Abstract
Purpose of Review
Pulmonary arterial hypertension (PAH) is a chronic, progressive, and incurable disease with significant morbidity and mortality. Despite increasingly available treatment options, PAH patients continue to experience disease progression and increased rates of hospitalizations due to right heart failure. Physician’s ability to comprehensively assess PAH patients, determine prognosis, and monitor disease progression and response to treatment remains critical in optimizing outcomes.
Recent Findings
Risk assessment in PAH should include a range of clinical, hemodynamic, and exercise parameters, performed in a serial fashion over the course of treatment. Approaches to risk assessment in PAH patients include the use of risk variables, scores, and equations that stratify the impact of both modifiable (e.g., 6-min walk distance, functional class, brain natriuretic peptide), and non-modifiable (e.g., age, gender, PAH etiology) risk factors. Such tools allow physicians to better determine prognosis, allocate treatment resources, and enhance the consistency of treatment approaches across providers.
Summary
Comprehensive and accurate risk prediction is essential to make individualized treatment decisions and optimizing outcomes in PAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a chronic and rapidly progressive disease that is characterized by extensive narrowing of the pulmonary vasculature, leading to an increase in pulmonary vascular resistance (PVR), subsequent right ventricular (RV) dysfunction, and eventual death [1]. Currently, there are multiple approved drugs that can be used as single or combination therapies—that have improved outcome and functionality in PAH [2]. However, despite improvement in short-term survival, PAH remains an incurable disease with a median survival of 7 years [3]. As such, means to predict outcome in this disease, including both mortal and morbid events, is an essential part of patient management.
There is no single variable that predicts outcomes in PAH patients. Therefore, risk assessment in PAH patients should include a range of clinical, hemodynamic, and exercise parameters performed in a serial fashion to reflect a patient’s course during the disease [4••, 5]. Accurate risk assessments allow clinicians to determine patients’ prognoses, identify treatment goals, help patients make informed decisions, and monitor disease progression. Risk assessment is especially important in settings where clinical PAH experience is not available, as it could facilitate timely referral to a PAH center and/or lung transplantation [5]. Approaches for assessing risk in PAH patients include the use of risk variables, as recommended in the 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines, and the application of risk equations and scores, such as those developed by the French, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA), and the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) [4••, 6••, 7, 8••]. The following review explores the development and use of risk assessment tools and prognostic scores, their respective merits and limitations, as well as considerations for the future of risk stratification in PAH.
Risk Assessment in PAH
The seminal NIH Registry was the first large study to describe the natural history of disease progression and ultimately delineate patient prognosis in the era prior to the availability of PAH specific therapies [7]. With the advent of epoprostenol and subsequently a variety of other drugs for the treatment of PAH, there is an increasing interest in identifying clinical variables to risk stratify patients and select appropriate therapies [9, 10]. A variety of individual factors exist, which are associated with outcomes in PAH. These may be distilled roughly into several categories, including clinical demographics, functional capacity (both subjective and objective), and parameters derived from diagnostic testing, such as imaging, laboratory testing, and invasive hemodynamics.
Demographics and Etiology of PAH
Though PAH has a marked female predominance, male gender is strongly implicated with adverse outcomes in PAH. In the REVEAL registry, older male patients had substantially higher mortality rates compared to female patients, particularly those older than 65 [11]. This finding was confirmed by data from the French PAH registry, wherein male gender was strongly associated with increased mortality. Etiology of PAH is also strongly linked to prognosis [11,12,13]. Patients who have porto-pulmonary hypertension, as well as patients with connective tissue disease, in particular scleroderma, appear to have worse survival in comparison with PAH patients overall [11, 14,15,16]. Patients with “familial” PAH, especially those with “heritable” PAH, related to BMPR2 loss of function mutations typically have more recalcitrant pulmonary hypertension with higher pulmonary arterial (PA) pressures and PVR at presentation and less robust response to therapy [11, 17, 18]. Conversely, patients with congenital heart disease-related pulmonary hypertension, in general, have improved survival relative to idiopathic PAH, although this association was less robust and not clinically different than IPAH when adjusted for other relevant clinical risk factors [11, 19]. Although race has not historically been associated with outcome in PAH, a recent study form the U.S. National PH Biobank demonstrated that non-Hispanic Black patients had a lower mean pulmonary arterial pressure (mean PAP), lower PVR, and shorter 6-min walk distance (6MWD) compared with Hispanics and non-Hispanic Whites, but other hemodynamic parameters were similar [20]. Similarly, epidemiologic data from the USA between 2001 and 2010 showed a higher death rate for non-Hispanic Blacks [21]. This observation was supported by a small study with idiopathic, familial, or anorexigen-induced PAH, in which a multivariate survival analysis showed an association between Black or Asian race and increased risk of death [22]. Interestingly, analysis of REVEAL data showed dissimilar findings with an increased 5-year mortality in non-Hispanic White patients [23]. However, in this analysis, race was not weighted against other potential determinants of survival, including subtype of PAH.
Assessment of Functional Capacity
Both subjective and objective assessments of functional capacity have been strongly associated with future outcomes in PAH. The most commonly employed assessment of functional capacity is a subjective assessment usually performed by clinical providers, such as New York Heart Association (NYHA) or World Health Organization (WHO) functional class, graded on a scale of I–IV with higher grades associated with more severe symptoms. These are objectively assessed using the 6-min walk test (6MWT), a widely used, simple, and typically reproducible method for assessing functional capacity. Numerous studies have shown a strong association between more advanced functional class at presentation and long-term survival. In addition, changes in functional class assessment at follow-up have been associated with concomitant improvements and decrement in survival rates [7, 8••, 11, 24]. Though a continuum exists of 6MWT distances, the concept of threshold levels associated with improved or decreased survival has gained some traction in the literature. For example, a 6MWT distance threshold of 440 m was associated with low risk of mortality in a variety of contemporary risk stratification algorithms [11, 12, 24]. In the REVEAL registry, a 6MWT distance less than 165 m was associated with significantly higher mortality risk [11]. An improvement in 6MWT distance has not been clearly associated with improvement in long-term survival; however, a decrease in walk distance after diagnosis was associated with worse prognosis (Fig. 1) [25, 26]. Maximal exercise testing using cardiopulmonary exercise testing may provide additional prognostic information. For example, peak VO2 less than 15 mL/kg per minute or a peak VE/VCO2 slope greater than 36 has been shown to be a marker of adverse prognosis. Similarly, reduced end tidal CO2 levels have also been associated with adverse outcomes in PAH patients [24, 27,28,29].
Kaplan-Meier survival estimates based on the percentage change in the 6-min walk distance (6MWD) ± 10 percentage points for all patients with a baseline 6MWD and follow-up 6MWD within 1 year. Reprinted without changes from Elsevier, Farber et al., Predicting outcomes in pulmonary arterial hypertension based on the 6-min walk distance, Mar 1, 2015, volume 34, issue 3, page 7, under the Creative Commons open access license agreement (CC BY-NC-ND 4.0) [25]
Direct and Indirect Non-invasive Imaging Measures of Right Ventricular Function
RV imaging is a crucial component of assessment of patient’s with PAH. Indeed, pulmonary vascular remodeling principally raises RV afterload, which has secondary impacts on RV size, hypertrophy, and function. Consequently, RV function is a critical determinant of outcomes in PAH.
In clinical practice, the right ventricle is most commonly imaged with transthoracic echocardiography. Though right ventricular ejection fraction (RVEF) cannot be easily obtained from 2D echocardiography, a variety of surrogates of RV systolic function can be derived, including tricuspid annular plane systolic exertion (TAPSE), RV fractional area change (RVFAC), and RV global longitudinal strain. TAPSE values less than 1.8 cm have been associated with reduced survival rates in PAH [30,31,32,33]. Recent studies have suggested that follow-up TAPSE values, after initial therapy, are even more predictive for long-term prognosis than baseline TAPSE values [34]. Other indirect echocardiographic measures of RV function associated with outcomes in PAH include right atrial area, with right atrial area greater than 18 cm2 connected to increased risk of mortality [24, 35]. Lastly, in multiple studies, pericardial effusion is associated with worse survival than patients without pericardial effusion [11, 36,37,38]. Cardiac magnetic resonance imaging (CMRI) may provide an even more robust assessment of RV function through three-dimensional imaging [39]. CMRI is being increasingly utilized as a clinical and research tool in PH. RV and LV end diastolic volume indices, RV mass index, RV stroke volume index, RVEF, and RV to LV ratios are all associated with other hemodynamic and functional surrogates of survival and importantly with survival itself in PAH [40,41,42,43,44]. Among these CMRI parameters, decrease in RVEF by 5% was the strongest predictor of mortality in PAH [45].
Laboratory Parameters
In the REVEAL registry, renal insufficiency was strongly associated with worse survival, and this was subsequently confirmed in the updated REVEAL calculator [11, 46] and a ≥ 10% decline in eGFR from baseline over a year was significantly increased in risk of death [47]. Natriuretic peptides, such as brain natriuretic peptide (BNP) and N-terminal fragment of pro-brain natriuretic peptide (N-terminal proBNP), are biomarkers that have been studied in PH and have been included as endpoints in multiple PAH clinical trials. Absolute levels of NT proBNP and BNP are correlated with worse clinical outcomes, both at baseline at follow-up [11, 46, 48]. Elevated cardiac troponin, at any detectable level, is associated with RV dysfunction and worse outcomes in PAH patients [49, 50]. As with patients with left-sided heart failure, hyponatremia predicts worse outcomes in PH patients even after adjusting for other parameters, including right atrial pressure, cardiac index, and diuretic use [51]. Lower serum albumin and sodium levels were associated with increasing death, while increases in total bilirubin and creatinine were strongly predictors of death [52]. Other biochemical variables, such as blood urea nitrogen (BUN) and uric acid, are also associated with increased risk [45]. Importantly, uric acid appears to be not only a significant risk factor for 1-year mortality but also time to clinical worsening and decrease in 6MWD. Coupled with these correlations is the finding that uric acid levels are reduced by PAH specific therapies [53]. Markers of inflammation like CRP, thrombosis, and platelet activation are also associated with outcome in PAH [54, 55]. Lastly, markers of impaired glucose metabolism and increased insulin resistance, like HgbA1C, are new markers of outcome in this disease [56].
Hemodynamic Variables
In the seminal NIH registry, PA pressures were significantly associated with survival, but this finding has not been subsequently confirmed in more contemporary studies after adjusting for other variables [7, 11, 12, 57]. However, a variety of hemodynamic parameters remain significantly associated with long-term PAH patient survival. In both the REVEAL registry and French PAH registry, elevated right atrial pressure (RAP) was associated with worse survival rates [11, 12]. In REVEAL, severely elevated PVR over 32 Wood Units was associated with worse outcomes and PVR’s < 5 Wood Units correlate with increased survival [11, 58••]. Mixed venous oxygen saturation < 65% and cardiac index (CI) < 2.5 L min−1 m−2 at baseline and decrease in these parameters over time have been associated with worse outcomes [59]. Decrease in stroke volume index (SVI) is also associated with worse survival and has a stronger predictive value than CI [60]. In fact, a lower SVI discriminated patients at a higher risk of death or transplantation, even among those who were in NYHA class I or II with CI ≥ 2.5 L min−1 m−2 at follow-up [60]. In this same report, change in mean PAP, SVI, PVR, and pulmonary artery compliance (PCa) was significantly associated with death or lung transplantation regardless of the baseline quartile (Fig. 2). However, in a recent hemodynamic sub-study of the SERAPHIN trial, the absolute value of CI and RAP at 6 months, but not the changes from baseline, predicted the risk of disease progression [61]. Use of absolute values, rather than changes in values, is easier to implement in scoring systems for PAH. A high RV index as a combination of RAP, PVR, and SVO2 was also reported as a strong predictor of patient outcomes [62]. Lastly, viewing right and left ventricular morphometrics (RVEDV:LVEDV ratio) with hemodynamic parameters, particularly PVR, can add to the predictive value of each parameter individually (Fig. 3) [40].
Predicted survival estimates according to percent change from baseline for hemodynamic variables. a Stroke volume index (SVI), b right atrial pressure (RAP), c cardiac index (CI), d heart rate (HR), e pulmonary vascular resistance (PVR), and f pulmonary arterial compliance (PCa). Reprinted without modification, with permission from Wolters Kluwer Health, Inc., Jason Weatherald, Athénaïs Boucly, Denis Chemla, et al., Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension, Circulation, volume 137, issue 7, pages 693–704, https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.117.029254
Plot of competing outcomes by RVEDV/LVEDV ratio. Reprinted without changes from SAGE Publishing, Benza et al., Bosentan-based, treat-to-target therapy in patients with pulmonary arterial hypertension: results from the COMPASS-3 study, Pulmonary Circulation, Vol 8, Issue 1, DOI: https://doi.org/10.1177/2045893217741480, under the Creative Commons open access license agreement (CC BY 4.0) [40]
Pulmonary Function Testing
A reduction in diffusion capacity for carbon monoxide (DLCO) is characteristic of pulmonary vascular disease, particularly in combination with normal or near normal spirometry. Indeed, an isolated reduction in DLCO on pulmonary function testing is often one of the first clinical clues to the presence of PAH on routine diagnostic testing [63, 64]. However, the severity of reduction in DLCO may in and of itself be prognostic. In the REVEAL registry, reduction in DLCO below 32% was independently associated with worse survival, whereas DLCO above 80% predicted was associated with better prognosis [11, 63].
Hospitalization
Hospitalization has been used as part of a combined clinical endpoint in several recent large, event-driven studies of pharmacotherapies in PAH. However, there is increasing recognition that hospitalization is strongly associated with future prognosis in PAH. Several studies have shown that an all-cause hospitalization is associated with high risk of long-term mortality. In a multivariate analysis from the REVEAL registry data, all-cause hospitalization within the past 6 months remained an independent predictor of death and recurrent hospitalization [65, 66]. More recently, the impact of hospitalization and other morbidity events on subsequent mortality in SERAPHIN and GRIPHON studies was analyzed using the landmark method [67]. There was an increased risk of death up to the end of the study among patients who experienced a primary endpoint event of hospitalization for worsening of PAH compared with those who did not (HR 6.55; 95% CI 4.02 to 10.67).
Risk Scores and Calculators
Risk assessment in PAH should be performed, utilizing the full spectrum of the above-mentioned parameters. Ideally, a risk assessment tool should be evidence based, easy to use, with excellent discrimination, having been derived from contemporary data, while being dynamic and accurate throughout the course of the disease.
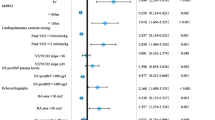
The NIH equation was one of the earliest prognosis equations for patients with PAH [7]. The equation utilized patient data (n = 194) from the US-based NIH registry to predict survival based purely on hemodynamics, including mPAP, mean RAP, and CI. The Scottish Composite Index developed by the Scottish Pulmonary Vascular Unit, UK is constructed from a multivariate Cox model from 182 PAH patients using age, sex, etiology, right atrial pressure, cardiac output, and 6MWT distance to predict survival [68]. More contemporary multivariable indices of risk include the REVEAL risk equation and scores [11] [69, 70], the French risk equation [12], later low-risk stratification algorithm [8••], COMPERA [6••], and the Swedish PAH Registry (SPAHR) risk score [71]. The COMPERA, SPAHR, and the French risk scores are all derived from the ESC/ERS guidelines risk table (Fig. 4). This table is an expert derived matrix, which summarizes and silos contemporary low, intermediate, and high-risk clinical variables into a clinically useful resource reference (4••).
European Respiratory Society guidelines for risk assessment in PAH. Reproduced with permission of the © 2019 European Society of Cardiology & European Respiratory Society. European Respiratory Journal 46 (4) 903–975; DOI: https://doi.org/10.1183/13993003.01032-2015 Published 30 September 2015 [4••]
Derived from 2529 newly and previously diagnosed PAH patients, the original REVEAL risk equation was created to stratify patient risk into five intervals: better-than-average, average, worse-than-average, poor, and extremely poor survival [11]. REVEAL enrolled adult and pediatric patients with WHO group 1 PAH who met hemodynamic criteria (mean PAP > 25 mmHg at rest or > 30 mmHg with exercise, mean PA wedge pressure or left ventricular end-diastolic pressure of ≤ 18 mmHg, and PVR of > 3 Wood Units) and were receiving treatment for PAH at the time of enrollment. The equation was derived using step-wise multivariate regression and uses 13 parameters to derive a survival outcome (c-index of 0.74). A simplified risk score based on the prognostic equation was then designed to be easy to use tool in everyday clinical practice (Fig. 5) [70]. The REVEAL equation and subsequent calculator were validated internally in both newly diagnosed patients [72] and serially in patients throughout their disease progression [69], as well as in external patient registries and clinical trials [69, 73,74,75,76], demonstrating utility for both patient risk assessment and treatment decisions. Recently, an updated REVEAL 2.0 risk score calculator (REVEAL 2.0, internally validated c-index of 0.76) was published with additional variables of all-cause hospitalization, a revised renal function measured by estimated glomerular filtration rate along with increased cut points for 6MWD and natriuretic peptides levels (Fig. 6) [58••]. Cut-points and values of other existing variables were also re-assessed to optimize clinical relevance and to achieve better discrimination between risk categories. In REVEAL 2.0 “low-risk” was defined as a score of ≤ 6 (12-month mortality risk of ≤ 2.6%), “intermediate-risk” was defined as a score of 7 or 8 (12-month mortality risk of 6.2 and 7.0%, respectively), and “high-risk” was defined as a score of ≥ 9 (12-month mortality risk of ≥ 10.7%) (Fig. 7a). The score was also able to discriminate between risk categories for 5-year mortality (Fig. 7b). REVEAL 2.0 can also be used to predict clinical worsening in addition to mortality (Fig. 8).
One-year Kaplan-Meier survival estimate for the REVEAL development cohort with predicted risk score. Reprinted with permission from Chest Journal, Benza et al., The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension, Vol. 141, Issue 2, pages 354–362, 2012, with permission from Elsevier (License # 4536030779445) [72]
REVEAL 2.0 Updated Risk Score Calculator. Risk score ranges from 0 (lowest) to 23 (highest). Reprinted without changes from Chest Journal, Benza et al., Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies, in press, 2019, under the Creative Commons open access license agreement (CC BY-NC-ND 4.0) [58••]
Kaplan-Meier survival estimates by REVEAL 2.0 risk scores calculated at 1-year post-enrollment (baseline for this analysis). a 12-Month survival estimate. b 60-Month survival estimate. Reprinted without changes from Chest Journal, Benza et al., Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies, in press, 2019, under the Creative Commons open access license agreement (CC BY-NC-ND 4.0) [58••]
Kaplan-Meier estimates of clinical worsening by REVEAL 2.0 risk scores calculated at 1-year post-enrollment (baseline for this analysis). Reprinted without changes from Chest Journal, Benza et al., Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies, in press, 2019, under the Creative Commons open access license agreement (CC BY-NC-ND 4.0) [58••]
The French risk prediction equation was developed from incident and prevalent PAH patients, irrespective of etiology, in the French Pulmonary Hypertension Registry (FPHR) registry between 2002 and 2003 [13]. The equation used three independent prognostic markers for survival: sex, 6MWT distance, and cardiac output. In the internal validation on the FPHR data, the equation performed with a c-index of 0.57. It was externally validated in the REVEAL registry with a ROC AUC of 0.72 and good discrimination of high and low risk groups, but less so in the middle strata at 1 year after diagnosis [73].
In light of the recognized importance of risk prediction, the latest 2015 ESC/ERS PH guidelines included updated recommendations for patient assessment, risk stratification, and treatment goals based on risk assessment specifically for patients with PAH [4••]. A regular assessment of patients with PAH and the role of expert centers in these assessments is highly emphasized in these recommendations. In an attempt to distill the common elements of risk prediction in an easy to visualize format, a risk table was developed based on an expert opinion (Fig. 4) [4••]. PAH patients are categorized as low, intermediate, or high risk of death or transplant-free survival based whether they fall into several color-coded risk silos comprising various risk parameters and cutoffs, with estimated 1-year mortality of < 5, 5–10, and > 10%, respectively. This table borrows many common risk elements from the earlier REVEAL equation and score, including WHO functional class, natriuretic peptide levels, 6MWD cutoffs, presence of pericardial effusion, and RAP. The ESC/ERS risk stratification table is meritorious in its simplicity of approach. However, it is recognized that patients may have variables that fall into different risk categories at the same time point, limiting its “real-world” applicability. An attempt to solve this important limitation resulted in three important publications from the French, Swedish, and COMPERA registries, described in subsequent paragraphs.
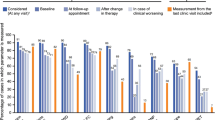
Using the French registry, Boucly and colleagues derived a simplified risk assessment tool from the ERS/ECS risk table using the sum of four possible low-risk criteria to determine if the patient is low risk according to thresholds prescribed in the 2015 ESC/ERS guidelines [8••]. Low-risk criteria included WHO/NYHA functional class I or II, 6MWD > 440 m, RAP < 8 mmHg, and cardiac index ≥ 2.5 L min−1 m−2. The performance of the tool was assessed with n = 1017 newly diagnosed patients with IPAH, HPAH, and drug-induced PAH from the FPHR (Fig. 9). The tool is designed to give a prognosis at baseline and first reassessment with the latter reflecting a more accurate depiction of risk. Although this tool can identify several level of risk, its main utility is to identify the “low risk” patient, which is the treatment goal set forth by contemporary thinking [77]. It is not yet externally validated.
Kaplan-Meier curves of transplant-free survival according to the number of low-risk criteria at a time of pulmonary arterial hypertension diagnosis and b first re-evaluation within the first year after diagnosis using the French risk calculator. Reproduced with permission of the ©ERS 2019: European Respiratory Journal 50 (2) 1700889; DOI: https://doi.org/10.1183/13993003.00889-2017 Published 3 August 2017 [8••]
The COMPERA risk assessment strategy categorizes patients into low, intermediate, or high risk of death [6••]. The overall risk category is determined by computing the mean of the risk grades from available variables (WHO functional class, 6MWD, BNP or NT-proBNP, right atrial pressure, cardiac index, and mixed venous oxygen saturation) for each patient and rounding to the nearest integer. In the COMPERA registry, treatment-naïve, newly diagnosed PAH population (n = 1588) were observed to have mortality rates 1 year after diagnosis at 2.8% in the low risk cohort, 9.9% in the intermediate risk cohort, and 21.2% in the high-risk cohort, respectively (Fig. 10).
Kaplan–Meier survival estimates in patients with all forms of PAH combined per individual risk stratification at baseline using the COMPERA risk calculator. Reproduced with permission of the ©ERS 2019: European Respiratory Journal 50 (2) 1700740; DOI: https://doi.org/10.1183/13993003.00740-2017 Published 3 August 2017 [6••]
Comparison and Limitations of Risk Stratification Tools
Although promising in their consistent findings, the contemporary risk models require prospective validation among a wider variety of WHO group I patients to determine utility and generalizability. Demonstrating the utility of a risk score in a mixed cohort of patients is important, particularly one in which previously diagnosed patients predominate, as this latter population is most representative of routine clinical practice. The REVEAL risk calculator is broadly applicable to the general PAH population as it predicts risk in newly diagnosed and previously diagnosed patients with various PAH etiologies. The predictive strength of REVEAL is also maintained when patient data are missing for some variables included in the calculator. The COMPERA and the FPHR scores were derived from populations, which were newly diagnosed and in FPHR, restricted to patients with idiopathic, heritable, or drug-induced PAH. When compared within the REVEAL dataset, the REVEAL 2.0 provided better characterization of the actual risk of mortality at 12 months than either COMPERA or FPHR. It was noted that COMPERA and FPHR overestimated and underestimated mortality risk in a primary US population when compared with REVEAL 2.0, which could have significant impact on clinical decision making (Table 1) [58••]. In addition, although the French method was designed to primarily identify the low risk patient, it tended to overestimate risk in this category. REVEAL 2.0 also predicts clinical worsening at 1 year of follow-up, which enhances its utility, and may prompt more intensive intervention earlier in the disease course.
Although all these scores appropriately identified high and low risk patients, they had poorer discrimination for those in between/ at intermediate risk. For clinicians using these tools at bedside, it is important to appreciate if it has been calibrated to the time of diagnosis or further along the disease progression. For example, variables used in COMPERA are better at delineation of risk at 1 year than the French score, although the French risk score was better at delineating a true low risk patient than COMPERA [78]. A similar drawback (clustering of risk) was noted in the original REVEAL risk calculator-based Kaplan–Meier survival estimates. This clustering effect was remedied in the updated calculator (see Fig. 6). Whether this early clustering of risk is related to study inclusion criterion in unclear. Lastly, the risk strategies derived from the ESC/ERS guidelines do not utilize weighting of variables, which limits their calibration, particularly in the intermediate-risk range.
All the present risk assessments for PAH patients have been derived used traditional statistical methods or expert opinion. Both this and efforts to over simplify risk-prediction have resulted in lack of robustness with respect to predicting outcome in complex disease. In clinical practice, patients present with some features that are indicative of low risk, and others that indicate intermediate or high risk. These analyses do not cover the full spectrum of PAH subgroups. Also, clinically relevant variables, such as rate of disease progression, currently remain unaccounted for. Not surprisingly, only a minority of patients in analysis cohorts during application of risk scores achieved the intended goal of low risk status at follow-up. Hence, the risk scores themselves need to undergo periodic refinements to incorporate new data on predictors of disease progression and mortality and, thereby, maintain their clinical utility.
Machine learning methods can address these shortcomings by evaluating data with consideration of causality between variables. Bayesian networks have been used to evaluate patient mortality in similar complex cases, such as heart failure patient selection for left ventricular assist devices [55]. This method is robust to missing data when evaluating individual patient risk and allows for incorporation of expert input alongside machine learned parameters. For an orphan disease state like PAH, the further advantage of BNs over traditional statistical methods is that they can adapt dynamically with the evolution of treatment options and expansion of the patient cohort [79].
Using Risk Stratification in PAH
Along with advancing treatment options, appropriate and accurate risk prediction is essential to halt disease progression and make individualized treatment plans in PAH disease management. Early and accurate risk prediction allows for the identification of patients who are more likely to progress rapidly, which is especially relevant in settings where tertiary PAH care is not readily available. Assessment of prognosis allows physicians to educate and inform patients of their prognosis, thereby allowing them to make informed decisions about treatment options (Fig. 11) [1]. If widely adopted, appropriate risk prediction will enhance consistency of treatment approaches across practitioners and assist in the timely referral for lung transplantation. Lastly, risk models can enhance clinical study design both by selecting the appropriate study cohort and serving as a study end-point.
Theoretic risk decision tree for PAH using multifaceted tools. Reprinted with permission from the Pulmonary Hypertension Association, Benza et al., Risk assessment in pulmonary arterial hypertension patients: the long and short of it. advances in pulmonary hypertension: 2018, volume 16, issue 3, pages 125–135. [1]
Conclusion
Current ESC/ERS guidelines recommend regular risk assessment of patients with PAH, with an overall treatment goal of achieving a low-risk status. All risk assessments should be considered in terms of the individual patient, their pertinent history and current PAH-specific therapy. Regular risk assessment allows clinicians to gauge the individual patient response to each treatment option over various follow-up time-points, allowing for timely referral for lung transplantation. PAH risk assessment tools have proven to be effective in accurately predicting survival in multiple patient cohorts and may provide a surrogate end point in future clinical trials. Therefore, it is important that risk assessment be considered while managing PAH therapies as it provides guidance for individualized clinical decision-making and optimizing patient outcomes.
References
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Benza RL, Lohmueller LC, Kraisangka J, Kanwar M. Risk assessment in pulmonary arterial hypertension patients: the long and short of it. Advances in Pulmonary Hypertension. 2018;16(3):125–35.
Kanwar MK, Thenappan T, Vachiery JL. Update in treatment options in pulmonary hypertension. J Heart Lung Transplant. 2016;35(6):695–703.
Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest. 2012;142(2):448–56.
•• Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75 Most recent guidelines published in the field of PH which summarizes the approach to risk stratification.
Benza RL, Farber HW, Selej M, Gomberg-Maitland M. Assessing risk in pulmonary arterial hypertension: what we know, what we don’t. Eur Respir J. 2017;50(2).
•• Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2) Risk stratification in the COMPERA registry.
D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–9.
•• Boucly A, Weatherald J, Savale L, Jais X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2) French Risk equation.
McGoon MD, Krichman A, Farber HW, Barst RJ, Raskob GE, Liou TG, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923–31.
Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40(4):780–8.
Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164–72.
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–63.
Humbert M, Sitbon O, Yaici A, Montani D, O'Callaghan DS, Jais X, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36(3):549–55.
Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54(9):3043–50.
Mathai SC, Hassoun PM. Pulmonary arterial hypertension in connective tissue diseases. Heart Fail Clin. 2012;8(3):413–25.
Sithamparanathan S, Nair A, Thirugnanasothy L, Coghlan JG, Condliffe R, Dimopoulos K, et al. Survival in portopulmonary hypertension: outcomes of the United Kingdom National Pulmonary Arterial Hypertension Registry. J Heart Lung Transplant. 2017;36(7):770–9.
Evans JD, Girerd B, Montani D, Wang XJ, Galie N, Austin ED, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. 2016;4(2):129–37.
Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177(12):1377–83.
Lowe BS, Therrien J, Ionescu-Ittu R, Pilote L, Martucci G, Marelli AJ. Diagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomes. J Am Coll Cardiol. 2011;58(5):538–46.
Al-Naamani N, Paulus JK, Roberts KE, Pauciulo MW, Lutz K, Nichols WC, et al. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ. 2017;7(4):793–6.
George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest. 2014;146(2):476–95.
Kawut SM, Horn EM, Berekashvili KK, Garofano RP, Goldsmith RL, Widlitz AC, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95(2):199–203.
Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest. 2015;148(4):1043–54.
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119.
Farber HW, Miller DP, McGoon MD, Frost AE, Benton WW, Benza RL. Predicting outcomes in pulmonary arterial hypertension based on the 6-minute walk distance. J Heart Lung Transplant. 2015;34(3):362–8.
Farber HW. Validation of the 6-minute walk in patients with pulmonary arterial hypertension: trying to fit a square PEG into a round hole? Circulation. 2012;126(3):258–60.
Blumberg FC, Arzt M, Lange T, Schroll S, Pfeifer M, Wensel R. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur J Heart Fail. 2013;15(7):771–5.
Oudiz RJ. The role of exercise testing in the management of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2005;26(4):379–84.
Oudiz RJ, Midde R, Hovenesyan A, Sun XG, Roveran G, Hansen JE, et al. Usefulness of right-to-left shunting and poor exercise gas exchange for predicting prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2010;105(8):1186–91.
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–41.
Ghio S, Klersy C, Magrini G, D'Armini AM, Scelsi L, Raineri C, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140(3):272–8.
Haeck ML, Scherptong RW, Marsan NA, Holman ER, Schalij MJ, Bax JJ, et al. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5(5):628–36.
Murata I, Kihara H, Shinohara S, Ito K. Echocardiographic evaluation of pulmonary arterial hypertension in patients with progressive systemic sclerosis and related syndromes. Jpn Circ J. 1992;56(10):983–91.
Mazurek JA, Vaidya A, Mathai SC, Roberts JD, Forfia PR. Follow-up tricuspid annular plane systolic excursion predicts survival in pulmonary arterial hypertension. Pulm Circ. 2017;7(2):361–71.
Bustamante-Labarta M, Perrone S, De La Fuente RL, Stutzbach P, De La Hoz RP, Torino A, et al. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. J Am Soc Echocardiogr. 2002;15(10 Pt 2):1160–4.
Austin C, Burger C, Kane G, Safford R, Blackshear J, Ung R, et al. High-risk echocardiographic features predict mortality in pulmonary arterial hypertension. Am Heart J. 2017;189:167–76.
Fenstad ER, Le RJ, Sinak LJ, Maradit-Kremers H, Ammash NM, Ayalew AM, et al. Pericardial effusions in pulmonary arterial hypertension: characteristics, prognosis, and role of drainage. Chest. 2013;144(5):1530–8.
Hemnes AR, Gaine SP, Wiener CM. Poor outcomes associated with drainage of pericardial effusions in patients with pulmonary arterial hypertension. South Med J. 2008;101(5):490–4.
Benza R, Biederman R, Murali S, Gupta H. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;52(21):1683–92.
Benza RL, Raina A, Gupta H, Murali S, Burden A, Zastrow MS, et al. Bosentan-based, treat-to-target therapy in patients with pulmonary arterial hypertension: results from the COMPASS-3 study. Pulm Circ. 2018;8(1):2045893217741480.
Grapsa J, O'Regan DP, Pavlopoulos H, Durighel G, Dawson D, Nihoyannopoulos P. Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: comparison with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2010;11(1):64–73.
Peacock AJ, Vonk NA. Cardiac magnetic resonance imaging in pulmonary arterial hypertension. Eur Respir Rev. 2013;22(130):526–34.
van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58(24):2511–9.
Peacock AJ, Crawley S, McLure L, Blyth KG, Vizza CD, Poscia R, et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging. 2014;7(1):107–14.
Baggen VJ, Leiner T, Post MC, van Dijk AP, Roos-Hesselink JW, Boersma E, et al. Cardiac magnetic resonance findings predicting mortality in patients with pulmonary arterial hypertension: a systematic review and meta-analysis. Eur Radiol. 2016;26(11):3771–80.
Leuchte HH, El Nounou M, Tuerpe JC, Hartmann B, Baumgartner RA, Vogeser M, et al. N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest. 2007;131(2):402–9.
Chakinala MM, Coyne DW, Benza RL, Frost AE, McGoon MD, Hartline BK, et al. Impact of declining renal function on outcomes in pulmonary arterial hypertension: a REVEAL registry analysis. J Heart Lung Transplant. 2018;37(6):696–705.
Frantz RP, Farber HW, Badesch DB, Elliott CG, Frost AE, McGoon MD, et al. Baseline and serial brain natriuretic peptide level predicts 5-year overall survival in patients with pulmonary arterial hypertension: data from the REVEAL registry. Chest. 2018;154(1):126–35.
Heresi GA, Tang WH, Aytekin M, Hammel J, Hazen SL, Dweik RA. Sensitive cardiac troponin I predicts poor outcomes in pulmonary arterial hypertension. Eur Respir J. 2012;39(4):939–44.
Schuuring MJ, van Riel AC, Vis JC, Duffels MG, van Straalen JP, Boekholdt SM, et al. High-sensitivity troponin T is associated with poor outcome in adults with pulmonary arterial hypertension due to congenital heart disease. Congenit Heart Dis. 2013;8(6):520–6.
Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177(12):1364–9.
Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant. 2011;30(9):982–9.
Dhaun N, Vachiery JL, Benza RL, Naeije R, Hwang LJ, Liu X, et al. Endothelin antagonism and uric acid levels in pulmonary arterial hypertension: clinical associations. J Heart Lung Transplant. 2014;33(5):521–7.
Quarck R, Nawrot T, Meyns B, Delcroix M. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53(14):1211–8.
Lannan KL, Phipps RP, White RJ. Thrombosis, platelets, microparticles and PAH: more than a clot. Drug Discov Today. 2014;19(8):1230–5.
Belly MJ, Tiede H, Morty RE, Schulz R, Voswinckel R, Tanislav C, et al. HbA1c in pulmonary arterial hypertension: a marker of prognostic relevance? J Heart Lung Transplant. 2012;31(10):1109–14.
Sandoval J, Bauerle O, Palomar A, Gomez A, Martinez-Guerra ML, Beltran M, et al. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation. 1994;89(4):1733–44.
•• Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019; Most recent publication in the field of PAH risk stratification with the updated risk calculator.
Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, Westerkamp V, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;39(3):589–96.
Weatherald J, Boucly A, Chemla D, Savale L, Peng M, Jevnikar M, et al. Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation. 2018;137(7):693–704.
Galie N, Jansa P, Pulido T, Channick RN, Delcroix M, Ghofrani HA, et al. SERAPHIN haemodynamic substudy: the effect of the dual endothelin receptor antagonist macitentan on haemodynamic parameters and NT-proBNP levels and their association with disease progression in patients with pulmonary arterial hypertension. Eur Heart J. 2017;38(15):1147–55.
Sinning C, Harbaum L, Schrage B, Rubsamen N, Magnussen C, Waschki B, et al. Right ventricular index for risk stratification of patients with pulmonary arterial hypertension. Respiration. 2018;96(3):249–58.
Chandra S, Shah SJ, Thenappan T, Archer SL, Rich S, Gomberg-Maitland M. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J Heart Lung Transplant. 2010;29(2):181–7.
van der Lee I, Zanen P, Grutters JC, Snijder RJ, van den Bosch JM. Diffusing capacity for nitric oxide and carbon monoxide in patients with diffuse parenchymal lung disease and pulmonary arterial hypertension. Chest. 2006;129(2):378–83.
Burger CD, Long PK, Shah MR, McGoon MD, Miller DP, Romero AJ, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest. 2014;146(5):1263–73.
Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest. 2013;144(5):1521–9.
McLaughlin VV, Hoeper MM, Channick RN, Chin KM, Delcroix M, Gaine S, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol. 2018;71(7):752–63.
Lee W-TN, Ling Y, Sheares K, Pepke-Zaba J, Peacock AJ, Johnson MK. Predicting survival in pulmonary arterial hypertension in the United Kingdom. Eur Respir J. 2012:erj01966–2011.
Benza RL, Miller DP, Foreman AJ, Frost AE, Badesch DB, Benton WW, et al. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant. 2015;34(3):356–61.
Benza RL EC, Farber HW, Foreman AJ, Frost AE, Gomberg-Maitland M, McGoon MD, Pasta DJ, Selej M, Frantz RP. American Thoracic Society. Updated Risk Score Calculator For Patients With Pulmonary Arterial Hypertension (PAH) In The Registry To Evaluate Early And Long-Term PAH Disease Management (REVEAL). The Journal of Respiratory and Critical Care Medicine. 2017;In C106. Poster Discussion Session at the Annual Meeting of the American Thoracic Society:A6899-A.
Kylhammar D, Kjellstrom B, Hjalmarsson C, Jansson K, Nisell M, Soderberg S, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–81.
Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, et al. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354–62.
Sitbon O, Benza RL, Badesch DB, Barst RJ, Elliott CG, Gressin V, et al. Validation of two predictive models for survival in pulmonary arterial hypertension. Eur Respir J. 2015;46(1):152–64.
Kane GC, Maradit-Kremers H, Slusser JP, Scott CG, Frantz RP, McGoon MD. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest. 2011;139(6):1285–93.
Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40(3):596–603.
Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186(8):790–6.
Weatherald J, Boucly A, Sahay S, Humbert M, Sitbon O. The low-risk profile in pulmonary arterial hypertension. Time for a paradigm shift to goal-oriented clinical trial endpoints? Am J Respir Crit Care Med. 2018;197(7):860–8.
Hoeper MM, Pittrow D, Opitz C, Gibbs JSR, Rosenkranz S, Grunig E, et al. Risk assessment in pulmonary arterial hypertension. Eur Respir J. 2018;51(3).
Kanwar M, Lohmueller L, Correa P, Kraisangka J, Druzdzel M, Antaki J, et al. Application of Bayesian model to predict outcomes in pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37(4):S207.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. KANWAR reports grants from NIH and personal fees from Bayer Inc., outside the submitted work; Dr. Raina reports personal fees and non-financial support from Actelion, personal fees and non-financial support from United Therapeutics, personal fees from Bayer, non-financial support from Liquidia, and non-financial support from Bellerophon, outside the submitted work. Drs. Lohmueller, Kraisangka, and Benza declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pulmonary Hypertension
Rights and permissions
About this article
Cite this article
Kanwar, M., Raina, A., Lohmueller, L. et al. The Use of Risk Assessment Tools and Prognostic Scores in Managing Patients with Pulmonary Arterial Hypertension. Curr Hypertens Rep 21, 45 (2019). https://doi.org/10.1007/s11906-019-0950-y
Published:
DOI: https://doi.org/10.1007/s11906-019-0950-y