Abstract
In the last two decades transcranial sonography (TCS) has developed as a valuable, supplementary tool in the diagnosis and differential diagnosis of movement disorders. In this review, we highlight recent evidence supporting TCS as a reliable method in the differential diagnosis of parkinsonism, combining substantia nigra (SN), basal ganglia and ventricular system findings. Moreover, several studies support SN hyperechogenicity as one of most important risk factors for Parkinson’s disease (PD). The advantages of TCS include short investigation time, low cost and lack of radiation. Principal limitations are still the dependency on the bone window and operator experience. New automated algorithms may reduce the role of investigator skill in the assessment and interpretation, increasing TCS diagnostic reliability. Based on the convincing evidence available, the EFNS accredited the method of TCS a level A recommendation for supporting the diagnosis of PD and its differential diagnosis from secondary and atypical parkinsonism. An increasing number of training programmes is extending the use of this technique in clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decade, transcranial sonography (TCS) has become an important tool for supporting a diagnosis of parkinsonism. The association between substantia nigra hyperechogenicity (SN+) and Parkinson’s disease (PD), first described in 1995 [1], has stimulated clinical research studies aiming at evaluating the meaning of SN+ in diseased and in healthy individuals. Recently, further application of TCS to the investigation of other neurological disorders beyond parkinsonism has led to publication of interesting findings in Huntington’s disease and multiple sclerosis.
The present review, however, focuses on the value of TCS in the differential diagnosis of parkinsonism. This is still the most studied and discussed issue in the field, as evidenced by the inclusion of TCS in the European Federation of Neurological Societies (EFNS) guidelines for PD diagnosis [2••]. After a short overview of the methodology, we discuss limitations and advantages of this technique as well as its diagnostic accuracy. Supported by an expanding literature focused on the use of TCS in clinical practice, we further review its role in early PD diagnosis and the presence of SN+ as a risk marker for PD.
Methods and Procedure
Obtaining a clear image resolution has been a challenge since the first use of TCS for the assessment of small deep brain structures [3]. However, improvement in the B-mode technique, which utilises a 1.5- to 3.5-MHz phased array transducer and advances in signal processing have refined the image resolution [4•]. Thus, TCS systems now available can produce an image of higher resolution than those produced by the early TCS systems, even comparable with images obtained by MRI [5].
Ultrasound pulses are reflected at tissue interface and thus display tissue inhomogeneity. The returning signals are processed based on time from emission of the ultrasound wave to its reception at the probe and provide a 2D image of the brain. It needs to be stressed that system-specific image-processing technologies influence distinct measurements such as the assessment of echogenic areas of small brain structures. Normal ranges, especially for echogenic areas (such as the reflected signals of the substantia nigra (SN)), need to be separately validated for each system used. The application of tissue harmonic imaging may improve tissue contrast and can enable an easier delineation of small echogenic structures. However, this technique is more dependent on the acoustic bone window and limited by insonation depths [6]. Smaller structures are generally overrated in size so this method is not used for numerical measurement.
While image brightness and time-gain compensation should be adapted to individuals during the procedure, a penetration depth of 14–16 cm and a dynamic range between 45 and 55 DB are the preset parameters recommended to visualise the brain parenchyma.
The procedure should be performed with the individual laying in supine position. The probe is placed at the preauricular site to use the transtemporal approach. An important precondition for obtaining valid TCS findings is the identification of the optimal bone window for insonation, which needs to be maintained throughout the whole examination. In order to define the optimal window, the midline structures and the contralateral skull bone should be visualised in their whole extension in the imaging sector. Small adjustments of the transducer near the anterior helix of the ear are needed to find the individual position with the best possible visualisation of brain structures. In 10–20 % of individuals a poor bone window has been reported, especially in elderly female subjects and individuals from Asia [5, 7, 8].
Brainstem Structures
The brainstem, basal ganglia, and ventricles are imaged at standardised axial scanning planes, described in details in previous reviews [9] (Fig. 1).
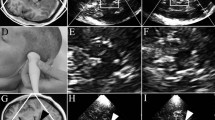
Most important scanning planes and structures in the assessment of movement disorders in a healthy adult. a With the probe placed to the posterior or middle temporal bone, the mesencephalic plane parallel to the orbitomeatal line and the third ventricular plane obtained by tilting the probe 10–20° upwards are visualised. b Axial TCS image of the mesencephalic plane. The butterfly-shaped hypoechogenic brain stem (dotted line) is surrounded by echogenic basal cisterns. Within the brain stem, the slightly echogenic substantia nigra and part of the slightly echogenic round-shaped red nucleus is delineated, which is found closer to the midline. The midline raphe is shown as a continuous echogenic structure (arrow) dividing the brain stem into two parts. c Slightly oblique third ventricular plane: the contralateral skull is well visualised due to its hyperechogenicity. The frontal horns of the lateral ventricles and the hypoechogenic thalami are illustrated by dotted lines. The parallel echogenic borders of the third ventricle can be seen between the thalami. The normoechogenic nucleus lentiformis and the slightly echogenic nucleus caudatus are also depicted (echogenicity of these structures is determined contralateral to the probe). The calcificated pineal gland (white arrow), which is a landmark for orientation, and the calcificated choroid plexus of the lateral ventricle (arrow head) are marked. NC nucleus caudatus, NL nucleus lentiformis, T thalamus
The scanning procedure usually starts in the axial plane parallel to the orbitomeatal line. At this level, the butterfly-shaped midbrain, surrounded by the echogenic basal cisterns (cisterna ambiens, cisterna quadrigemina, cisterna suprasellaris) can be visualised and used as quality criteria for the bone window.
Echogenicity of the ipsilateral SN, red nucleus and median midbrain raphe are assessable in this plane.
The midbrain raphe is usually seen as a highly echogenic line and should be evaluated in the lower midbrain where the red nuclei are also located and generally visible. Raphe echogenicity is classified as reduced when it is interrupted or not visible from either side, although the bone window is sufficient to delineate small structures within the brainstem.
SN echosignal shape, usually patchy or band-like, may vary even in the same individual when the scanning plane is slightly altered because of the arched SN anatomic structure [10•]. As the reflecting echosignal may interfere with echosignals originating from other structures like the basal cisterns, the echogenicity of the SN is assessed only ipsilaterally to the insonation side. Several methods have been applied in order to quantify signal brightness of the SN [11•, 12] and make it less dependent on the investigator’s subjective experience and the quality of bone windows (see section “Novel Technologies and new directions”) [10•, 11•, 13]. However, future validation is necessary before recently developed methods can be recommended for broader clinical application. To date, the best assessment is still the combination of a scoring system based on grading of the echogenicity and manual planimetric measurement [7].
Grading is based on the estimation of the sonographer when comparing SN echogenicity with the surrounding midbrain: in this semiquantitative approach, the SN is classified as either isoechogenic, mildly echogenic or hyperechogenic compared to the hypoechogenic brainstem. Of note, not only brightness but also size of the SN is incorporated in this rating. To better delineate adjacent structures (like the red nucleus), the rating should be performed after freezing of the image and a 2- to 4-fold magnification. This magnification is also necessary for the second approach: exact quantitative measurement of the small structure of the SN. In the frozen, magnified image, the ipsilateral SN is then traced manually and measured (SN planimetry in cm2) (Fig. 2).
TCS findings in healthy individuals and movement disorders. Zoomed images of the brain stem in a a control person and in a b PD patient. a Ipsilateral substantia nigra with normal size and echogenicity lateral of the round-shaped, partly visible red nucleus (dotted lines). b Ipsilateral substantia nigra with larger area of echogenicity and slightly increased brightness. c TCS image of the third ventricular plane in a patient with enlarged ventricular system. Enlarged frontal horn measured contralateral to the insonating probe perpendicularly from the septum pellucidum to the tip of the anterior horn and of the third ventricle measured between the ependymal layers as shown by the arrows. d Contralateral (large arrow) and ipsilateral (narrow arrow) hyperechogenicity of nucleus lentiformis (arrow). T thalamus (here lower part)
SN planimetric measurement has been shown to be accurate and reliable with little inter-rater variability [7]. To date different cut-offs values have been used and proposed to differentiate normal (SN−) and hyperechogenic (SN+) according to different devices and protocols [14–17].
Given the pre and post-processing variability, each laboratory should validate its own cut-off using a standard population of at least 100 parkinsonism-free individuals.
All further measurements then need to be related to this cohort: SN is defined as normal if the area measured is below the 75th percentile, as moderately hyperechogenic if the value is between the 75th and the 90th percentile and as markedly hyperechogenic if the area exceeds the 90th percentile. For the ultrasound system used in initial large studies (Sonoline Elegra, Siemens, Germany) sizes between 0.20 and 0.25 cm2 were classified as moderately enlarged, and 0.25 cm2 represents the limit for a markedly enlarged SN [18]. Recently, in line with these findings, Van de Loo et al. proposed an optimal cut-off of 0.24 cm2 for differentiating normal controls from patients with enlarged SN using the Siemens Antares System (Siemens, Germany) [7].
Basal Ganglia and Ventricular System
The ultrasound plane through the thalami and basal ganglia is visualised by tilting the ultrasound probe 10–20° upwards from the mesencephalic plane.
At this level, even with a suboptimal bone window, the weakly echogenic thalami and the intensive echogenic pineal gland, at the dorsal part of the image, are the most important landmarks. Between the thalami, the third ventricle appears as a highly echogenic double line. Both hyperechogenic lines reflecting the tissue interface of the ventricular border need to be visualised. After freezing and magnifying the image the maximal width of third ventricle should be measured as this is the most reliable measurement using TCS to date [7].
The comma-shaped frontal horn of the contralateral ventricle should be measured perpendicularly to the axial plane, bridging the distance from the septum pellucidum to the lateral ventricles walls.
Normal ranges of ventricle widths have been evaluated and are strongly dependent on age. Thus, in individuals under/over the age of 60, widths of <7/<10 mm (third ventricle) and <17/<20 mm (frontal horn) are regarded as normal [16].
Once the thalami and frontal horns are visualised, the location of the lenticular and caudate nuclei can be inferred from their anatomical position. Usually, these structures cannot be discerned from the surrounding white matter. Each region of circumscribed increased echogenicity should be semi-quantitatively evaluated and considered as possibly abnormal [18]. It should be emphasised, however, that several structures can cause misdiagnosis of basal ganglia hyperechogenicity due to lateral imaging artefacts. Thus, alterations in basal ganglia should be re-evaluated by slightly tilting and twisting the transducer in different positions.
Substantia Nigra Hyperechogenicity and the Diagnosis of Parkinson’s Disease
After the first description of SN hyperechogenicity in Parkinson’s disease by Becker et al., reports from independent groups all over the world (summarised in several reviews on this topic, e.g. [19]) confirmed the relevance of SN+ in PD. The expanding use of the technique required rules for a standardised procedure, leading to guidelines for TCS application in movement disorders [20, 21]. Moreover, based on extensive literature the EFNS awarded a level A recommendation for the method of TCS in support of the diagnosis of PD and its differential diagnosis from secondary and atypical parkinsonism [2••]. This recommendation applies only to adequately trained sonographers as this technique—like all ultrasound techniques–is still operator dependent [2••, 20, 22•]. Several recent studies assessed the applicability of TCS to different populations, underlining its high accuracy and value in PD diagnosis [7, 23]. The widespread use of brain parenchyma sonography in routine diagnostics is, however, still limited due to the frequent belief that TCS findings are highly examiner-dependent and of uncertain diagnostic value. With adequately trained sonographers sufficient diagnostic accuracy for a diagnosis of PD can be achieved as demonstrated in a study by Van de Loo and colleagues, in which four expert neurologists blinded to clinical diagnosis and the ultrasound results of one another assessed the echogenicity of the SN by planimetry and semiquantitative scoring in the same PD patients and controls. The study demonstrated that the inter-observer variability of SN planimetry is low in the hands of experienced investigators, diagnostic accuracy reached a sensitivity of 79 % and a specificity of 81 %) against a clinical standard of truth [7].
SN hyperechogenicity is characteristically seen in 70 to 98 % of Parkinson’s disease (PD) patients [18, 24] and has been reported to be independent of motor severity/disease stages [25]. This signal stability has recently been questioned as newer studies have found an association between the area of SN echogenicity and disease severity [26, 27]. A final decision regarding the stability of the echosignal over time can only be made after the technique is applied in sufficiently powered longitudinal studies. In a first small longitudinal study encompassing 5 years follow-up, no significant change of the quantitatively assessed SN could be found [28]. However, better resolution, larger cohorts and longer follow-up may facilitate the detection of subtle changes.
Atypical Parkinsonism
Diagnosis of atypical parkinsonism is still a challenge, particularly during the early phases, as the typical alterations seen with structural MR imaging techniques are only evident with advanced neurodegeneration and atrophy. Functional imaging, including pre- and postsynaptic imaging of the dopaminergic system and evaluation of brain metabolic profiles (FDG-PET) are more sensitive but not routinely available. In this situation, transcranial sonography can be of value, helping support the differential diagnosis of rarer parkinsonian conditions with results comparable to FDG-PET [29••].
Several studies have shown that SN hyperechogenicity is rarely present in MSA-P and has been seen in less than a third of the patients with PSP [30–33]. Moreover, hyperechogenicity of the lentiform nucleus is frequently present in MSA-P and PSP but is seldom found in idiopathic PD [30, 31, 33]. As PSP progresses, specific changes in signal become evident on TCS. The enlargement of the third ventricle is a classical TCS feature in PSP patients [29••, 30], and a recent study suggests that the overall size of the mesencephalic brainstem, measured planimetrically, may constitute another specific PSP marker [34]. The different subtypes of PSP also differ with regard to the third ventricle’s width, especially in the early course. The SN is more frequently hyperechogenic in the Parkinsonian type of PSP than in the Richardson type [35•].
Only a few studies have evaluated transcranial ultrasound in the rarer corticobasal syndrome [32, 33]. Most CBD patients presented with marked bilateral SN hyperechogenicity, which could be used together with normal third ventricle width to discriminate CBD from PSP [32, 33]. The lentiform nucleus has been described as generally normal in CBD, but larger series with pathological confirmation are needed in order to understand the meaning of TCS findings, given the large pathological heterogeneity underlying CBS [32, 33, 36].
Differential Diagnosis: PD vs Secondary Parkinsonism
Brain imaging is crucial for the differentiation of PD from movement disorders associated with structural changes of the basal ganglia. In this respect, TCS has been demonstrated to be as sensitive as MRI in detecting structural brain alterations associated with the most frequent secondary movement disorders, especially in the early phases [30, 36].
Enlargement of the ventricular system, which can easily be displayed by TCS, associated with normal echogenicity of the SN is highly predictive for a hydrocephalus [37].
In idiopathic basal ganglia calcification (IBGC) symmetric hyperechogenic signals are seen corresponding to the calcifications detected by computer tomography [38, 39].
The association between Doppler or duplex sonography changes (indicating vascular lesions) in conjunction with normal SN echogenicity may also help differentiating vascular parkinsonism from idiopathic PD [40, 41].
In Wilson’s disease, lenticular nucleus hyperechogenicity is present, the size increasing with disease severity [42] and probably reflecting copper accumulation [43]. Moreover, some patients show hyperechogenicity of additional structures including the thalamus, SN [42] and ventral tegmental/periaqueductal grey matter [44].
Differential Diagnosis: PD vs Essential Tremor
SN echogenicity is usually normal in patients with essential tremor [45–48] and can differentiate PD from essential tremor (ET) with a sensitivity of 75–86 % [49] with DAT SPECT as the standard of truth [50]. A subgroup of patients with essential tremor, however, does show SN hyperechogenicity, often in association with non-motor symptoms typical of PD [51, 52•]. Recent studies indicate that this subgroup of ET-PD patients are quite likely to develop PD, whereas the majority of ET patients, who share neither the common non-motor symptoms nor the pathology or imaging findings with PD, stay PD-free during their lifetime [52•].
Differential Diagnosis: PD vs Atypical Parkinsonism
As previously reported, the assessment of the SN alone is not sufficient to distinguish between PD and atypical parkinsonian syndromes. However, even at a time when only minor symptoms are present, a combination of ultrasound markers, i.e. hyperechogenicity of the SN and normal echogenicity of the basal ganglia has a positive predictive value of 0.91 for idiopathic PD, whereas the combination of normal echogenicity of the SN and hyperechogenicity of the lenticular nucleus has a predictive value of at least 0.96 for either MSA-P or PSP [36]. This combination of markers can be supplemented by the results of the measurement of the third ventricle which dilates and the mesencephalic brainstem which atrophies in PSP at later stages.
Findings on the accuracy of early differential diagnosis of PD have been independently replicated by several groups with similar results [22•, 29••, 53•] (Table 1). In patients with dementia with Lewy bodies (DLB), SN hyperechogenicity is similar in prevalence and extension to idiopathic PD [54]], suggesting a common aetiology. However, there seems to be a difference when the extension of the echosignal of both sides is considered. One study assessing SN in DLB and later dementia associated with Parkinson’s disease (PDD) showed that an asymmetry index ≥1.15 of bilateral SN echogenic sizes, estimated by division of larger size by smaller size, was found in 69 % of PDD patients but only 20 % of DLB patients. This finding corresponds with the clinical picture, less asymmetry of motor symptoms being seen in DLB patients. Further studies are needed in order to confirm this finding in other populations.
Why is the SN Hyperechogenic?
Despite the large number of studies assessing TCS and SN hyperechogenicity in different neurological disorders, the cause of SN hyperechogenicity in PD is still only partly understood. Animal models and pathology studies suggested an association between iron content and SN hyperechogenicity [55–57]. This hypothesis is supported by the elevated iron content found postmortem in the SN of patients affected by PD. Iron has been suggested to play an active role in the generation of oxidative stress and in gliosis associated with disease progression [57, 58]. SN hyperechogenicity has been correlated with lower ceruloplasmin and iron levels in PD patients. Interestingly, in asymptomatic at risk subjects for PD low iron blood levels have been detected, supporting the idea that SN+ may be a sign of inherited alterations of iron metabolism. Microglial activation may also be a relevant mechanism for more intense SN echosignals, as it drives disease progression and is associated with iron deposition [28, 59].
The relevance of heavy metal accumulation for SN echogenicity is further underlined by the finding that SN and basal ganglia hyperechogenicity has been reported in several brain disorders with heavy metal accumulation including iron [38, 60] and copper [42, 43, 61].
Interestingly, patients with disorders associated with lower brain iron content, such as restless legs syndrome (RLS) patients, show decreased SN echogenicity compared to controls [62, 63]. The same findings have been demonstrated in other diseases with an alteration of brain iron distribution, such as Friedreich’s Ataxia [64, 65]. Several studies have assessed possible genetic contributions to the increased iron level in PD patients with SN hyperechogenicity. Point variations in the ceruloplasmin gene have been identified in occasional cases [66–70].
However, not all forms of increased tissue iron content lead to an increased reflection of the ultrasound waves. For example, the globus pallidus is generally hypoechogenic although it contains more iron than the SN. Moreover, injection of iron-loaded ferritin into the SN of rats did not lead to hyperechogenicity of this area, whereas free ferric iron did [55]. Therefore, iron bound to specific structures (other than ferritin) seems to be at least in part responsible for the echosignal. Further studies are needed in order to disentangle underlying pathophysiology of SN hyperechogenicity and the role of genetics and environmental factors.
SN Hyperechogenicity in Premotor and Prodromal PD
In cross-sectional studies, SN hyperechogenicity has been found in about 10 % of healthy subjects [55, 56] and the prevalence increases with age [71, 72]. Moreover, this alteration has been demonstrated to be more common in individuals with a positive family history of PD [73, 74]. The prevalence of SN hyperechogenicity in the normal population fits well with the reported 10 % prevalence of incidental Lewy bodies in asymptomatic persons aged over 50 years, which also indicates a predisposition to the clinical manifestation of PD or DLB [75].
Interestingly, the first TCS study described one healthy subject with SN hyperechogenicity who developed PD after 2 years of follow-up. Similar reports followed, suggesting SN+ represents a marker of individual predisposition for the development of PD. Several studies have subsequently addressed this issue. In healthy subjects with SN hyperechogenicity, an up to 60 % reduced presynaptic uptake of [18Fluoro] Dopa PET study was detected [56]. In another study, Behnke et al. demonstrated an abnormal F-dopa uptake and SN relaxation time in healthy subjects with SN+ compared to controls [76]. A recent study demonstrated that motor excitability, assessed by transcranial magnetic stimulation (TMS) is also altered in healthy subject with SN+ compared to controls, similar to PD findings obtained in patients [77]. SN hyperechogenicity in healthy individuals has also been associated with motor slowness, extrapyramidal symptoms [18] and an increased occurrence of non-motor PD symptoms, such as olfactory dysfunction [78] or depression [79, 80]. Moreover, SN+ has been detected to be prevalent in all monogenic forms of PD and, interestingly, in a high percentage of presymptomatic carriers [73, 81, 82].
These findings prompted a prospective 5-year follow- up study of more than 1800 individuals in three centres (Tubingen, Innsbruck and Homburg) in order to evaluate whether SN hyperechogenicity might be associated with an increased risk for PD in a healthy elderly population. Eleven cases of incident PD were diagnosed after 3 years and 21 after 5 years of follow-up [82, 83••]. Eight out of 10 (3 years) and 14 out of 17 (5 years) of these incident PD cases who had a sufficient bone window showed SN hyperechogenicity at baseline. The presence of baseline SN hyperechogenicity, therefore, increased the risk for incident PD by 17.37-fold (95 % CI, 3.71–81.34) and by 20.1-fold (95 % CI, 5.6–98.8) at 3 and 5 years, respectively, in individuals older than 50 years [83••, 84].
In idiopathic REM behavioural disorder (iRBD), the most specific prodromal feature for an alpha-synucleinopathy to date, SN+ can be found in up to half of all cases [85, 86]. In 2010, Iranzo and colleagues demonstrated that the combination of TCS and DAT SPECT can predict the conversion to parkinsonism in iRBD with a sensitivity of 100 % and a specificity of 55 % after 2.5 years [87].
In summary, several findings suggest SN hyperechogenicity to be a vulnerability factor of the nigrostriatal system for neuronal degeneration. The EFNS guidelines gave SN+ an “A” level of evidence for the detection of subjects at risk for PD [2••]. To date, several further longitudinal studies are investigating its value in premotor and prodromal phases of PD alone or in association with other non-motor markers.
Novel Technologies and New Directions
Despite the increasing number of centres assessing TCS in the diagnostic algorithm of parkinsonism, usage of this technique is still limited in clinical practice. The principal limitation of TCS assessment is the quality of the bone window, which limits resolution especially of small deep brain structures. Quality of images in less well-penetrable bone window can be partially improved by slightly lowering the insonation frequency to 1.7–2.0 MHz.
The detection and measurement of the SN is still largely dependent on the investigator’s expertise. In order to minimise this problem, several automatised methods have been evaluated, based on active contour algorithms [13], 3D SN detection based on random forests [11•, 88, 89] or with principal component analyses of artificial neural networks [90].
In a small clinical pilot study, Plate and colleagues, evaluated a 3D ultrasound technique applied by two independent investigators with different levels of experience [11•]. The 3D classification results were superior to classical measurement, independent from investigators’ experience (reaching a sensitivity of 91 % and specificity of 74 %). In another recent study, Skoloudík and colleagues placed a region of interest of 0.50 mm2 (based on histological images) on the SN in DICOM analyses [10•]. An algorithm computed the area for each voxel in greyscale intensity, using a 90th percentile of the controls cohort as normal cut-off value. The discriminative power was similar between manual and automatic measurement, reaching a sensitivity of 87 % and specificity of 92–96 % for the diagnosis of PD.
However, most of these new techniques are applied to off-line images and so are still dependent on the image’s quality assessed during the examination (and consequently on operator’s skills).
An interesting approach may be the combination and fusion of TCS findings with MRI or PET images. This method will probable enable the virtual navigation on TCS-MRI fused imaging, with an on-line tracking of transducer and imaging position. However, such advances will remove the principal advantages of TCS: the low cost and short investigation time.
In addition to its role in differential diagnosis, TCS may provide a useful tool for monitoring deep brain stimulation (DBS) electrodes positioning. In a recent small trial, TCS enabled the evaluation of DBS positioning as well as MRI [91].
The amelioration of spatial resolution of TCS will probably facilitate new measurements in the future, beyond standardised protocols of SN and ventricular system analyses, with potential application to other neurological disorders [92–94].
Conclusions
For almost 20 years, the use of TCS has extended our understanding of movement disorders and it is now considered a reliable tool for supporting early Parkinson’s disease diagnosis [2••].
Despite the advantages of this method, including lack of radiation, short investigation time and low cost, its use is still limited because of its reliance on examiner skill. In this review, we have highlighted recent evidence supporting TCS as a reliable method for the differential diagnosis of parkinsonism when substantia nigra, basal ganglia and ventricular signals are combined.
Moreover, several studies have implicated SN hyperechogenicity as the most important risk factor for PD to date (RR 20 at 5 years). Its utility in premotor and prodromal PD phases is now under investigation in several longitudinal studies. Despite the limits of TCS alone, its combination with other risk factors and prodromal symptoms will probably allow the identification of enriched-cohort of patients at high risk for developing PD and suitable of disease-modifying therapy.
New automatised algorithms may even enhance the diagnostic accuracy performance of TCS [7]. There is a need to standardise protocols and training programmes in order to use this technique correctly in routine practice. In the future, we hope that the dissemination of TCS in neurology will aid clinicians and patients understand parkinsonism and allow an earlier and more accurate diagnosis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K. Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology. 1995;45:182–4.
Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol. 2013;20:16–34. New European guidelines for the diagnosis of Parkinson’s Disease. TCS evaluation has a Level A of evidence in early and differential diagnosis of PD.
Becker G, Berg D. Neuroimaging in basal ganglia disorders: perspectives for transcranial ultrasound. Mov Disord. 2001;16:23–32.
Walter U, Skoloudík D. Transcranial sonography (TCS) of brain parenchyma in movement disorders: quality standards, diagnostic applications and novel technologies. Ultraschall Med. 2014;35:322–31. Extensive review focusing on TCS assessment and novel technologies.
Walter U, Kanowski M, Kaufmann J, Grossmann A, Benecke R, Niehaus L. Contemporary ultrasound systems allow high-resolution transcranial imaging of small echogenic deep intracranial structures similarly as MRI: a phantom study. Neuroimage. 2008;40:551–8.
Puls I, Berg D, Mäurer M, Schliesser M, Hetzel G, Becker G. Transcranial sonography of the brain parenchyma: comparison of B-mode imaging and tissue harmonic imaging. Ultrasound Med Biol. 2000;26:189–94.
Van de Loo S, Walter U, Behnke S, Hagenah J, Lorenz M, Sitzer M, et al. Reproducibility and diagnostic accuracy of substantia nigra sonography for the diagnosis of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:1087–92.
Kern R, Perren F, Kreisel S, Szabo K, Hennerici M, Meairs S. Multiplanar transcranial ultrasound imaging: standards, landmarks and correlation with magnetic resonance imaging. Ultrasound Med Biol. 2005;31:311–5.
Berg D, Becker G. Perspectives of B-mode transcranial ultrasound. Neuroimage [Internet]. 2002;15:463–73.
Skoloudík D, Jelínková M, Blahuta J, Cermák P, Soukup T, Bártová P, et al. Transcranial sonography of the substantia nigra: digital image analysis. AJNR Am J Neuroradiol. 2014;35:2273–8. Interesting application of novel technology using an automatized algorithm in the assessment of SN echogenicity.
Plate A, Ahmadi SA, Pauly O, Klein T, Navab N, Bötzel K. Three-dimensional sonographic examination of the midbrain for computer-aided diagnosis of movement disorders. Ultrasound Med Biol. 2012;38:2041–50. Small trial assessing SN hyperechogenicity with 3-D technology: the diagnostic classification was better compared with classical measurement and independent from investigators’ experience.
Chen L, Hagenah J, Mertins A. Feature analysis for Parkinson’s disease detection based on transcranial sonography image. Med Image Comput Comput Assist Interv. 2012;15:272–9.
Sakalauskas A, Lukoševičius A, Laučkaite K, Jegelevičius D, Rutkauskas S. Automated segmentation of transcranial sonographic images in the diagnostics of Parkinson’s disease. Ultrasonics. 2013;53:111–21.
Berg D, Godau J, Walter U. Transcranial sonography in movement disorders. Lancet Neurol. 2008;7:1044–55.
Hagenah J, König IR, Sperner J, Wessel L, Seidel G, Condefer K, et al. Life-long increase of substantia nigra hyperechogenicity in transcranial sonography. Neuroimage. 2010;51:28–32.
Go CL, Frenzel A, Rosales RL, Lee LV, Benecke R, Dressler D, et al. Assessment of substantia nigra echogenicity in German and Filipino populations using a portable ultrasound system. J Ultrasound Med. 2012;31:191–6.
Kim JY, Kim ST, Jeon SH, Lee WY. Midbrain transcranial sonography in Korean patients with Parkinson’s disease. Mov Disord. 2007;22:1922–6.
Berg D, Siefker C, Ruprecht-Dorfler P, Becker G. Relationship of substantia nigra echogenicity and motor function in elderly subjects. Neurology. 2001;56:13–7.
Berg D. Hyperechogenicity of the substantia nigra: pitfalls in assessment and specificity for Parkinson’s disease. J Neural Transm. 2011;118:453–61.
Berg D, Behnke S, Walter U. Application of transcranial sonography in extrapyramidal disorders: updated recommendations. Ultraschall Med. 2006;27:12–9.
Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Seidel G, et al. Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol. 2007;33:15–25.
Busse K, Heilmann R, Kleinschmidt S, Abu-Mugheisib M, Höppner J, Wunderlich C, et al. Value of combined midbrain sonography, olfactory and motor function assessment in the differential diagnosis of early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:441–7. Large study evaluating the different role of single and combined markers in the early diagnosis of Parkinson’s Disease.
Alonso-Canovas A, Lopez-Sendon JL, Buisan J, de Felipe-Mimbrera A, Guillan M, Garcia-Barragan N, et al. Sonography for diagnosis of Parkinson’s disease—from theory to practice: a study on 300 participants. J Ultrasound Med. 2014;33:2069–74.
Walter U, Wittstock M, Benecke R, Dressler D. Substantia nigra echogenicity is normal in non-extrapyramidal cerebral disorders but increased in Parkinson’s disease. J Neural Transm. 2002;109:191–6.
Spiegel J, Hellwig D, Möllers MO, Behnke S, Jost W, Fassbender K, et al. Transcranial sonography and [123I]FP-CIT SPECT disclose complementary aspects of Parkinson’s disease. Brain. 2006;129:1188–93.
Kolevski G, Petrov I, Petrova V. Transcranial sonography in the evaluation of Parkinson’s disease. J Ultrasound Med. 2007;26:509–12.
Weise D, Lorenz R, Schliesser M, Schirbel A, Reiners K, Classen J. Substantia nigra echogenicity: a structural correlate of functional impairment of the dopaminergic striatal projection in Parkinson’s disease. Mov Disord. 2009;24:1669–75.
Berg D, Merz B, Reiners K, Naumann M, Becker G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disord. 2005;20:383–5.
Hellwig S, Reinhard M, Amtage F, Guschlbauer B, Buchert R, Tüscher O, et al. Transcranial sonography and [18F]fluorodeoxyglucose positron emission tomography for the differential diagnosis of parkinsonism: a head-to-head comparison. Eur J Neurol. 2014;21:860–6. Perspective study evaluating TCS and PET in the differential diagnosis of parkinsonism: the results show that the two assessment have comparable accuracies.
Walter U, Dressler D, Probst T, Wolters A, Abu-Mugheisib M, Wittstock M, et al. Transcranial brain sonography findings in discriminating between parkinsonism and idiopathic Parkinson’s disease. Arch Neurol. 2007;64:1635–40.
Behnke S, Berg D, Naumann M, Becker G. Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J Neurol Neurosurg Psychiatry. 2005;76:423–5.
Sadowski K, Serafin-Król M, Szlachta K, Friedman A. Basal ganglia echogenicity in tauopathies. J Neural Transm. 2014;2003–5. doi:10.1007/s00702-014-1310-3
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D. Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology. 2003;60:74–7.
Sastre-Bataller I, Vázquez JF, Martínez-Torres I, Sahuquillo P, Rubio-Agustí I, Burguera JA, et al. Mesencephalic area measured by transcranial sonography in the differential diagnosis of parkinsonism. Parkinsonism Relat Disord. 2013;19:732–6.
Kostić VS, Mijajlović M, Smajlović D, Lukić MJ, Tomić A, Svetel M. Transcranial brain sonography findings in two main variants of progressive supranuclear palsy. Eur J Neurol. 2013;20:552–7.
Gaenslen A, Unmuth B, Godau J, Liepelt I, Di Santo A, Schweitzer KJ, et al. The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson’s disease: a prospective blinded study. Lancet Neurol. 2008;7:417–24.
Seidel G, Kaps M, Gerriets T, Hutzelmann A. Evaluation of the ventricular system in adults by transcranial duplex sonography. J Neuroimaging. 1995;5:105–8.
Brüggemann N, Wuerfel J, Petersen D, Klein C, Hagenah J, Schneider SA. Idiopathic NBIA—clinical spectrum and transcranial sonography findings. Eur J Neurol. 2011;18:2010–1.
Krogias C, Meves S, Schoellhammer M, Gold R, Andrich J. Sonographic detection of bilateral striopallidodentate calcinosis. J Neurol. 2009;256:266–7.
Tsai CF, Wu RM, Huang YW, Chen LL, Yip PK, Jeng JS. Transcranial color-coded sonography helps differentiation between idiopathic Parkinson’s disease and vascular parkinsonism. J Neurol. 2007;254:501–7.
Venegas-Francke P. Transcranial sonography in the discrimination of Parkinson’s disease versus vascular parkinsonism. Int Rev Neurobiol. 2010;90:147–56.
Walter U, Krolikowski K, Tarnacka B, Benecke R, Czlonkowska A, Dressler D. Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson’s disease. Neurology. 2005;64:1726–32.
Walter U, Skowrońska M, Litwin T, Szpak GM, Jabłonka-Salach K, Skoloudík D, et al. Lenticular nucleus hyperechogenicity in Wilson’s disease reflects local copper, but not iron accumulation. J Neural Transm. 2014;121:1273–9.
Martínez-Fernández R, Caballol N, Gómez-Choco M. MRI and transcranial sonography findings in Wilson’s disease. Mov Disord. 2013;28:740.
Bártová P, Kraft O, Bernátek J, Havel M, Ressner P, Langová K, et al. Transcranial sonography and (123)I-FP-CIT single photon emission computed tomography in movement disorders. Ultrasound Med Biol. 2014;40:2365–71.
Stockner H, Wurster I. Transcranial sonography in movement disorders. Int Rev Neurobiol. Elsevier; 2010.
Budisic M, Trkanjec Z, Bosnjak J, Lovrencic-Huzjan A, Vukovic V, Demarin V. Distinguishing Parkinson’s disease and essential tremor with transcranial sonography. Acta Neurol Scand. 2009;119:17–21.
Okawa M, Miwa H, Kajimoto Y, Hama K, Morita S, Nakanishi I, et al. Transcranial sonography of the substantia nigra in Japanese patients with Parkinson’s disease or atypical parkinsonism: clinical potential and limitations. Intern Med. 2007;46:1527–31.
Vlaar AMM, Bouwmans A, Mess WH, Tromp SC, Weber WEJ. Transcranial duplex in the differential diagnosis of parkinsonian syndromes: a systematic review. J Neurol. 2009;256:530–8.
Doepp F, Plotkin M, Siegel L, Kivi A, Gruber D, Lobsien E, et al. Brain parenchyma sonography and 123I-FP-CIT SPECT in Parkinson’s disease and essential tremor. Mov Disord. 2008;23:405–10.
Stockner H, Sojer M, KS K, Mueller J, Wenning GK, Schmidauer C, et al. Midbrain sonography in patients with essential tremor. Mov Disord. 2007;22:414–7.
Wurster I, Abaza A, Brockmann K, Liepelt-Scarfone I, Berg D. Parkinson’s disease with and without preceding essential tremor-similar phenotypes: a pilot study. J Neurol. 2014;261:884–8. This work suggested that TCS may identify essential tremor patients with SN+ at risk for developing Parkinson’s disease.
Behnke S, Hellwig D, Bürmann J, Runkel A, Farmakis G, Kirsch CM, et al. Evaluation of transcranial sonographic findings and MIBG cardiac scintigraphy in the diagnosis of idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:995–9. Longitudinal study evaluating MIBG and TCS in the early diagnosis of PD: the work showed TCS and MIBG as highly sensitive (79 and 81% respectively), particularly in combination (95%) for the early diagnosis of PD.
Walter U, Dressler D, Wolters A, Wittstock M, Greim B, Benecke R. Sonographic discrimination of dementia with Lewy bodies and Parkinson’s disease with dementia. J Neurol. 2006;253:448–54.
Berg D, Grote C, Rausch WD, Mäurer M, Wesemann W, Riederer P, et al. Iron accumulation in the substantia nigra in rats visualized by ultrasound. Ultrasound Med Biol. 1999;25:901–4.
Berg D, Roggendorf W, Schröder U, Klein R, Tatschner T, Benz P, et al. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol. 2002;59:999–1005.
Zecca L, Berg D, Arzberger T, Ruprecht P, Rausch WD, Musicco M, et al. In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Mov Disord. 2005;20:1278–85.
Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52:515–20.
Berg D, Seppi K, Liepelt I, Schweitzer K, Wollenweber F, Wolf B, et al. Enlarged hyperechogenic substantia nigra is related to motor performance and olfaction in the elderly. Mov Disord. 2010;25:1464–9.
Schneider SA, Hardy J, Bhatia KP. Syndromes of neurodegeneration with brain iron accumulation (NBIA): an update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Mov Disord. 2012;27:42–53.
Svetel M, Mijajlović M, Tomić A, Kresojević N, Pekmezović T, Kostić VS. Transcranial sonography in Wilson’s disease. Parkinsonism Relat Disord. 2012;18:234–8.
Godau J, Manz A, Wevers A-K, Gaenslen A, Berg D. Sonographic substantia nigra hypoechogenicity in polyneuropathy and restless legs syndrome. Mov Disord. 2009;24:133–7.
Kwon D-Y, Seo W-K, Yoon H-K, Park M-H, Koh S-B, Park K-W. Transcranial brain sonography in Parkinson’s disease with restless legs syndrome. Mov Disord. 2010;25:1373–8.
Synofzik M, Godau J, Lindig T, Schöls L, Berg D. Restless legs and substantia nigra hypoechogenicity are common features in Friedreich’s ataxia. Cerebellum. 2011;10:9–13.
Stockner H, Sojer M, Hering S, Nachbauer W, Seppi K, Schmidauer C, et al. Substantia nigra hypoechogenicity in Friedreich’s ataxia. Mov Disord. 2012;27:332–3.
Akbas N, Hochstrasser H, Deplazes J, Tomiuk J, Bauer P, Walter U, et al. Screening for mutations of the HFE gene in Parkinson’s disease patients with hyperechogenicity of the substantia nigra. Neurosci Lett. 2006;407:16–9.
Hochstrasser H, Bauer P, Walter U, Behnke S, Spiegel J, Csoti I, et al. Ceruloplasmin gene variations and substantia nigra hyperechogenicity in Parkinson’s disease. Neurology. 2004;63:1912–7.
Felletschin B, Bauer P, Walter U, Behnke S, Spiegel J, Csoti I, et al. Screening for mutations of the ferritin light and heavy genes in Parkinson’s disease patients with hyperechogenicity of the substantia nigra. Neurosci Lett. 2003;352:53–6.
Berg D, Hochstrasser H, Schweitzer KJ, Riess O. Disturbance of iron metabolism in Parkinson’s disease—ultrasonography as a biomarker. Neurotox Res. 2006;9:1–13.
Deplazes J, Schöbel K, Hochstrasser H, Bauer P, Walter U, Behnke S, et al. Screening for mutations of the IRP2 gene in Parkinson’s disease patients with hyperechogenicity of the substantia nigra. J Neural Transm. 2004;111:515–21.
Behnke S, Double KL, Duma S, Broe GA, Guenther V, Becker G, et al. Substantia nigra echomorphology in the healthy very old: correlation with motor slowing. Neuroimage. 2007;34:1054–9.
Liepelt I, Behnke S, Schweitzer K, Wolf B, Godau J, Wollenweber F, et al. Pre-motor signs of PD are related to SN hyperechogenicity assessed by TCS in an elderly population. Neurobiol Aging. 2011;32:1599–606.
Ruprecht-Dorfler P, Berg D, Tucha O, et al. Echogenicity of the substantia nigra in relatives of patients with sporadic Parkinson’s disease. Neuroimage. 2003;18:416–22.
Schweitzer KJ, Behnke S, Liepelt I, et al. Cross-sectional study discloses a positive family history for Parkinson’s disease and male gender as epidemiological risk factors for substantia nigra hyperechogenicity. J Neural Transm. 2007;114:1167–71.
Forno LS. Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc. 1969;17:557–75.
Behnke S, Schroeder U, Dillmann U, Buchholz HG, Schreckenberger M, Fuss G, et al. Hyperechogenicity of the substantia nigra in healthy controls is related to MRI changes and to neuronal loss as determined by F-Dopa PET. Neuroimage. 2009;47:1237–43.
Todd G, Taylor JL, Baumann D, Butler JE, Duma SR, Hayes M, et al. Substantia nigra echomorphology and motor cortex excitability. Neuroimage. Elsevier Inc.; 2010; 50:1351–6.
Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord. 2007;22:839–42.
Walter U, Hoeppner J, Prudente-Morrissey L, Horowski S, Herpertz SC, Benecke R. Parkinson’s disease-like midbrain sonography abnormalities are frequent in depressive disorders. Brain. 2007;130:1799–807.
Sommer U, Hummel T, Cormann K, Mueller A, Frasnelli J, Kropp J, et al. Detection of presymptomatic Parkinson’s disease: combining smell tests, transcranial sonography, and SPECT. Mov Disord. 2004;19:1196–202.
Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, Gasser T, et al. GBA-associated PD presents with nonmotor characteristics. Neurology. 2011;77:276–80.
Sierra M, Sánchez-Juan P, Martínez-Rodríguez MI, González-Aramburu I, García-Gorostiaga I, Quirce MR, et al. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson’s disease. Neurology. 2013;80:621–6.
Berg D, Behnke S, Seppi K, Godau J, Lerche S, Mahlknecht P, et al. Enlarged hyperechogenic substantia nigra as a risk marker for Parkinson’s disease. Mov Disord. 2013;28:216–9. Prospective longitudinal study with 5-years follow-up on 1271 subjects older than 50 years. During follow-up 21 subjects developed PD and subject with SN+ at baseline had a more than 20.6 times increased risk for developed PD compared with subjects SN−.
Berg D, Seppi K, Behnke S, Liepelt I, Schweitzer K, Stockner H, et al. Enlarged substantia nigra hyperechogenicity and risk for Parkinson’s disease: a 37-month 3-center study of 1847 older persons. Arch Neurol. 2011;68:932–7.
Stockner H, Iranzo A, Seppi K, Serradell M, Gschliesser V, Sojer M, et al. Midbrain hyperechogenicity in idiopathic REM sleep behavior disorder. Mov Disord. 2009;24:1906–9.
Iwanami M, Miyamoto T, Miyamoto M, Hirata K, Takada E. Relevance of substantia nigra hyperechogenicity and reduced odor identification in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11:361–5.
Iranzo A, Lomeña F, Stockner H, Valldeoriola F, Vilaseca I, Salamero M, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2010;9:1070–7.
Pauly O, Ahmadi S-A, Plate A, Boetzel K, Navab N. Detection of substantia nigra echogenicities in 3D transcranial ultrasound for early diagnosis of Parkinson’s disease. Med Image Comput Comput Assist Interv. 2012;15:443–50.
Ahmadi S-A, Baust M, Karamalis A, Plate A, Boetzel K, Klein T, et al. Midbrain segmentation in transcranial 3D ultrasound for Parkinson diagnosis. Med Image Comput Comput Assist Interv. 2011;14:362–9.
Blahuta J, Soukup T, Čermák P. The image recognition of brain-stem ultrasound images with neural network based on PCA. In: Savino M, Andria G, editors. 2011 I.E. International Symposium on Medical Measurements and Applications (MeMeA 2011) Proceedings Bari: IEEE; 2011: 134–142
Walter U, Kirsch M, Wittstock M, Müller J-U, Benecke R, Wolters A. Transcranial sonographic localization of deep brain stimulation electrodes is safe, reliable and predicts clinical outcome. Ultrasound Med Biol. 2011;37:1382–91.
Kiphuth IC, Huttner HB, Struffert T, Schwab S, Köhrmann M. Sonographic monitoring of ventricle enlargement in posthemorrhagic hydrocephalus. Neurology. 2011;76:858–62.
Saft C, Hoffmann R, Strassburger-Krogias K, Lücke T, Meves SH, Ellrichmann G, et al. Echogenicity of basal ganglia structures in different Huntington’s disease phenotypes. J Neural Transm. 2014. doi:10.1007/s00702-014-1335-7.
Walter U, Wagner S, Horowski S, Benecke R, Zettl UK. Transcranial brain sonography findings predict disease progression in multiple sclerosis. Neurology. 2009;73:1010–7.
Bor-Seng-Shu E, Pedroso JL, Felicio AC, Ciampi de Andrade D, Teixeira MJ, Braga-Neto P, et al. Substantia nigra echogenicity and imaging of striatal dopamine transporters in Parkinson’s disease: a cross-sectional study. Parkinsonism Relat Disord. 2014;20:477–81.
Prestel J, Schweitzer KJ, Hofer A, Gasser T, Berg D. Predictive value of transcranial sonography in the diagnosis of Parkinson’s disease. Mov Disord. 2006;21:1763–5.
Izawa MO, Miwa H, Kajimoto Y, Kondo T. Combination of transcranial sonography, olfactory testing, and MIBG myocardial scintigraphy as a diagnostic indicator for Parkinson’s disease. Eur J Neurol. 2012;19:411–6.
Acknowledgments
We would like to thank all participants of the studies assessing SN+ in longitudinal cohorts and all the staff members involved in applying and teaching this method at the moment, especially Ina Posner. We would also like to express our gratitude to Georg Becker who was the first to assess and promote TCS in movement disorders.
Andrea Pilotto and his work was supported by a Research-fellowhip program of DAAD (German Academic Exchange Service)
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Rezzak Yilmaz declares no conflict of interest.
Andrea Pilotto has received an honorarium payment from Research-Fellowship Programme of DAAD (German Academic Exchange Service. Rezzak Yilmaz declares no conflict of interest. Daniela Berg has received consultancy fees from UCB, Novartis, Lundbeck, GSK and TEVA, founding from UCB, Novartis, Lundbeck, GSK and TEVA and grant from Michael J. Fox Foundation, BmBF, dPV (German Parkinson’s Disease Association), Center of Integrative Neurosciences, Internationale Parkinson Fonds, Janssen Pharmaceutica, TEVA Pharma GmbH and UCB Pharma GmbH.
Daniela Berg has received consultancy fees from UCB, Novartis, Lundbeck, GSK and TEVA.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuroimaging
Rights and permissions
About this article
Cite this article
Pilotto, A., Yilmaz, R. & Berg, D. Developments in the Role of Transcranial Sonography for the Differential Diagnosis of Parkinsonism. Curr Neurol Neurosci Rep 15, 43 (2015). https://doi.org/10.1007/s11910-015-0566-9
Published:
DOI: https://doi.org/10.1007/s11910-015-0566-9