threshold elevation is coded from white to black (0–16 dB; right). Lower right panel The scotoma of 100% output intensity is shown together with the phosphene drawn at 50% of output intensity to demonstrate the topographical coincidence. At 70% stimulator output intensity the maximal threshold elevation was 3.2 dB, at 80% it was 7.2 dB, at 90% 9.0 dB, and at 100% 9.7 dB
At stimulator output intensities of 70%–100% a static perimetry was performed. TMS SOA was set to 115 ms, where the maximal suppressive effect was found as described in the companion contribution (Kammer et al. 2004). Changes in contrast threshold due to TMS within ±10° of the visual field are depicted in decibels using a gray scale. With 70% stimulator output intensity the changes were below 3.2 dB and were distributed over the whole visual field (lower left panel of Fig. 1). At higher stimulator intensities (80–100%) a circumscribed transient scotoma was found in the lower right quadrant at 1° and 4° eccentricity (middle panels of Fig. 1) with maximal threshold changes from 7.0 dB (80%) up to 9.7 dB (100%). The extent of this scotoma in the visual field was much smaller than the phosphene observed by the subject at the same intensities (cross-hatched fields). The right panel of Fig. 1 depicts the scotoma obtained at 100% stimulator output intensity overlapped by the phosphene obtained at 50% stimulator output intensity. The phosphene in the right lower quadrant just covers the scotoma.
In the three remaining subjects phosphene thresholds were also below the TMS output intensities, yielding effects in contrast threshold elevation. Table 1 presents phosphene thresholds of all four subjects as measured with the coil placed at the same stimulation site used for the extensive contrast threshold measurements reported in the companion contribution (Kammer et al. 2004). To compare phosphene thresholds with intensities from visual suppression in each subject linear regression was calculated for contrast threshold values over TMS intensities from Fig. 5b in the companion contribution, and intensities twice the individual control levels in the absence of TMS were calculated. In three subjects the ratio of phosphene thresholds over contrast elevation thresholds was 0.55, in the remaining subject 0.70 (mean 0.59±0.07).
Phosphene contours and static perimetry results from the stimulation sites used in the companion contribution are shown in Fig. 2 for all four subjects. The static perimetry was performed using the SOA with the maximal suppressive effect. In three subjects a sector within a lower quadrant of the visual field was that most strongly affected, whereas in subject TK elevations were observed in both lower quadrants. The maximum elevation was observed in the sector where the object was positioned for the contrast measurements in all subjects (see Fig. 2).
Phosphenes and scotomas in the visual field. The extent of the perceived phosphene is shown superimposed on the perimetric measurement for each subject (compare Fig. 1). The stimulation site was the same as that chosen for the detailed threshold measurements reported in the companion contribution (Kammer et al. 2004). TMS intensities and SOAs: ESP, 85%, 95 ms; SB, 70%, 85 ms; FS, 100%, 115 ms; TK, 80%, 105 ms
In subject ESP the phosphene was observed unilaterally in a visual sector covering the region with contrast threshold elevation. In the remaining three subjects bilateral phosphenes occurred, covering the unilateral contrast elevation sector in subject SB and FS and touching the bilateral contrast elevation sectors in subject TK.
Stimulation site and topography of visual cortex
In each subject the retinotopy of visual cortical areas was mapped using fMRI. Panels a and b of Fig.3 present a polar map of subject TK as measured by fMRI on the inflated hemispheres of the individually rendered cortical surface. The borders of the retinotopic areas V1, V2, V3, Vp, and V3a are indicated by lines. These lines are manually drawn following the directional change in retinal polar angle. Figure 3b shows the color code used for the identified retinotopic areas. Panels c–f of Fig. 3 show the functional anatomy of the retinotopic areas as measured by fMRI on the individual refolded cortical surfaces for all four subjects. The dorsal areas V1, V2, V3, and part of V3a at the occipital surface represent the two lower quadrants of the visual field. The ventral areas V1 (green), V2 (blue), and VP (light yellow) represent the two upper quadrants of the visual field. They are located rostrally along the interhemispheric cleft and the bottom part of the occipital pole facing the cerebellum resulting in a larger distance to the occipital pole compared to the dorsal areas. In all subjects the dorsal part of V1 covers the edge formed by the occipital pole and the interhemispheric cleft. The angle between the edge and the vertical plane is highly variable among the four subjects, as is the position of the visual areas with respect to the anatomical structures (Amunts et al. 2000; Brindley et al. 1972; Hasnain et al. 1998). The stimulation sites for the measurements shown in Fig. 2 are indicated by arrows tagging the midpoint of the magnetic coil with the maximum of the electric field induced. These stimulation sites were also used for the detailed threshold measurements reported in the companion contribution (Kammer et al. 2004). They were chosen in these experiments without topographic information from the fMRI image but instead by searching for a strong masking effect in test runs prior to the systematic experiments. In three subjects the chosen stimulation site was almost centered over the interhemispheric cleft, whereas in subject ESP a more lateral position along the edge of the polar pole was used. The maximum of the electric field was directed to V1 in two subjects (ESP, SB) and to V2 in one (FS). In the case of the TK subject the maximum was oriented to a cortex site from which no functional retinotopic response was available. Extrapolation from the retinotopic stripes yielded the maximum over the extrastriate areas V2 and V3.
Individual retinotopic maps and stimulation sites. a, b The polar map of subject TK is shown on the inflated occipital poles of the cortex. a fMRI results are color-coded for the corresponding polar angle in the visual field (insets); lines borders between the different retinotopic areas. b The areas are completely filled with arbitrarily chosen colors to differentiate the areas in the refolded cortical surfaces. V1v, representing the upper quadrants of the visual field, is visible behind V1d in the depth of the interhemispheric cleft. c The individual retinotopic areas are shown for the four subjects using the same color code as in b. Superimposed on the images are yellow pin-arrows indicating magnetic stimulation sites at which perimetric and phosphene measurements were performed (Fig. 2). Long handle of the pin-arrow indicates the axis perpendicular to the midpoint of the coil surface, and along which the maximum of the electric field is induced. Orientation of the pin-arrow indicates the induced current direction of the first upstroke of the biphasic pulse
In two subjects we measured phosphenes and transient scotoma, systematically varying the stimulation site. In Fig. 4 the stimulation sites are projected onto the individual cortical surfaces together with the phosphenes and scotomas measured at the corresponding sites. In subject SB the stimulation sites covered the right occipital pole from the interhemispheric cleft to a lateral position (Euclidean distance between the two outer sites: 46 mm), whereas in subject TK both hemispheres were covered (Euclidean distance between the two outer sites: 71 mm). Phosphenes were always dispersed in one or both lower quadrants of the visual field covering a segment that expands with increasing eccentricity. At the uppermost lateral positions (subject SB, right; subject TK, left) the phosphenes were restricted to the contralateral visual field; with all other position sites bilateral phosphenes were observed by the subjects. Despite different stimulation sites they always remained white or gray and did not change their texture but always resembled clouds or bubbles. The scotomas were always observed in quadrants of the visual field covered by a phosphene. In the case of subject SB the extent of the scotomas was consistently smaller than that of the corresponding phosphenes.
Effect of different magnetic stimulation sites on threshold elevation and phosphene perception. The occipital view on the cortical surface with the retinotopic areas is shown for two subjects as in Fig. 3. a Subject SB. b Subject TK. Magnetic coil positions of several measurements are indicated by yellow pin-arrows. Insets Threshold elevation as measured by perimetry and phosphene perception for the corresponding stimulation sites in horizontal order (compare Fig. 3)
Shifting the coil to lower positions did not change the general appearance of phosphenes. Figure 5 shows the phosphenes found with upper and lower coil positions for subject SB (maximum Euclidean distances: horizontal 29 mm, vertical 40 mm). They were always located in the two lower quadrants of the visual field and never reached the upper visual field. The upper and lower stimulation positions shown were the vertical borders for phosphene generation; when the coil was shifted above the upper or below the lower row it no longer evoked phosphenes. This stimulation at the border in the upper or lower rows elicited smaller phosphenes than stimulation at of central positions. Despite the large variation in coil positions the form of the phosphenes remained constant. The horizontal position of the coil determined whether phosphenes occurred in the left, the right, or both visual fields. Variation in the coil position in the vertical direction resulted in a slight change in polar angle with a rotation towards the horizontal meridian in lower coil positions and a rotation towards the vertical meridian in upper coil positions. No changes in polar angle of the phosphene segments was observed if the coil was shifted to more lateral positions across the borders of the retinotopic areas (data not shown).
Phosphenes observed from various stimulation sites along a vertical axis. The occipital view on the cortical surface with the retinotopic areas is shown for subject SB as in Fig. 3. Magnetic coil positions of several measurements are indicated by the colored pin-arrows. Red pins The phosphene observed in the right visual field; blue pins phosphene observed in the left visual field; yellow pins phosphenes observed in both visual fields. Phosphene contours are shown in a frame (36°×28°) as gray pixel graphs from the subject’s drawing
Coil positions in Figs. 3, 4, 5 were shown in projection to the polar maps of retinotopic mapping only because these maps allow the classification of the different visual areas. Careful analysis of the eccentricity maps did not reveal a systematic relationship between phosphene and scotoma position in the visual field and stimulation site of the coil (data not shown).
Discussion
The main findings of the present study are: (a) Phosphene thresholds are below the stimulation intensities needed for visual suppression. (b) Contours of phosphenes in the visual field cover the segments where visual suppression occurs. The core of the phosphene observed with lower stimulus intensity corresponds to the core of the transient scotoma induced with higher stimulus intensity. (c) Phosphenes and transient scotomas can be elicited from a large area over the occipital pole. Because of the large range of possible coil positions they cannot be related exclusively to a certain functionally defined visual area. They correspond only to the stimulated hemisphere. (d) Phosphene contours and texture remain quite stable with different coil positions over one hemisphere and do not change when the coil is focused on different visual areas.
Visibility of phosphenes
Whereas short trains of magnetic stimuli (two to five) seem to induce the perception of phosphenes in all subjects (Boroojerdi et al. 2002; Ray et al. 1998), the success rate of inducing phosphenes with single pulse TMS is highly variable: different investigators have reported success rates of almost zero (Amassian et al. 1989; Corthout et al. 2000; Kamitani and Shimojo 1999), 13% (Beckers and Hömberg 1991), 27% (Aurora et al. 1998), 58% (Bohotin et al. 2002), 67% (Meyer et al. 1991), 79% (Marg and Rudiak 1994), 80% (Sparing et al. 2002), 82% (Kastner et al. 1998), 89% (Afra et al. 1998), and up to 100% in smaller samples (Cowey and Walsh 2000; Kammer 1999; Kammer and Nusseck 1998; Stewart et al. 2001). In our experience the ability to detect phosphenes must be “learned,” much as with gestalt processes. To confirm that subjects perceive phosphenes induced by TMS the following criteria had to be fulfilled: (a) dependence on the stimulated hemisphere, i.e., perception in the left visual field with stimulation at the right occipital pole and vice versa (Meyer et al. 1991); (b) visibility with eyes both open and closed (Kammer and Beck 2002); (c) dependence on gaze direction (Meyer et al. 1991). About 80% of all our subjects investigated in our laboratory so far reported the perception of phosphenes in the very first test session, while the remaining subjects have required up to four training sessions until phosphenes were reliably detected by the subject, and none failed if they were willing to come again for another session. Once perceived, the subject always saw TMS-induced phosphenes immediately in any subsequent session. This is reminiscent of gestalt processes viewing pictures such as the famous dalmatian dog (Gregory 1970). However, the stability of phosphene thresholds (Kammer and Beck 2002; Kammer et al. 2001a; Stewart et al. 2001) indicates that the induction of phosphenes reflects a basal electrophysiological process. The observation that the impression of phosphenes is reduced in the visual suppression experiments seems to contradict the notion that phosphene induction is reliable. An explanation could be that an attention process at a higher level filters the perception of phosphenes depending on the task. However, it was recently reported that visual mental imagery lowers phosphene thresholds (Sparing et al. 2002). It remains unknown whether the form and extent of phosphenes changes with different attentional states.
Kastner et al. (1998) noted that with higher stimulus strength phosphenes are less visible or disappear completely. Our subjects never reported such an impression. However, we found that with increasing stimulus strength phosphenes covered a larger area in the visual field (see Fig. 1). Since Kastner et al. (1998) used a round coil depolarizing a larger part of the cortex, the observation of phosphene disappearance is suggestive for the following hypothesis: phosphene visibility is based on the detection of phosphene edges. A huge phosphene area might then be more difficult to be detected since attention must be drawn to the periphery of the overall visual field.
Brain structures involved
The coincidence of phosphene contours and transient scotomas in retinotopic coordinates suggests that the same brain structures are involved in the generation of phosphenes and in the suppression of visual perception. Candidate structures for both are (a) the striate cortex V1 (Amassian et al. 1994; Corthout et al. 1999; Cowey and Walsh 2000; Kastner et al. 1998; Meyer et al. 1991; Sparing et al. 2002), (b) extrastriate areas V2/V3 (Epstein et al. 1996; Kastner et al. 1998; Potts et al. 1998), (c) the optic radiation as a subcortical structure (Marg and Rudiak 1994), and (d) corticocortical tracts projecting from V2/V3 back to V1 (Kammer et al. 2001a).
Since the strength of the induced current dissipates with the square of the distance (Epstein et al. 1990; Ilmoniemi et al. 1999), target structures as close as possible to the coil are most likely to be depolarized by a TMS pulse. With the occipital stimulation site these are the dorsal parts of the occipital pole representing the lower parts of the visual field, as confirmed by the fMRI mapping. In consequence the observed phosphenes and scotomas were restricted to the lower visual hemifield. In the case of scotomas a similar restriction has been reported by Kamitani and Shimojo (1999). Scotomas observed by Kastner et al. (1998) also dominated the lower visual field, but in some cases they invaded into the central part of the upper visual field (1°-3° eccentricity). The most likely explanation for this invasion is the larger electric field induced by the round coil the authors used.
Considering the maximum peak of the electric field under the figure-of-eight coil, our data indicate both the striate and extrastriate cortex as generator structure. However, the contours of phosphenes and scotomas in the present study do not strictly follow the retinotopy of the visual areas on which the coil was focused. Therefore it seems unlikely that distinct focal regions of both V1 and extrastriate areas are the generator structures for the effects that we observed. Furthermore, much as with Cowey and Walsh (2000), we failed to observe any qualitative differences between phosphenes from extrastriate target sites (V2/V3) and phosphenes from striate sites. These differences have been reported from direct electrical stimulation of the cortical surface of striate and extrastriate areas (Lee et al. 2000; Penfield and Rasmussen 1950). A simple reason for the lack of qualitative differences might be the size of the cortical area depolarized by a TMS pulse. Depending on the stimulator strength this can reach a diameter of 20–50 mm, thus depolarizing more than one functionally characterized visual area. We further cannot exclude that areas close to the lateral wings of the figure-of-eight coil were stimulated in addition to the area in the center of the coil (Jalinous 1991). A modeling approach might be able to clarify this issue.
From the coil position data, i.e., the orientation of the maximum induced electric field, we cannot infer with certainty the real stimulation site causing phosphenes and visual suppression. If the structures at the occipital pole (cortical layers, axons from corticocortical connections and subcortical axons) differ considerably in their threshold for the tangentially oriented currents induced by TMS, a low-threshold structure might even be depolarized when the coil is not focused on that structure. In general such a low-threshold structure can be formed by a bend in a fiber (Amassian et al. 1992; Maccabee et al. 1993). Both remaining structures, the optic radiation and the back-projection fibers (Felleman and Van Essen 1991; Hupe et al. 1998), fulfill the criteria for a low-threshold structure since both are bent somewhere at the dorsal parts of the occipital pole (Fig. 6).
Diagram of possible target structures for phosphene and scotoma generation (modified from Kammer et al. 2001a). A horizontal section through the right occipital pole is shown (Talairach and Tournoux 1988) Visual areas V1-V3 are color coded as in Fig. 3. The fibers of the optical radiation (lines) terminate in the dorsal (V1d) and ventral part (V1v) of the area striata in the calcarine sulcus. The dorsal portion of the fibers passes V2 in the white matter. Stippled lines Back-projections from V2 and V3 to V1
The lack of a strict dependence of phosphenes and scotomas on the retinotopy of the area under the focus of the coil could be explained by a mixed depolarization of both the tips of the optic radiation projecting from the lateral geniculate nucleus to V1 and the back-projecting fibers from extrastriate areas to V1. Since the dorsal part V1d representing the lower visual quadrants is situated at the occipital surface just beyond the skull (see Fig. 3), the fibers from the optic radiation nearing the skull in the white matter also represent lower visual quadrants. The same holds true for the back-projecting fibers since the parts of V2 and V3 close to the skull exclusively represent the lower visual quadrants. However, a local coincidence of retinotopic representation in both fibers is unlikely since the back-projecting fibers stem from retinotopic areas with receptive fields larger than those of the fibers from the lateral geniculate nucleus. Thus our finding of quite stereotypic segmental forms in both the scotomas and phosphenes could be explained best by both the optic radiation and the back-projecting fibers being depolarized. Since both fiber tracts terminate in V1, this view is consistent with the notion that an intact V1 is indispensable for the perception of phosphenes (Cowey and Walsh 2000; Gothe et al. 2002).
Mechanisms causing phosphenes and transient scotomas
The electrophysiological basis of the TMS effect is still not completely understood. From the motor system we know that excitation of interneurons and, depending on intensity and geometry of the induced electric field, excitation of pyramid cells takes place with TMS (Rothwell 1997). The indirect or direct depolarization of the pyramid cells causes muscle twitches via the corticospinal projections. An inhibitory phase of the cortex follows the excitation (Fuhr et al. 1991; Inghilleri et al. 1993). The inhibition is most likely generated as an active network reaction to the strong coherent excitation. An additional contribution might stem from a direct stimulation of inhibitory interneurons. Although a TMS pulse may additionally hyperpolarize some neuronal structures in dependence of the orientation of the axons within the electric field, until now there is no hint that any kind of TMS-induced inhibition stems from a direct hyperpolarization of neurons. In the visual system we consider phosphenes as the electrophysiological equivalent to the induced muscle twitches caused by TMS of the motor cortex, i.e., based on excitation. In the first part of the study presented in the companion contribution (Kammer et al. 2004) we concluded that visual suppression is based on the inhibitory response to a TMS pulse, concurring with Amassian et al. (1989). This conclusion is in line with the finding that phosphene thresholds lie below a calculated threshold for visual suppression by a factor of 0.59. If one assumes that visual suppression is based on direct hyperpolarization of neurons, one would expect to observe it with stimulation intensities below excitation, the opposite of what we found. Taken together, it is most likely that phosphenes and transient scotoma are generated in the same cortical structure and reflect the excitatory and inhibitory effect of a TMS pulse.
References
Afra J, Mascia A, Gerard P, Maertens de Noordhout A, Schoenen J (1998) Interictal cortical excitability in migraine—a study using transcranial magnetic stimulation of motor and visual cortices. Ann Neurol 44:209–215
Amassian VE, Cracco RQ, Maccabee PJ (1989) Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol 74:401–416
Amassian VE, Eberle L, Maccabee PJ, Cracco RQ (1992) Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol 85:291–301
Amassian VE, Maccabee PJ, Cracco RQ, Cracco JB, Somasundaram M, Rothwell JC, Eberle L, Henry K, Rudell AP (1994) The polarity of the induced electric field influences magnetic coil inhibition of human visual cortex: implications for the site of excitation. Electroencephalogr Clin Neurophysiol 93:21–26
Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K (2000) Brodmann’s areas 17 and 18 brought into stereotaxic space—where and how variable? Neuroimage 11:66–84
Aurora SK, Ahmad BK, Welch KMA, Bhardhwaj P, Ramadan NM (1998) Transcranial magnetic stimulation confirms hyperexcitability of occipital cortex in migraine. Neurology 50:1111–1114
Beckers G, Hömberg V (1991) Impairment of visual perception and visual short term memory scanning by transcranial magnetic stimulation of occipital cortex. Exp Brain Res 87:421–432
Beckers G, Zeki S (1995) The consequences of inactivating areas V1 and V5 on visual motion perception. Brain 118:49–60
Bohotin V, Fumal A, Vandenheede M, Gerard P, Bohotin C, de Noordhout AM, Schoenen J (2002) Effects of repetitive transcranial magnetic stimulation on visual evoked potentials in migraine. Brain 125:912–922
Boroojerdi B, Meister IG, Foltys H, Sparing R, Cohen LG, Töpper R (2002) Visual and motor cortex excitability: a transcranial magnetic stimulation study. Clin Neurophysiol 113:1501–1504
Brindley GS, Donaldson PE, Falconer MA, Rushton DN (1972) The extent of the region of occipital cortex that when stimulated gives phosphenes fixed in the visual field. J Physiol (Lond) 225:57P–58P
Corthout E, Uttl B, Walsh V, Hallett M, Cowey A (1999) Timing of activity in early visual cortex as revealed by transcranial magnetic stimulation. Neuroreport 10:2631–2634
Corthout E, Uttl B, Juan CH, Hallett M, Cowey A (2000) Suppression of vision by transcranial magnetic stimulation: a third mechanism. Neuroreport 11:2345–2349
Cowey A, Walsh V (2000) Magnetically induced phosphenes in sighted, blind and blindsighted observers. Neuroreport 11:3269–3273
Epstein CM, Zangaladze A (1996) Magnetic coil suppression of extrafoveal visual perception using disappearance targets. J Clin Neurophysiol 13:242–246
Epstein CM, Schwartzberg DG, Davey KR, Sudderth DB (1990) Localizing the site of magnetic brain stimulation in humans. Neurology 40:666–670
Epstein CM, Verson R, Zangaladze A (1996) Magnetic coil suppression of visual perception at an extracalcarine site. J Clin Neurophysiol 13:247–252
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex 1:1–47
Fuhr P, Agostino R, Hallett M (1991) Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 81:257–262
Goebel R, Khorramsefat D, Muckli L, Hacker H, Singer W (1998) The constructive nature of vision—direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci 10:1563–1573
Gothe J, Brandt SA, Irlbacher K, Roricht S, Sabel BA, Meyer BU (2002) Changes in visual cortex excitability in blind subjects as demonstrated by transcranial magnetic stimulation. Brain 125:479–490
Gregory RL (1970) The intelligent eye. McGraw-Hill, New York
Hasnain MK, Fox PT, Woldorff MG (1998) Intersubject variability of functional areas in the human visual cortex. Hum Brain Mapp 6:301–315
Hupe JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J (1998) Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature 394:784–787
Ilmoniemi RJ, Ruohonen J, Karhu J (1999) Transcranial magnetic stimulation—a new tool for functional imaging of the brain. Crit Rev Biomed Eng 27:241–284
Inghilleri M, Berardelli A, Cruccu G, Manfredi M (1993) Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol (Lond) 466:521–534
Jalinous R (1991) Technical and practical aspects of magnetic nerve stimulation. J Clin Neurophysiol 8:10–25
Kamitani Y, Shimojo S (1999) Manifestation of scotomas created by transcranial magnetic stimulation of human visual cortex. Nat Neurosci 2:767–771
Kammer T (1999) Phosphenes and transient scotomas induced by magnetic stimulation of the occipital lobe: their topographic relationship. Neuropsychologia 37:191–198
Kammer T, Beck S (2002) Phosphene thresholds evoked by transcranial magnetic stimulation are insensitive to short-lasting variations in ambient light. Exp Brain Res 145:407–410
Kammer T, Nusseck HG (1998) Are recognition deficits following occipital lobe TMS explained by raised detection thresholds? Neuropsychologia 36:1161–1166
Kammer T, Erb M, Beck S, Grodd W (2000) Multimodal mapping of the visual cortex: comparison of functional MRI and stereotactic TMS. Eur J Neurosci 12 [Suppl] 11:192
Kammer T, Beck S, Erb M, Grodd W (2001a) The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin Neurophysiol 112:2015–2021
Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H (2001b) Motor thresholds in humans. A transcranial magnetic stimulation study comparing different pulseforms, current directions and stimulator types. Clin Neurophysiol 112:250–258
Kammer T, Puls K, Strasburger H, Hill NJ, Wichmann FA (2004) TMS in the visual system. I. The psychophysics of visual suppression. Exp Brain Res (http://dx.doi.org/10.1007/s00221-004-1991-1)
Kastner S, Paul I, Ziemann U (1998) Transient visual field defects induced by transcranial magnetic stimulation over the occipital lobe. Exp Brain Res 118:19–26
Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM (1999) The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science 284:167–170
Lee HW, Hong SB, Seo DW, Tae WS, Hong SC (2000) Mapping of functional organization in human visual cortex—electrical cortical stimulation. Neurology 54:849–854
Maccabee PJ, Amassian VE, Eberle LP, Cracco RQ (1993) Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol (Lond) 460:201–219
Marg E, Rudiak D (1994) Phosphenes induced by magnetic stimulation over the occipital brain: description and probable site of stimulation. Optom Vis Sci 71:301–311
Meyer BU, Diehl RR, Steinmetz H, Britton TC, Benecke R (1991) Magnetic stimuli applied over motor cortex and visual cortex: influence of coil position and field polarity on motor responses, phosphenes, and eye movements. Electroencephalogr Clin Neurophysiol Suppl 43:121–134
Pascual-Leone A, Walsh V (2001) Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292:510–512
Penfield W, Rasmussen T (1950) The cerebral cortex of man: a clinical study of localization and function. Macmillan, New York
Potts GF, Gugino LD, Leventon ME, Grimson WEL, Kikinis R, Cote W, Alexander E, Anderson JE, Ettinger GJ, Aglio LS, Shenton ME (1998) Visual hemifield mapping using transcranial magnetic stimulation coregistered with cortical surfaces derived from magnetic resonance images. J Clin Neurophysiol 15:344–350
Ray PG, Meador KJ, Epstein CM, Loring DW, Day LJ (1998) Magnetic stimulation of visual cortex: factors influencing the perception of phosphenes. J Clin Neurophysiol 15:351–357
Rothwell JC (1997) Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods 74:113–122
Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RBH (1995) Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268:889–893
Sparing R, Mottaghy FM, Ganis G, Thompson WL, Töpper R, Kosslyn SM, Pascual-Leone A (2002) Visual cortex excitability increases during visual mental imagery—a TMS study in healthy human subjects. Brain Res 938:92–97
Stewart LM, Walsh V, Rothwell JC (2001) Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia 39:415–419
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. Thieme, Stuttgart
Acknowledgements
We thank Sandra Beck, Hans-Günther Nusseck, and Kuno Kirschfeld, for support and for many fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kammer, T., Puls, K., Erb, M. et al. Transcranial magnetic stimulation in the visual system. II. Characterization of induced phosphenes and scotomas. Exp Brain Res 160, 129–140 (2005). https://doi.org/10.1007/s00221-004-1992-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-1992-0