Abstract
Purpose of Review
To review the current evidence and modalities for treating pulmonary hypertension (PH) in heart failure with preserved ejection fraction (HFpEF).
Recent Findings
In recent years, several therapies have been developed that improve morbidity in HFpEF, though these studies have not specifically studied patients with PF-HFpEF. Multiple trials of therapies specifically targeting the pulmonary vasculature such as phosphodiesterase (PDE) inhibitors, prostacyclin analogs, endothelin receptor antagonists (ERA), and soluble guanylate cyclase stimulators have also been conducted. However, these therapies demonstrated lack of consistency in improving hemodynamics or functional outcomes in PH-HFpEF.
Summary
There is limited evidence to support the use of pulmonary vasculature-targeting therapies in PH-HFpEF. The mainstay of therapy remains the treatment of the underlying HFpEF condition. There is emerging evidence that newer HF therapies such as sodium-glucose transporter 2 inhibitors and angiotensin-receptor-neprilysin inhibitors are associated with improved hemodynamics and quality of life of patients with PH-HFpEF. There is also a growing realization that more robust phenotyping PH and right ventricular (RV) function may hold promise for therapeutic strategies for patients with PH-HFpEF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is at least as common as heart failure with reduced ejection fraction (HFrEF) and generally carries a similar prognosis with a high burden of morbidity and mortality [1]. The prevalence of HFpEF is only projected to increase [2, 3]. Pulmonary hypertension (PH) is frequently co-morbid with HFpEF. The prevalence of PH in HFpEF may be up to 80% [4], yet its definition and prevalence vary widely between studies [3, 5]. PH-HFpEF is classified under the World Symposium on Pulmonary Hypertension (WSPH) group 2 PH, or PH due to left heart disease. The latter is defined hemodynamically as mean pulmonary artery pressure (mPAP) > 20 mmHg along with pulmonary artery wedge pressure (PAWP) > 15 mmHg [6]. It is further subclassified as isolated post-capillary pulmonary hypertension (IpcPH) when PVR < 2 Woods Unit (WU) or combined pre- and post-capillary pulmonary hypertension (CpcPH) when PVR ≥ 2WU [7]. The recent lowering of both the mPAP as well as PVR cut points was based on large populations studies of normative data [8]. IpcPH is more prevalent than CpcPH, with some studies reporting IpcPH at least twice as prevalent [9, 10]. CpcPH, however, is associated with pulmonary congestion, worse right ventricle (RV) function, more impairment in oxygen delivery with hypoxemia during exertion [9, 11], and ultimately higher risk of mortality [11].
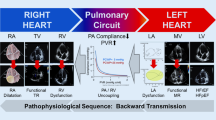
Because PH is both a marker of disease severity in HFpEF and has hemodynamic characteristics shared with pulmonary arterial hypertension (PAH), it naturally follows that targeting the pulmonary vasculature may constitute a therapeutic target in patients with HFpEF. The goal of this review is to provide an update on the management of PH in the context of HFpEF (Fig. 1).
Therapeutic options for Pulmonary Hypertension in Heart Failure with Preserved Ejection Fraction (HFpEF). Created with Biorender.com
Management of PH in HFpEF
The first goal of treating PH in HFpEF is to target the underlying heart failure syndrome and its potential causes. HFpEF is associated with multiple comorbidities such as type 2 diabetes mellitus, systemic hypertension, atrial fibrillation (AF), obstructive sleep apnea (OSA), and obesity. Controlling these comorbidities with dietary modification, weight loss, aerobic exercise, and even drug therapy improves outcomes in HFpEF [3].
Loop Diuretics
In the ACC/AHA heart failure guidelines, loop diuretics have a class I indication to achieve and maintain euvolemia [12]. Normalization of left heart filling pressures will also lead to a reduction in pulmonary pressures. Additionally, reduction in left heart filling pressures results in both an increase in pulmonary artery compliance (calculated as the ratio of stroke volume to pulmonary artery pulse pressure) and a decrease in PVR—the net effect is a reduction of RV afterload [13]. RV function is a critical determinant of mortality in HFpEF [14]. The benefits of loop diuretics on PH were demonstrated in studies that involved pulmonary artery (PA) pressure monitoring devices (cardioMEMS) and tailored diuresis. Titrating loop diuretics based on PA pressure reduced HF hospitalizations [15, 16].
Even though some small studies suggested the benefits of torsemide compared with other loop diuretics on myocardial fibrosis and ventricular remodeling [17, 18], there are no studies to compare between different loop diuretics in the context of PH-HFpEF. The recently published open-label, pragmatic clinical trial TRANSFORM-HF (Torsemide Comparison With Furosemide for Management of Heart Failure) compared torsemide and furosemide in N = 2859 patients with heart failure of which 25% had HFpEF. There was no difference in hospitalization rates at 12 months, regardless of LVEF [18]. The trial did not specifically account for PH.
Therefore, loop diuretics should be used as needed for volume management in patients with PH-HFpEF. No current evidence suggests the superiority of one loop diuretic over the other in this context.
The utility of cardioMEMS to guide diuresis in PH associated with left heart disease was described in a subsequent analysis of the CHAMPION trial (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients). Benza et al. found that patients with PH (17.5% with preserved LVEF) had a significant 36% decrease in HF hospitalizations but a non-significant difference in mortality [19]. This reduction in HF hospitalizations was consistent across subgroups of patients with PVR ≥ 3 WU and PVR < 3 WU. Assmus et al. investigated the effects of cardioMEMS using the MEMS-HF data where 35% of patients with PH had preserved LVEF [20]. The authors observed a significant and comparable reduction in HF hospitalizations in patients with IpcPH (55% reduction) and CpcPH (63% reduction) as well as a meaningful improvement in health-related quality-of-life surveys.
Mineralocorticoid Receptor Antagonists
The use of mineralocorticoid receptor antagonists (MRA) is currently given a class IIB recommendation in the ACC/AHA heart failure guidelines for treating HFpEF [12]. Spironolactone for the treatment of HFpEF was studied in a randomized, double-blind, placebo-controlled trial of N = 3445 patients with HFpEF (TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist). Spironolactone was associated with reduction in HF hospitalizations but did not significantly reduce the incidence of the primary composite outcome of death from cardiovascular causes, aborted cardiac arrest, or HF hospitalization [21]. Notably, a post hoc analysis examined the regional differences in outcomes in participants from the Americas (USA, Canada, Brazil, and Argentina) to participants from Eastern Europe (Russia and the Republic of Georgia) [22]. As compared to placebo, spironolactone reduced primary outcome in the Americas but not in Russia/the Republic of Georgia. This was accompanied with an overall low event rate of primary outcome for both spironolactone and placebo in participants from Russia/the Republic of Georgia 2.5 and 2.3 per 100 patient-years, respectively, compared to 10.4 and 12.6 per 100 patient-years in the Americas, respectively. In addition, an analysis of spironolactone metabolite in the urine revealed the absence of urine metabolite was more common in subjects from Russia and the Republic of Georgia (30% vs 3%) casting doubts over compliance with the trial drug [23].
While MRA may be considered for treatment of HFpEF regardless of the presence of PH [3], there is some evidence to suggest MRA may have direct impacts on the pulmonary vasculature. MRA has been shown to reverse aldosterone inhibiting effect on endothelin-type B in endothelial cells within pulmonary vessels. Endothelin-type B has a major vasodilatory effect on pulmonary artery endothelial cells [24, 25]. An experimental animal study showed that spironolactone and epleronone did not reduce PA pressure or reverse vascular remodeling, yet higher drug levels correlated with lower RV systolic pressures and lower PVR in rats with PAH and RV dysfunction. Notably, there was no significant difference between spironolactone and epleronone [26]. The potential benefit of MRA on PAH was investigated in a retrospective review of four large databases (N = 1229 patients). The authors did not find survival nor clinical benefit with MRA [24]. A large retrospective study by Lahm and colleagues [27] found that MRA use did not improve survival but was rather a marker of disease severity in patients with PH due to left heart disease.
In summary, while spironolactone showed benefits for the treatment of HFpEF, its potential benefit in PH-HFpEF is only speculative based on the reversal of the aldosterone vasoconstricting effect on pulmonary vessels.
Angiotensin Receptor-Neprilysin Inhibitors (ARNi)
The mortality benefit of Angiotensin-converting enzyme inhibitors (ACE-I) and Angiotensin receptor blockers (ARB) are well known in patients with HFrEF [12]. However, their effectiveness in HFpEF did not yield comparable results across various clinical trials, giving ARB a class IIB recommendation in the ACC/AHA guidelines for the treatment of HFpEF, benefiting mostly patients with a LVEF at the lower end of the spectrum [12, 28,29,30]. Yet, the possible benefit of ACE-I or ARB in PH-HFpEF is derived from a large retrospective Veterans Affair study that reported that ACE-I or ARB use in PH, especially group 2 PH, was associated with lower mortality [27].
The role of Angiotensin Receptor-Neprilysin Inhibitor (ARNi) in HFpEF is controversial. It is currently a class IIB recommendation in the ACC/AHA guidelines [12]. The PARAGON-HF trial (Prospective Comparison of ARNI with ARB Global Outcomes in HF with preserved ejection fraction) was a randomized, double-blind, active-comparator trial [31]. After a run-in phase which excluded 925 patients, N = 4822 patients were randomly assigned to ARNi or ARB. ARNi failed to show statistical superiority over ARB with the primary endpoint of total HF hospitalizations and cardiovascular death (P = 0.06). In a post hoc analysis combining the data of PARAGON-HF and PARADIGM-HF (Prospective Comparison of ARNi with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial), the authors identified a group of patients with lower LVEF (women with LVEF < 60% and men with LVEF < 45%) who are more likely to benefit from ARNi [32]. Unfortunately, data on the presence of PH nor RV function was not presented in either analysis.
Over the past couple of years, evaluation of ARNi in the treatment of PH has gained interest given its potential effect on pulmonary vascular vasodilation and remodeling. This effect was suggested in a PH rat model which found that a 6-week course of ARNi reduced pulmonary vascular thickness, RV pressure, and RV hypertrophy and reduced collagen deposition compared to placebo [33]. Moreover, the combination of ARNi and bosentan (an endothelin receptor antagonist) had more improvement in PH and pulmonary vascular remodeling compared to bosentan (or ARNi) alone [34]. In an attempt to delineate the impact of ARNi on PH associated with left heart disease, a meta-analysis by Zhang and colleagues reviewed N = 875 patients with HFrEF in ten retrospective observational studies. The authors observed a reduction in mPAP (weighted mean difference, 2.92 mm Hg; 95% CI, 0.66–5.19 mm Hg; P < 0.05), a reduction in PA systolic pressure (PASP), and an increase in tricuspid annular plane systolic excursion TAPSE after initiation of ARNi. Findings were suggestive of an effect of ARNi on PH and RV not exclusively dependent on improvement in left heart function [35].
Codina and colleagues also sought to examine ARNi in PH associated with HFpEF [36]. In this single-arm, investigator-initiated, interventional study, N = 14 ambulatory patients with CardioMEMS were followed over a total of 18 weeks divided into 3 periods of 6 weeks each, pre-ARNi, ARNi-ON, and ARNi-OFF. Between pre-ARNi vs ARNi-ON, mPAP significantly decreased by 4.99 mmHg [95% CI, −5.55 to −4.43]. When ARNi was stopped (ARNi-OFF), mPAP significantly increased by +2.84 mmHg [95% CI +2.26 to +3.42]. Similarly, ARNi met the secondary endpoints of increasing 6MWD compared to pre-ARNi and ARNi-OFF periods, reducing B-line on ultrasound (no significant worsening with ARNi-OFF), and improving quality of life assessed by KCCQ and EuroQol-visual analogue scales (with significant worsening of KCCQ with ARNi OFF). Notably, loop diuretic management did not differ between periods.
Overall, the role of ARNi in treating HFpEF remains controversial to date but recent observations of their potential benefit on PH-HFpEF warrant further and larger cohort investigations. Given their impact on systemic blood pressure, care should be taken to avoid systemic hypotension, particularly in the setting of PH and RV dysfunction.
Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2i)
SGLT2i are now considered a mainstay therapy for HFpEF [37]. Two large randomized controlled trials, EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) and DELIVER trial (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) showed that SGLT2i reduced cardiovascular death and heart failure-related hospitalizations, regardless of the presence of diabetes mellitus [38,39,40,41]. The mechanisms behind the therapeutic benefit of SGLT2i are not well understood. Although there is clearly a component of natriuresis, SGLT2i may reduce myocardial fibrosis and result in myocardial remodeling. In patients with diabetes, SGLT2i reduce diastolic filling pressure and have a favorable effect on vascular stiffness [42]. In addition, the use of SGLT2i in patients with diabetes had a favorable effect on kidney function, weight loss, and hypertension control which all have beneficial effects on the course of HFpEF.
Some experimental observations also suggest SGLT2i could have a favorable effect on the pulmonary vasculature. Uthman and colleagues found that empagliflozin and dapagliflozin reduced reactive oxygen species (ROS) and restored nitric oxide (NO) availability in endothelial cells in a study on human coronary arteries [43]. In an experimental rat model, Dai and colleagues found that dapagliflozin reduced the pathological process of pulmonary vascular remodeling by inhibiting inflammasome pathway mainly toll-like receptor 4/nuclear transcription factor-κB/NACHT, LRR, and PYD domain-containing protein 3 (TLR4/NF-κB/NLRP3) [44].
A recent randomized controlled trial by Nassif and colleagues examined the impact of empagliflozin compared to placebo on PA pressures by studying patients with cardioMEMS devices [45]. The study included 65 patients of which 50% had HFpEF. Compared to placebo, empagliflozin 10 mg daily reduced PA diastolic, systolic, and mPAP as early as the first week. The improvement was sustained through pre-specified follow-up intervals (weeks 8 and 12). Even though subgroup analysis was limited by a small sample size, the decrease in PA pressures was comparable in HFrEF and HFpEF. Notably, loop diuretic use was also comparable between empagliflozin and placebo groups implying that PA pressure lowering happened independently of SGLT2i “diuretic” effect. The CAMEO-DAPA trial (Evaluation of the Cardiac and Metabolic Effects of Dapagliflozin in Heart Failure With Preserved Ejection Fraction) was a phase II, prospective, double-blind study that aims to compare dapagliflozin 10 mg daily to placebo in patients with HFpEF and elevated PAWP during exercise [46••]. Borlaug and colleagues found that treatment with dapagliflozin led to reductions in both resting PAWP (−3.5 mm Hg [95% CI, −6.6 to −0.4]; P = 0.029) and exercise PAWP (−5.7 mm Hg [95% CI, −10.8 to −0.7]; P = 0.027). This reduction in pressure was accompanied by beneficial effects on plasma volume and body weight.
In summary, just like for the treatment of HFpEF, SGLT2i seem to have reproducible benefits on PH-HFpEF and hold promise in treating PH-HFpEF with larger studies needed.
Atrial Fibrillation
Left atrial (LA) myopathy and AF may arise from LA enlargement, a consequence of elevated filling pressures in HFrEF. LA myopathy/AF in HFpEF may also be related to systemic or local inflammatory processes such as obesity and epicardial fat [47]. In the early stages of HFpEF, there is a decrease in LA compliance and reservoir function followed by a decrease in LA contractility with ensuing LA enlargement. With AF, LA pressure further increases, both increasing pulmonary artery pressure [48].
In experimental studies, SGLT2i led to reduction in atrial fibrosis and cardiac electrical remodeling [44]. A more recent retrospective study also observed that SGLT2i use in patients with type 2 diabetes mellitus reduced the recurrence of AF after AF catheter ablation [49].
While observations derived from larger trials suggest a trend favoring rhythm control for AF in the setting of HF, this benefit is not consistent across all studies [47, 50]. Considering timing of therapy since AF onset, one study showed that adopting an early rhythm control strategy (< 1 year since AF onset) over less strict rhythm control reduced the composite outcome of cardiovascular death, stroke, hospitalization for acute coronary syndrome or worsening HF (5.7 per 100 patient-years vs 7.9 per 100 patient-years, P = 0.03) [45]; whereas, another study demonstrated no difference between pharmacologic rhythm or rate control in terms of survival or cardiovascular hospitalization in patients with AF onset within 6 months of enrollment [51]. Notably, when considering a rate control strategy for AF in patients with HFpEF, a more lenient approach may be favored over a strict rate control given possible harm that translates into reduction in functional capacity with no mortality benefit with the more strict approach [52]. When it comes to rhythm control strategies for AF in HFpEF, data derived from larger trials show some trend favoring catheter ablation over pharmacologic rhythm control [53, 54].
In a more recent randomized, prospective, single-blinded, controlled trial dedicated to AF in the setting of HFpEF, Chieng et al. compared catheter ablation versus medical therapy for management of AF in N = 31 patients with HFpEF [55]. After a 4-week run-in period where all participants underwent antiarrhythmic therapy to achieve an AF ventricular rate of less than 100 beats per minute, participants were randomized 1:1. With the caveat of the relatively small sample size of the study, catheter ablation led to reduction in primary endpoint which was peak exercise PAWP at 6 months. While right atrial (RA) pressure was also reduced in the catheter ablation group, peak PA pressure was unchanged from baseline. Additionally, there was an improvement in peak O2 consumption and MLHF (Minnesota Living with Heart Failure) and a decrease in N-terminal pro–B-type natriuretic (NT pro-BNP) peptide levels. Very interestingly, following catheter ablation, 50% of the patients no longer met the exercise PAWP criteria for HFpEF, suggesting the potential benefits of ablation in patients with AF and HFpEF.
In summary, although robust data for management of AF in the setting of PH-HFpEF is lacking, a rhythm control strategy, particularly catheter ablation, may be appropriate in some cases. The decision should be individualized and follow the ACC/AHA guidelines in addressing AF with HF [12] until more data emerge.
Obesity
The use of drug therapy in addressing obesity in HFpEF was cemented in the recently published randomized, double-blind, placebo-controlled STEP-HFpEF [56]. Patients (N = 529) with body mass index ≥ 30 kg/m2 were randomized to semaglutide 2.4 mg once weekly vs usual care. Semaglutide is a glucagon-like peptide 1 receptor agonist approved for weight loss [57]. At 52 weeks, patients who received semaglutide met the dual primary endpoint of improved functional status assessed by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS; 16.6 vs 8.7 in placebo) and body weight loss (−13.3% vs −2.6% in placebo). Similarly, the semaglutide cohort met secondary endpoints by improving 6-min walking distance (6MWD) and hierarchical composite outcome of death, heart failure events, differences in the change in the KCCQ-CSS and 6MWD, and the change in the C-reactive protein level with a win ratio of 1.72 (95% confidence interval (CI), 1.37 to 2.15; P < 0.001). Serious adverse events were more common in the placebo group (26.7% vs 13.3%). The most common serious adverse event in the semaglutide group was gastrointestinal events which was the main reason for discontinuation of semaglutide. Whether the benefits observed in STEP-HFpEF were all secondary to weight loss versus other mechanisms remain unknown. Significant weight loss can be realized through bariatric surgery. Recent observations suggest that bariatric surgery in patients with obesity and PH led to reduction in pulmonary pressures and improved RV function by echocardiographic assessment [58, 59]. While bariatric surgery led to fewer cardiac ischemic events and in-hospital mortality in patients with PH, bariatric surgery was associated with higher odds of atrial fibrillation and acute pulmonary embolism [60].
Obstructive Sleep Apnea
OSA remains prevalent and underdiagnosed in patients with HF [61]. It is associated with increased mortality, HF readmissions, and healthcare costs in patients with HFpEF [62]. HFpEF and OSA share mutual risk factors and comorbidities such as obesity and hypertension. Multiple mechanisms like increased arterial stiffness and impaired diastolic function could explain the relationship between OSA and HFpEF. Most of all, hypoxemia associated with OSA not only triggers systemic inflammation but also promotes pulmonary vasculature constriction and remodeling, both of which constitute a link between PH and HFpEF. In addition, sleep-related hypoxia (oxygen saturation < 90%) was shown to be associated with RV dysfunction in patients with PAH [63].
When it comes to addressing OSA in patients with PH-HFpEF, positive airway pressure (PAP) was shown to improve diastolic function markers such as left atrial volume index, early mitral inflow velocity/mitral annular early diastolic velocity (E/E’) in patients with HFpEF [64]. Moreover, PAP was shown to reduce pulmonary systolic pressures in patients with PH [65]. In a recent retrospective propensity-matched study of N = 4327 patients with HFpEF and OSA (25.6% with PH), Cistulli et al. report that those who were adherent to PAP devices had a 26% decrease in emergency department visits and a 57% decrease in hospitalizations compared to the year before PAP initiation [66]. Of note, PAP adherence was defined as ≥ 4 h/night for > 70% of nights over a consecutive period of 30 days, in the first 90 days of therapy. Similarly, PAP-adherent patients had fewer health care resource use compared to nonadherent patients.
Pulmonary Vasculature Targets
With the increase in PVR in the context of CpcPH, therapies targeting pulmonary vasculature, like in PAH, have been of keen interest. Pathways that are known to lead to pulmonary vasculature remodeling include increased endothelin-1 (ET-1) production, reduced prostacyclin, and NO production as well as reduced soluble guanylate cyclase (sGC) activity in pulmonary vascular endothelium [67]. Targeting these pathways could have hypothetical benefits on the pre-capillary component of CpcPH in patients with HFpEF.
Prostacyclin Analogs
Prostacyclin analogs can cause pulmonary vascular vasodilation with clear benefits in patients with pulmonary arterial hypertension [68]. While these drugs showed benefits in PAH, the FIRST (Flolan International Randomized Survival Trial) trial found that epoprostenol (Flolan) infusion in patients with HF with LVEF < 25% was associated with worse mortality, prompting early termination [69]. Unfortunately, 20 years later, the SOUTHPAW (Study to Evaluate the Safety and Efficacy of Oral Treprostinil in Subjects With Pulmonary Hypertension and Heart Failure With Preserved Ejection Fraction—ClinicalTrials.gov Identifier–NCT03037580) study, which aimed to evaluate the effect of oral treprostinil on change in 6MWD in patients with CpcPH, was terminated prematurely due to slow enrollment.
Endothelin Receptor Antagonists
Endothelin receptor antagonists (ERA) are approved for the treatment of PAH. ET-1—which leads to pulmonary vasoconstriction—is elevated in HF and higher levels are associated with worse outcomes including mortality [70]. The role of ERA in HFpEF has been investigated in several randomized trials (Table 1). In a randomized trial, sitaxsentan met its primary endpoint of improving exercise capacity in patients with HFpEF by increasing treadmill time (90 s vs 37 s, P = 0.03). Sitaxsentan was similar to placebo in terms of secondary endpoints including change in NYHA (New York Heart Association) functional class, death, or HF hospital stay as well as adverse reactions. Notably, the study did not account for the presence of PH [71]. A subsequent randomized, placebo-controlled trial studied bosentan in patients with HFpEF and PH diagnosed by right heart catheterization (RHC) with mPAP > 25 mmHg, PAWP > 15 mmHg at rest [72]. Compared to placebo, bosentan treatment did not improve 6MWD over 12 weeks. Additionally, bosentan did not improve NT pro-BNP, echocardiographic, hemodynamic parameters, nor quality of life.
The MELODY-1 trial (Macitentan in subjects with combined prE- and post-capiLlary pulmOnary hypertension due to left ventricular DYsfunction) failed to show the benefit of macitentan over placebo on the composite outcome of fluid retention or worsening NYHA class in patients with CpcPH, of which 76% had HFpEF [73]. Macitentan and placebo had comparable reduction in PVR. MELODY-1 was one of the first trials to enroll a patient population with clear evidence of a pre-capillary PH component [74]. Importantly, macitentan led to numerically more serious adverse events related to edema and volume overload. A larger follow-up study was terminated early (SERENADE; ClinicalTrials.gov–Identifier NCT03153111). In the existing SERENADE data, there was no difference in primary or secondary endpoints between macitentan and placebo, but as seen in MELODY-1, numerically more serious adverse events in macitentan compared to placebo (40.9% vs 32.4%), mainly fluid retention and electrolytes disturbances. With this higher rate of adverse events and lack of benefit on NT-proBNP or functional status, the subsequent SERENADE open label aiming to further assess long-term safety and efficacy was closed (Clinicaltrials.gov Identifier–NCT03714815).
In summary, ERA should not be used in the treatment of PH-HFpEF and may be associated with harm.
Nitric Oxide Pathway
The proinflammatory conditions associated with HFpEF—like obesity and insulin resistance—lead to microvascular inflammation involving endothelial cells. The increase in oxidative stress leads to reduction in NO which in turn leads to reduction in intracellular cyclic guanosine 3′,5′-monophosphate (cGMP) production by soluble guanylate cyclase (sGC). The cGMP is broken down by phosphodiesterase (PDE) mainly PDE type 5 (PDE-5). The reduction in cGMP leads to reduction in vascular compliance and increased stiffness culminating in increased PVR. Multiple trials have examined PDE-5 inhibitors and sGC stimulators in HFpEF, but fewer in PH-HFpEF.
Phosphodiesterase Inhibitors
The most studied PDE inhibitor subclass is PDE-5 inhibitors, mainly sildenafil. PDE-3 inhibitors such as milrinone have recently become of interest as well, as these drugs have vasodilatory properties by increasing cyclic adenosine monophosphate levels (cAMP).
PDE-5 Inhibitors
Given both success in PAH and animal studies, significant excitement centered around the use of PDE-5 inhibitors in PH-HFpEF (Table 1). In a single-center, double-blind, placebo-controlled trial, patients with PH-HFpEF (EF > 50% and PH diagnosed by echocardiography) were randomized to sildenafil 50 mg three times a day vs placebo [75]. Patients who received sildenafil had a significant decrease in systemic blood pressure as well as a decrease in mPAP and PVR at 6 and 12 months. In addition, sildenafil substantially increased cardiac index and reduced RA and RV end-diastolic pressure from baseline and compared to placebo. These positive findings were unfortunately not replicated in a subsequent double-blind, placebo-controlled trial where sildenafil treatment over 12 weeks did not meet its primary endpoint of reducing mPAP in patients with PH-HFpEF with NYHA class II to IV [76]. The lack of benefit was observed in patients with higher PVR (> 240 dynes/s/cm−5 reflective of CpcPH) as well as in patients with lower PVR (≤ 240 dynes/s/cm−5 reflective of IpcPH), the latter of which were the majority of patients. Sildenafil did not reduce PAWP, RA, or RV pressure and did not improve cardiac output. Compared to placebo, sildenafil also did not improve peak oxygen consumption.
A larger study of patients with HFpEF also failed to show improvements in peak oxygen consumption [77]. The study did not specifically account for the presence of PH, yet baseline echocardiographic assessment showed that median PASP was 41 mmHg with interquartile range [33–51]. Furthermore, sildenafil did not improve 6MWD nor quality of life.
The impact of sildenafil on 6MWD as a primary endpoint was further delineated in a single-center, randomized, placebo-controlled trial [78]. Patients with HFpEF and CpcPH were randomized in a 3:2 ratio to receive either sildenafil or placebo for 6 months. CpcPH was derived using an echocardiogram as PASP > 40 mmHg, PVR > 3 WU, and/or transpulmonary gradient (TPG) > 15 mmHg. Contrary to the findings of the RELAX trial, Belyavskiy and colleagues found that 6MWD increased by +50 m (95% CI, 36 to 64 m) in the sildenafil group at 6 months, whereas no significant improvement was seen in the placebo group +18 m (95% Cl −6 to +41 m). In addition, sildenafil improved NYHA functional class (more patients went from NYHA II and III to NYHA I) and increased exercise duration at 6 months compared to baseline +75 s (95% Cl +23 to +130 s). Moreover, sildenafil reduced PVR and PASP. Kramer and colleagues investigated both sildenafil and tadalafil in a retrospective analysis of N = 40 patients with HFpEF and CpcPH who received either drug for 12 months; their findings supported that PDE-5 inhibitors had positive effects on 6MWD, World Health Organization functional class, and resulted in fewer HF hospitalizations [79].
In most trials where safety was assessed, sildenafil and placebo had similar rates of total adverse events [76, 77]. Sildenafil had more common vascular adverse events including hypotension, headache, flushing, and dizziness. For instance, in the RELAX trial, vascular events were 20% in sildenafil vs 8% placebo (P = 0.011) even though the change in systemic mean arterial pressure was comparable in both groups. Serious adverse events tended to occur more commonly in sildenafil groups without reaching statistical significance [76, 77]. The notable exception to this is the SIOVAC (Sildenafil for Improving Outcomes After Valvular Correction) study which enrolled patients with persistent PH 1 year after valvular surgery (91% mitral interventions). In this study, patients with mPAP ≥ 30 mmHg were randomized to receive sildenafil vs placebo. Compared to placebo, the sildenafil cohort had a worse composite clinical score (any cause of death, HF hospitalization, WHO functional class, or changes in global self-assessment) [80]. The presence of elevated PVR did not modify the findings.
The ESC/ERS PH guidelines currently give no recommendations regarding the use of PDE-5 inhibitors for significant CpcPH. However, use in IpcPH is a class III recommendation (should not be used) [81].
PDE-3 Inhibitor
Milrinone, a PDE-3 inhibitor, increases cAMP therefore increasing myocardial inotropy and reducing systemic vascular resistance. In a relatively small trial, Nanayakkara and colleagues examined the safety and benefit of extended-release oral milrinone in N = 23 patients with HFpEF and NYHA III symptoms without specifically accounting for the presence of PH [82]. Milrinone was overall safe with no significant decrease in systemic blood pressure or increase in HR. Compared to placebo, milrinone had a significantly greater improvement in KCCQ score (+10 ± 13 vs −3 ± 15; P = 0.04) including the quality of life subdomain. Similarly, milrinone improved 6MWD (10 ± 62 m) compared to placebo (−42 ± 77 m) without reaching statistical significance (P = 0.092). There was no significant decrease in NT-proBNP and no improvement in filling pressure on the echocardiogram. A randomized, crossover trial assessing cilostazol on functional status and NT-proBNP in patients with HFpEF and NYHA II-IV is currently ongoing (ClinicalTrials.gov Identifier–NCT05126836).
Soluble Guanylate Cyclase Stimulators
In a double-blind, randomized, placebo-controlled, parallel-group phase 2a study, three doses of riociguat 0.5 mg, 1 mg, and 2 mg were compared to placebo in patients with HFpEF and PH defined by RHC, mPAP ≥ 25 mmHg, PAWP > 15 mmHg at rest [83]. Elevated PVR was not an inclusion criterion. The aim of the study was to explore the acute hemodynamics effects of riociguat. At 6 h, there was no difference between 2 mg riociguat and placebo in the primary endpoint of decreasing peak mPAP. While riociguat 2 mg did not impact PAWP, PVR, or TPG, riociguat led to a significant increase in stroke volume and cardiac index (+0.4 L/min/m2; 95% CI 0.2 to 0.7; P = 0.001) without affecting heart rate. In addition, riociguat 2 mg was associated with decreased systemic vascular resistance, systolic (−11.7 mmHg; 95% CI −22.4 to −0.9) and diastolic blood pressure (−6.3 mmHg; 95%CI −11.8 to −0.7), and RV end-diastolic area. Adverse events of riociguat were consistent with a drop in mean arterial pressure below 60 mmHg, yet, patients did not present with symptoms of hypotension. More recently, the DYNAMIC trial was conducted, though was terminated prematurely due to enrollment issues. In this trial, riociguat (up to 1.5 mg three times daily) resulted in an increase of cardiac output (by 0.37 ± 1.26 L/min) at 26 weeks compared with placebo (−0.11 ± 0.92 L/min)—(least-squares mean difference, 0.54 L/min; P = 0.014) [84]. Riociguat was also associated with mild reductions in TPG and PVR. However, there was no difference in 6MWD, QOL measures, NT-proBNP, or WHO functional class. Drug-related adverse events, including hypotension, were more common in the riociguat arm as was the dropout rate. Thus, it remains unclear if the modest hemodynamic benefits of riociguat are clinically relevant.
Two randomized, placebo-controlled trials examined the impact of vericiguat on functional capacity in HFpEF using patient-reported outcomes, without accounting for the presence of PH. In the SOCRATES-PRESERVED trial (vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF), vericiguat failed to meet the two primary endpoints of improving NT-proBNP or decreasing LA volume, yet higher doses of vericiguat [2.5–5 mg] and [5–10 mg] led to meaningful increase in KCCQ [85]. However, in the larger VITALITY-HFpEF trial (Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction) involving N = 789 patients, two different doses of vericiguat (10 mg and 15 mg) failed to improve KCCQ and 6MWD at 24 weeks compared to placebo [86].
Although most large studies have only enrolled HFpEF patients, there is no compelling evidence (Table 1) to suggest the benefits of sGC stimulators in PH-HFpEF.
Sotatercept
Sotatercept is a fusion protein that inhibits certain members of the transforming growth factor β family; this action is thought to promote apoptosis and reduce inflammation within the pulmonary vasculature. Sotatercept recently showed dramatic benefits in PAH patients on dual PAH therapy in the STELLAR study [87••]. Compared to placebo, sotatercept resulted in an increase in 6MWD (40.8 m) compared to placebo. Eight of the nine secondary endpoints also favored sotatercept. Nonserious bleed was the most common adverse event, occurring in 21.5% of the sotatercept group and in 12.5% in placebo. Sotatercept is currently being investigated for CpcPH due to HFpEF in the CADENCE study (ClinicalTrials.gov Identifier–NCT04945460).
Pulmonary Artery Denervation
Pulmonary artery denervation (PADN), a catheter-based technique, aims to abolish baroreceptor reflex in PA and its bifurcations. Zhang and colleagues compared PADN to sildenafil plus sham PADN in N = 98 patients with CpcPH in HF of which 39% had HFpEF [88]. Compared to sildenafil plus sham, PADN reached its primary endpoint of increasing 6MWD (83 m vs 15 m; P < 0.001) at 6 months. PADN also improved mPAP and lowered PVR and PAWP at 6 months. PADN had less clinical worsening (16.7% vs 40%; P = 0.014) and less worsening of HF (14.6% vs 36%; P = 0.016). The PADN procedure was mostly safe. The recent 3-year follow data was also promising [89]. These aspects are currently being investigated in the ongoing TReatment of Pulmonary Hypertension Group II (TROPHY-II) Study (ClinicalTrials.gov Identifier–NCT03611270). More data is certainly needed to determine the safety and efficacy of this therapy in PH-HFpEF.
Is Targeting the Pulmonary Vasculature the Right Approach?
Despite enthusiasm to specifically target the pulmonary vasculature, it remains possible that this approach has limited efficacy. For example, higher ET-1 levels in volunteers free of cardiovascular disease appear protective of future heart failure events and LV dilation/function [90]. Thus, it remains possible that the development of pre-capillary PH occurs principally to reduce LV preload. However, Omote et al. observed during exercise that patients with CpcPH had a higher incidence of pulmonary congestion quantified by Kerley B lines on ultrasound, lower arterial oxygen saturation, and more ventilation–perfusion mismatch when compared with IpcPH. Lung congestion was inversely proportional to PVR at rest in patients with CpcPH [9]. It remains unknown, however, if these patients would have even more congestion in the absence of an elevated PVR.
The role of vasodilator testing (inhaled NO or nitroprusside) during an invasive hemodynamic assessment to understand the link between the elevation in PVR and HFpEF is an important concept. Al-Naamani and colleagues found that testing with iNO in patients with PH-HFpEF (IpcPH and CpcPH included) was well tolerated, with half of the patients having an increase in PAWP between 1 and 16 mmHg. While only a small minority met the criteria for a positive response to iNO, the responders were more likely to be women and obese patients. A positive response to iNO was not a predictor of survival [91]. The most recent retrospective single-center experience showed that iNO in patients with CpcPH reduced PVR by 1.1 ± 1.4 WU and increased PAWP by 1.3 ± 3.7 mmHg. There was no significant difference in the effect of iNO on patients thought to have predominantly PAH with component of left heart disease compared to those thought to have left heart disease with pre-capillary component. While a more increased PAWP was associated with more decrease in PVR, this effect of iNO was not correlated with a tolerance to PAH-specific medications [92].
Other Therapies
Levosimendan has PDE-3 inhibiting properties, activates potassium channels, and sensitizes myofilaments to calcium. In phase 2 clinical trial including N = 37 patients with HFpEF with NYHA class II-III, and PH defined by RHC (mPAP > 35 mmHg, PAWP > 20 mmHg), once weekly levosimendan injection for 6 weeks failed to meet its primary end point of reducing exercise PAWP (–1.4 mm Hg; 95% CI, –7.8 to 4.8; P = 0.65). Yet, compared to placebo, levosimendan reduced PAWP measured across all exercise stages (P = 0.047) and improved 6MWD (P = 0.033) [93]. The potential benefits of levosimedan seemed more related to venodilation and reduction in stressed blood volume than the inotropic properties of the drug [94].
Early animal studies showed that in a rodent model of PH-HFpEF with features of metabolic syndrome, oral nitrite, and metformin reduced pulmonary arterial pressures and improved pulmonary vascular remodeling by activating the SIRT3-AMP-Activated Protein Kinase pathway [95]. Nitrite which is transformed into nitric oxide was tested as an inhaled, nebulized treatment for HFpEF. In a multicenter, double-blind, placebo-controlled, 2-treatment, crossover trial of N = 105 patients with HFpEF, inhaled nitrite over 4 weeks did not improve the primary outcome of improving mean peak oxygen consumption compared to placebo (13.5 vs 13.7 mL/kg/min; P = 0.27) [96]. Inhaled nitrite did not meet secondary endpoints such as increasing daily activity levels, improving KCCQ-12, or decreasing NT pro-BNP. Several ongoing studies are exploring this therapy with different delivery methods. Metformin is also currently under investigation (ClinicalTrials.gov Identifier–NCT03629340) as the potential therapy for PH-HFpEF.
Conclusion
With the increasing prevalence of HFpEF, the quest for new therapies is ongoing. The importance of characterizing PH in the context of HFpEF is critical for prognosis and has implications for therapeutic strategies. Prior investigations of PAH therapies have not shown consistency in improving HF outcomes or functional status in patients with PH-HFpEF. Ongoing and future studies targeting a well-defined phenotype of patients with PH-HFpEF, such as CpcPH with RV dysfunction, as well as developing therapies that better target the underlying HFpEF condition may ultimately prove to be beneficial.
Availability of Data and Materials
This work constitutes a narrative review, thus access to data or materials is not applicable.
Change history
05 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11906-024-01305-4
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–617.
Moles VM, Grafton G. Pulmonary hypertension in heart failure with preserved ejection fraction. Cardiol Clin. 2022;40(4):533–40.
Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329(10):827–38.
Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–26.
Inampudi C, Silverman D, Simon MA, Leary PJ, Sharma K, Houston BA, et al. Pulmonary hypertension in the context of heart failure with preserved ejection fraction. Chest. 2021;160(6):2232–46.
Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897. https://doi.org/10.1183/13993003.01897-2018. PMID: 30545974; PMCID: PMC6351334.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61(1):2200879. https://doi.org/10.1183/13993003.00879-2022. PMID: 36028254.
Maron BA, Kovacs G, Vaidya A, Bhatt DL, Nishimura RA, Mak S, et al. Cardiopulmonary hemodynamics in pulmonary hypertension and heart failure: JACC review topic of the week. J Am Coll Cardiol. 2020;76(22):2671–81.
Omote K, Sorimachi H, Obokata M, Reddy YNV, Verbrugge FH, Omar M, et al. Pulmonary vascular disease in pulmonary hypertension due to left heart disease: pathophysiologic implications. Eur Heart J. 2022;43(36):3417–31.
Sera F, Ohtani T, Tamaki S, Yano M, Hayashi T, Nakagawa A, et al. Pulmonary hypertension with a precapillary component in heart failure with preserved ejection fraction. Heart. 2023;109(8):626–33. https://doi.org/10.1136/heartjnl-2022-321565. PMID: 36543519.
Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68(23):2525–36.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032.
Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, et al. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail. 2018;11(4):e004436. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004436. PMID: 29643065; PMCID: PMC5901761.
Inciardi RM, Abanda M, Shah AM, Cikes M, Claggett B, Skali H, et al. Right ventricular function and pulmonary coupling in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2023;82(6):489–99.
Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(6):935–44.
Heywood JT, Jermyn R, Shavelle D, Abraham WT, Bhimaraj A, Bhatt K, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135(16):1509–17.
Buggey J, Mentz RJ, Pitt B, Eisenstein EL, Anstrom KJ, Velazquez EJ, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169(3):323–33.
Mentz RJ, Anstrom KJ, Eisenstein EL, Sapp S, Greene SJ, Morgan S, et al. Effect of torsemide vs furosemide after discharge on all-cause mortality in patients hospitalized with heart failure: the TRANSFORM-HF randomized clinical trial. JAMA. 2023;329(3):214–23.
Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, et al. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant. 2015;34(3):329–37.
Assmus B, Angermann CE, Alkhlout B, Asselbergs FW, Schnupp S, Brugts JJ, et al. Effects of remote haemodynamic-guided heart failure management in patients with different subtypes of pulmonary hypertension: insights from the MEMS-HF study. Eur J Heart Fail. 2022;24(12):2320–30.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) Trial. Circulation. 2015;131(1):34–42.
De Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, et al. Spironolactone metabolites in TOPCAT-new insights into regional variation. N Engl J Med. 2017;376(17):1690–2.
Safdar Z, Cho E. Effect of spironolactone use in pulmonary arterial hypertension-analysis from pivotal trial databases. Pulm Circ. 2021;11(4):20458940211045616.
Maron BA, Waxman AB, Opotowsky AR, Gillies H, Blair C, Aghamohammadzadeh R, et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials). Am J Cardiol. 2013;112(5):720–5.
Lu M, Chen LY, Gairhe S, Mazer AJ, Anderson SA, Nelson JNH, et al. Mineralocorticoid receptor antagonist treatment of established pulmonary arterial hypertension improves interventricular dependence in the SU5416-hypoxia rat model. Am J Physiol Lung Cell Mol Physiol. 2022;322(3):L315–32.
Lahm T, Hess E, Barón AE, Maddox TM, Plomondon ME, Choudhary G, et al. Renin-angiotensin-aldosterone system inhibitor use and mortality in pulmonary hypertension: insights from the veterans affairs clinical assessment reporting and tracking database. Chest. 2021;159(4):1586–97.
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–81.
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–67.
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338–45.
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–20.
Solomon SD, Vaduganathan MBLC, Packer M, Zile M, Swedberg K, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141(5):352–61.
Clements RT, Vang A, Fernandez-Nicolas A, Kue NR, Mancini TJ, Morrison AR, et al. Treatment of pulmonary hypertension with angiotensin II receptor blocker and neprilysin inhibitor sacubitril/valsartan. Circ Heart Fail. 2019;12(11):e005819.
Chaumais MC, Djessas MRA, Thuillet R, Cumont A, Tu L, Hebert G, et al. Additive protective effects of sacubitril/valsartan and bosentan on vascular remodelling in experimental pulmonary hypertension. Cardiovasc Res. 2021;117(5):1391–401.
Zhang J, Du L, Qin X, Guo X. Effect of sacubitril/valsartan on the right ventricular function and pulmonary hypertension in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2022;11(9):e024449.
Codina P, Domingo M, Barceló E, Gastelurrutia P, Casquete D, Vila J, et al. Sacubitril/valsartan affects pulmonary arterial pressure in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2022;9(4):2170–80.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627–39. https://doi.org/10.1093/eurheartj/ehad195. Erratum in: Eur Heart J. 2024 Jan 1;45(1):53. PMID: 37622666.
Kosiborod MN, Bhatt AS, Claggett BL, Vaduganathan M, Kulac IJ, Lam CSP, et al. Effect of dapagliflozin on health status in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol. 2023;81(5):460–73.
Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27(11):1954–60.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98.
Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2(9):1025–9.
Uthman L, Homayr A, Juni RP, Spin EL, Kerindongo R, Boomsma M, et al. Empagliflozin and dapagliflozin reduce ROS generation and restore NO bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell Physiol Biochem. 2019;53(5):865–86.
Dai C, Kong B, Shuai W, Xiao Z, Qin T, Fang J, et al. Dapagliflozin reduces pulmonary vascular damage and susceptibility to atrial fibrillation in right heart disease. ESC Heart Fail. 2023;10(1):578–93.
Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. 2021;143(17):1673–86.
•• Borlaug BA, Reddy YNV, Braun A, Sorimachi H, Omar M, Popovic D, et al. Cardiac and metabolic effects of dapagliflozin in heart failure with preserved ejection fraction: the CAMEO-DAPA trial. Circulation. 2023;148(10):834–44. https://doi.org/10.1161/CIRCULATIONAHA.123.065134. Epub 2023 Aug 3. PMID: 37534453; PMCID: PMC10529848. Findings from the study suggest potential benefit of SGLT-2 inhibitors on pulmonary hypertension in patients with heart failure with preserved ejection fraction.
Reddy YNV, Borlaug BA, Gersh BJ. Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation. 2022;146(4):339–57.
Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9(3).
Abu-Qaoud MR, Kumar A, Tarun T, Abraham S, Ahmad J, Khadke S, et al. Impact of SGLT2 inhibitors on AF recurrence after catheter ablation in patients with type 2 diabetes. JACC Clin Electrophysiol. 2023;9(10):2109–18. https://doi.org/10.1016/j.jacep.2023.06.008. Epub 2023 Aug 9. PMID: 37565953.
Camm AJ, Naccarelli GV, Mittal S, Crijns HJGM, Hohnloser SH, Ma C-S, et al. The increasing role of rhythm control in patients with atrial fibrillation. J Am Coll Cardiol. 2022;79(19):1932–48.
Yang E, Tang O, Metkus T, Berger RD, Spragg DD, Calkins HG, et al. The role of timing in treatment of atrial fibrillation: an AFFIRM substudy. Heart Rhythm. 2021;18(5):674–81.
Mulder BA, Van Veldhuisen DJ, Crijns HJ, Tijssen JG, Hillege HL, Alings M, et al. Lenient vs strict rate control in patients with atrial fibrillation and heart failure: a post-hoc analysis of the RACE II study. Eur J Heart Fail. 2013;15(11):1311–8.
Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143(14):1377–90.
Parkash R, Wells GA, Rouleau J, Talajic M, Essebag V, Skanes A, et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation. 2022;145(23):1693–704.
Chieng D, Sugumar H, Segan L, Tan C, Vizi D, Nanayakkara S, et al. Atrial fibrillation ablation for heart failure with preserved ejection fraction: a randomized controlled trial. JACC Heart Fail. 2023;11(6):646–58.
Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023.
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002.
Valera RJ, Fonnegra CB, Cogollo VJ, Sarmiento-Cobos M, Rivera C, Lo Menzo E, et al. Impact of rapid weight loss after bariatric surgery in systemic inflammatory response and pulmonary hemodynamics in severely obese subjects with pulmonary hypertension. J Am Coll Surg. 2023;236(2):365–72.
Salman AA, Salman MA, Shaaban HE-D, Abdelsalam A, Tourky M, Lotfy SM, et al. Effect of bariatric surgery on the cardiovascular system in obese cases with pulmonary hypertension. Obes Surg. 2021;31(2):523–30.
Esparham A, Shoar S, Mehri A, Modukuru VR. Bariatric surgery and cardiovascular disease risk in patients with pulmonary hypertension: a propensity score matched analysis of US national inpatient sample. Obes Surg. 2023;33(10):3230–6. https://doi.org/10.1007/s11695-023-06799-6. Epub 2023 Aug 28. PMID: 37639208.
Jackson GR, Singh A. Novel approaches to sleep apnea in heart failure. Heart Fail Clin. 2024;20(1):29–38.
Healy WJ, Khayat R, Kwon Y. Breathe better and preserve heart. J Am Heart Assoc. 2023;12(14):e030806.
Lowery MM, Hill NS, Wang L, Rosenzweig EB, Bhat A, Erzurum S, et al. Sleep-related hypoxia, right ventricular dysfunction, and survival in patients with group 1 pulmonary arterial hypertension. J Am Coll Cardiol. 2023;82(21):1989–2005.
Yoshihisa A, Suzuki S, Yamaki T, Sugimoto K, Kunii H, Nakazato K, et al. Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail. 2013;15(5):543–50.
Arias MA, García-Río F, Alonso-Fernández A, Martínez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J. 2006;27(9):1106–13.
Cistulli PA, Malhotra A, Cole KV, Malik AS, Pépin JL, Sert Kuniyoshi FH, et al. Positive airway pressure therapy adherence and health care resource use in patients with obstructive sleep apnea and heart failure with preserved ejection fraction. J Am Heart Assoc. 2023;12(14):e028733.
Guazzi M, Ghio S, Adir Y. Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol. 2020;76(9):1102–11.
Sitbon O, Noordegraaf AV. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur Respir Rev. 2017;26(143):160055.
Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134(1):44–54.
Pousset F, Isnard R, Lechat P, Kalotka H, Carayon A, Maistre G, et al. Prognostic value of plasma endothelin-1 in patients with chronic heart failure. Eur Heart J. 1997;18(2):254–8.
Zile MR, Bourge RC, Redfield MM, Zhou D, Baicu CF, Little WC. Randomized, double-blind, placebo-controlled study of sitaxsentan to improve impaired exercise tolerance in patients with heart failure and a preserved ejection fraction. JACC Heart Fail. 2014;2(2):123–30.
Koller B, Steringer-Mascherbauer R, Ebner CH, Weber T, Ammer M, Eichinger J, et al. Pilot study of endothelin receptor blockade in heart failure with diastolic dysfunction and pulmonary hypertension (BADDHY-Trial). Heart Lung Circ. 2017;26(5):433–41.
Vachiéry JL, Delcroix M, Al-Hiti H, Efficace M, Hutyra M, Lack G, Papadakis K, Rubin LJ. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51(2):1701886. https://doi.org/10.1183/13993003.01886-2017. PMID: 29437943.
Hsu S, Tedford RJ. Will we be singing a different tune on combined post- and pre-capillary pulmonary hypertension? Eur Respir J. 2018;51(2):1702589. https://doi.org/10.1183/13993003.02589-2017. PMID: 29437950.
Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124(2):164–74.
Hoendermis ES, Liu LCY, Hummel YM, Van Der Meer P, De Boer RA, Berger RMF, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36(38):2565–73.
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–77.
Belyavskiy E, Ovchinnikov A, Potekhina A, Ageev F, Edelmann F. Phosphodiesterase 5 inhibitor sildenafil in patients with heart failure with preserved ejection fraction and combined pre- and postcapillary pulmonary hypertension: a randomized open-label pilot study. BMC Cardiovasc Disord. 2020;20(1):408.
Kramer T, Dumitrescu D, Gerhardt F, Orlova K, Ten Freyhaus H, Hellmich M, et al. Therapeutic potential of phosphodiesterase type 5 inhibitors in heart failure with preserved ejection fraction and combined post- and pre-capillary pulmonary hypertension. Int J Cardiol. 2019;283:152–8.
Bermejo J, Yotti R, García-Orta R, Sánchez-Fernández PL, Castaño M, Segovia-Cubero J, et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J. 2018;39(15):1255–64.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur Heart J. 2022;43(38):3618–731.
Nanayakkara S, Byrne M, Mak V, Carter K, Dean E, Kaye DM. Extended-release oral milrinone for the treatment of heart failure with preserved ejection fraction. J Am Heart Assoc. 2020;9(13):e015026.
Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146(5):1274–85.
Dachs TM, Duca F, Rettl R, Binder-Rodriguez C, Dalos D, Ligios LC, et al. Riociguat in pulmonary hypertension and heart failure with preserved ejection fraction: the haemoDYNAMIC trial. Eur Heart J. 2022;43(36):3402–13.
Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38(15):1119–27.
Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O’Connor CM, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA. 2020;324(15):1512–21.
•• Hoeper MM, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M, McLaughlin VV, et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478–90. Encouraging results as a therapeutic option for pulmonary arterial hypertension, now with ongoing investigation for combined pre- and post-capillary pulmonary hypertension.
Zhang H, Zhang J, Chen M, Xie DJ, Kan J, Yu W, et al. Pulmonary artery denervation significantly increases 6-min walk distance for patients with combined pre- and post-capillary pulmonary hypertension associated with left heart failure: the PADN-5 study. JACC Cardiovasc Interv. 2019;12(3):274–84.
Zhang H, Kan J, Zhang J, Xie D, Li X, Zhou W, et al. 3-year outcome in patients with combined precapillary and postcapillary pulmonary hypertension: results from PADN-5 trial. JACC Heart Fail. 2023;11(8 Pt 2):1135–46.
Leary PJ, Jenny NS, Bluemke DA, Kawut SM, Kronmal RA, Lima JA, et al. Endothelin-1, cardiac morphology, and heart failure: the MESA angiogenesis study. J Heart Lung Transplant. 2020;39(1):45–52.
Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail. 2015;3(6):467–74.
Krishtopaytis E, Ampnti SA, Obeidat M, Ramahi N, Lane J, Toth D, et al. Can inhaled nitric oxide response predict tolerance to therapies and survival in patients with combined precapillary and postcapillary pulmonary hypertension? Am J Cardiol. 2023;207:363–9.
Burkhoff D, Borlaug BA, Shah SJ, Zolty R, Tedford RJ, Thenappan T, et al. Levosimendan improves hemodynamics and exercise tolerance in PH-HFpEF: results of the randomized placebo-controlled HELP trial. JACC Heart Fail. 2021;9(5):360–70.
Brener MI, Hamid NB, Sunagawa K, Borlaug BA, Shah SJ, Rich S, et al. Changes in stressed blood volume with levosimendan in pulmonary hypertension from heart failure with preserved ejection fraction: insights regarding mechanism of action from the HELP trial. J Card Fail. 2021;27(9):1023–6.
Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, et al. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133(8):717–31.
Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA. 2018;320(17):1764–73.
Udelson JE, Lewis GD, Shah SJ, Zile MR, Redfield MM, Burnett J Jr, et al. Effect of praliciguat on peak rate of oxygen consumption in patients with heart failure with preserved ejection fraction: the CAPACITY HFpEF randomized clinical trial. JAMA. 2020;324(15):1522–31.
Andersen MJ, Ersbøll M, Axelsson A, Gustafsson F, Hassager C, Køber L, et al. Sildenafil and diastolic dysfunction after acute myocardial infarction in patients with preserved ejection fraction: the Sildenafil and Diastolic Dysfunction After Acute Myocardial Infarction (SIDAMI) trial. Circulation. 2013;127(11):1200–8.
Liu LC, Hummel YM, van der Meer P, Berger RM, Damman K, van Veldhuisen DJ, et al. Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health-related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. Eur J Heart Fail. 2017;19(1):116–25.
Funding
This work received no funding.
Author information
Authors and Affiliations
Contributions
EK, ERA NSD wrote the main manuscript text, prepared the table. EK prepared the figure. RJT revised the manuscript, table and figure. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Tedford reports no direct conflicts of interest related to this manuscript. He is co-chair of the PH due to left heart disease task force for the 7th World Symposium on Pulmonary Hypertension. He reports general disclosures to include consulting relationships with Medtronic, Abbott, Acorai, Aria CV Inc., Acceleron/Merck, Alleviant, Cytokinetics, Edwards LifeSciences, Gradient, Lexicon Pharmaceuticals, and United Therapeutics. Dr. Tedford serves on steering committees for Merck, Edwards, and Abbott as well as a research advisory board for Abiomed. He also does hemodynamic core lab work for Merck. All other authors report no disclosures related to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kozaily, E., Akdogan, E.R., Dorsey, N.S. et al. Management of Pulmonary Hypertension in the Context of Heart Failure with Preserved Ejection Fraction. Curr Hypertens Rep 26, 291–306 (2024). https://doi.org/10.1007/s11906-024-01296-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-024-01296-2