Abstract
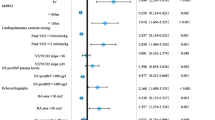
Which combination of clinical parameters improves the prediction of prognosis in patients with pulmonary arterial hypertension (PAH) remains unclear. We examined whether combined assessment of pulmonary vascular resistance and right ventricular function by echocardiography is useful for classifying risks in PAH. In 41 consecutive patients with PAH (mean age of 48.9 ± 17.3 years, 31 females), a 6-min walk test, pulmonary function test, and echocardiography were performed at baseline and during PAH-specific therapies. The study endpoint was defined as a composite of cardiovascular death and hospitalization for PAH and/or right ventricular failure. During a follow-up period of 9.2 ± 8.7 months, 18 patients reached the endpoint. Multivariate regression analysis showed that the ratio of tricuspid regurgitation pressure gradient to the time–velocity integral of the right ventricular outflow tract (TRPG/TVI) and tricuspid annular plane systolic excursion (TAPSE) during PAH-specific treatment were independent prognostic predictors of the endpoint. Using cutoff values indicated by receiver operating characteristic analysis, the patients were divided into four subsets. Multivariate analyses by Cox's proportional hazards model adjusted for age, sex and body mass index indicated that subset 4 (TRPG/TVI ≥ 3.89 and TAPSE ≤ 18.9 mm) had a significantly higher event risk than did subset 1 (TRPG/TVI < 3.89 and TAPSE > 18.9 mm): HR = 25.49, 95% CI 4.70–476.97, p < 0.0001. Combined assessment of TRPG/TVI and TAPSE during adequate PAH-specific therapies enables classification of risks for death and/or progressive right heart failure in PAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While specific therapies for pulmonary arterial hypertension (PAH) have been considerably improved in the past decade [1], prognosis of PAH still remains dismal and difficult to predict. It is becoming clear that no single parameter can fulfill the role of a reliable prognostic indicator in PAH [2]. Although risk score calculators by composite predictive parameters have been proposed on the basis of results of large-scale registry studies [3], the prognostic equations have not been sufficiently validated as clinically relevant predictors that are associated with long-term outcome. Echocardiography is routinely used for screening and diagnosis of PAH, with systolic pulmonary arterial pressure being estimated from the peak tricuspid regurgitation pressure gradient (TRPG) and right atrial pressure [4]. However, correlations between hemodynamic data estimated by TRPG and those measured by right heart catheterization (RHC) are not strong [5], and thus the utility of TRPG in definitive diagnosis and follow-up of patients with PAH is limited [6].
The right ventricle (RV) initially compensates for an increase in pulmonary vascular resistance (PVR) through adaptive hypertrophy and remodeling. However, RV dysfunction eventually occurs when RV pressure overload is sustained for a prolonged period, though there is a significant variability in RV adaptation to chronic pressure overload [7]. In light of the natural history of PAH, an approach to a better classification of event risks in PAH is combined assessment of PVR and functional reserve in the RV. We hypothesized that the hemodynamic status of PAH could be classified into four subsets with different event risks, like subsets in the Forrester classification of acute heart failure, if we could find appropriate cutoff values of indices of PVR and RV function. Lately, it has been reported that the ratio of TRPG to the time-velocity integral of the right ventricular outflow tract (TVI) correlates well with PVR and is a possible surrogate marker of long-term outcome [8]. Tricuspid annular plane systolic excursion (TAPSE) is a simple and reproducible parameter of RV systolic function and has been recognized to have important prognostic implications for PAH patients [9, 10]. Hence, in this study, we examined whether serial assessment of RV function and PVR by echocardiographic methods is useful for characterization of cardiopulmonary hemodynamic status and for risk classification in PAH patients who undergo adequate PAH-specific therapies.
Methods
The present study was approved by the institutional review board of Sapporo Medical University Hospital (approval number, 292-7) and conducted in accordance with the World Medical Association Declaration of Helsinki.
Patient population
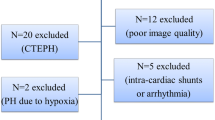
Of patients referred to Sapporo Medical University Hospital between November 2009 and December 2017 for evaluation of suspected pulmonary hypertension, 124 patients were diagnosed as PAH based on RHC data for mean pulmonary arterial pressure ≥ 25 mmHg and pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg at rest and were enrolled in this study. We excluded 83 patients based on exclusion criteria: (1) congenital systemic-to-pulmonary shunts, (2) the presence of other causes of pre-capillary pulmonary hypertension such as pulmonary hypertension due to lung diseases, chronic thromboembolic pulmonary hypertension or other rare diseases, (3) post-capillary pulmonary hypertension defined as mean pulmonary arterial pressure ≥ 25 mmHg at rest and PAWP > 15 mmHg and (4) poor acoustic window in echocardiographic examination. The rationale for exclusion of post-capillary pulmonary hypertension was possible overestimation of PVR by TRPG/TVI because of increased TRPG being attributable to high PAWP rather than high transpulmonary pressure gradient. After the exclusion, 41 patients with PAH (mean age of 48.9 ± 17.3 years, 31 females) contributed to the present analyses (Fig. 1).
All of the patients underwent baseline laboratory blood tests (routine hematology, serum biochemistry, and biomarkers) and echocardiography. A 6-min walk distance test (6MWT) was performed in 31 patients with tolerable exercise capacity. Diffusing capacity for carbon monoxide (DLCO) was measured in 30 patients available for a pulmonary function test. Data for blood parameters, 6MWT, %DLCO, and echocardiographic parameters were collected again at a stable clinical condition under a sufficient period of PAH-specific treatment (under-Tx measurements). A timing of under-Tx measurements was defined as the time point when regular outpatient treatment with optimal dose adjustment was achieved after a sufficient period of specific PAH-targeted treatments, including upfront/sequential triple combination therapies and epoprostenol infusion (Table 2). The optimal dose adjustment was based on clinical symptoms consistent with clinical deterioration or the occurrence of adverse events, exercise capacity and hemodynamic measurements in accordance with 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension [2]. After collection of data for under-Tx measurements, all 41 patients were further followed on a regular basis by outpatients’ visits (Fig. 1). The mean follow-up period for evaluation of the prognostic factors after under-Tx measurements was 9.2 ± 8.7 months.
Echocardiography
Transthoracic echocardiography was performed in accordance with the American Society of Echocardiography [4] and the European Association of Echocardiography recommendations [11], using Vivid E9 or Vivid 7 (GE Healthcare, Tokyo, Japan) with an M5S transducer.
Left ventricular (LV) end-diastolic and end-systolic dimensions were determined in the parasternal long axis view. LV ejection fraction (LVEF) was calculated using the modified Simpson’s biplane method. The LV eccentricity index was calculated as 100 × D2/D1, where D2 is the minor-axis dimension of the left ventricle parallel to the interventricular septum and D1 is the minor-axis dimension perpendicular to and bisecting the interventricular septum, and used as an index of septal geometric abnormality caused by RV diastolic pressure overload. An abnormal value for the LV eccentricity index is considered to be more than 100% in accordance with the recommendation of the American Society of Echocardiography [4]. Transmitral flow velocities were determined by pulsed-wave Doppler echocardiography, and mitral flow parameters, including early ventricular filling velocity (E) and late ventricular filling velocity (A), were measured and the E/A ratio was calculated. A tissue Doppler echocardiogram from the apical four-chamber view was recorded. A sample volume was placed at the medial annulus in the apical four-chamber view, and peak myocardial velocity during early diastole (e’) was measured and E/e’ was calculated. TRPG was calculated by applying the simplified Bernoulli equation. Right atrial area at end systole was measured in the apical four-chamber view. RV outflow Doppler was obtained to measure TVI. The ratio of TRPG to TVI of the RV outflow tract was then calculated as a reliable estimation of PVR, as reported previously [8]. As an index of systolic function of the RV, TAPSE was measured in M-mode tracing obtained in the apical four-chamber view.
Clinical endpoints
Follow-up data were retrospectively collected from medical charts. The clinical endpoint was defined as a composite of cardiovascular death and hospitalization for PAH and/or RV failure. The composite endpoint was used since cardiac death alone is unlikely to fully reflect prognosis in the present patient population, which is composed of heterogeneous groups with regard to causative conditions.
Statistical analysis
Continuous variables are summarized by mean values ± SD, and categorical variables are shown as absolute counts and percentages. Mean values at baseline and under-Tx measurements were compared using the paired t test. Categorical variables were analyzed using the Chi-square test and comparison of two proportions was used when appropriate. Time to an adverse event was calculated as the interval from the date of echocardiography to the event. p values < 0.05 were considered statistically significant. Hazard ratios (HRs) for the clinical endpoint were estimated by Cox proportional hazard analysis. Variables that were significantly associated with the event in univariate analyses were incorporated in multivariate Cox models. A receiver operating characteristic (ROC) curve was used to determine cutoff values of TRPG/TVI and TAPSE. The difference in event-free survival curves between the groups was tested by the Kaplan–Meier method and log-rank test. The difference in multiple comparison procedures was tested by Bonferroni correction. All statistical analyses were performed using JMP software (version 13, SAS Institute, Cary, NC, USA).
Results
Clinical characteristics of patients
Table 1 summarizes the clinical features and baseline hemodynamics measured by RHC in the study population. The patient group consisted of 23 patients with idiopathic or heritable PAH, 16 with connective tissue disease including 5 with systemic sclerosis, and 2 with other causes of PAH. The mean duration between RHC and baseline echocardiography was 0.3 ± 6.5 months. The mean interval between baseline measurement and under-Tx measurement was 22.1 ± 19.9 months.
Comparison of responses to PAH therapies in patients with and those without adverse events
During a mean follow-up period of 9.2 ± 8.7 (range 0.2–30.2) months (Fig. 1), 4 patients died of cardiac causes and 14 patients were hospitalized for progression of heart failure. Mean follow-up periods in patients with and those without adverse events were 9.4 ± 8.9 and 9.2 ± 9.1 months, respectively. As shown in Table 2, at the time of collection of data from baseline measurements, nine patients had received a prostaglandin I2 analogue including an oral selective prostacyclin-receptor agonist, seven patients had received an endothelin receptor antagonist, five patients had received a phosphodiesterase 5 inhibitor, and three patients had received triple combination therapies. During the PAH-specific treatment period, all of the 41 patients received additional PAH-specific therapies with epoprostenol, a selective prostacyclin-receptor agonist, an endothelin receptor antagonist, a phosphodiesterase 5 inhibitor, a soluble guanylate cyclase stimulator, or triple combination therapies.
Clinical characteristics in patients who developed adverse events and patients who were event-free are presented in Table 2. In patients with adverse events, diastolic systemic blood pressure was significantly increased after PH-specific treatments compared with that at baseline. In patients without adverse events, heart rate and levels of serum uric acid and brain natriuretic peptide were significantly reduced after PH-specific treatments. Favorable changes were also observed in echocardiographic parameters after PAH-specific treatments in patients without adverse events; E/e’, TRPG, TRPG/TVI, and RA area were significantly reduced and E/A and TAPSE were significantly increased.
Relationships of clinical and echocardiographic parameters with adverse events
Univariate Cox proportional hazard risk analysis was performed to identify variables that predict the clinical composite endpoint in patients with PAH. As shown in Table 3, hazard risk for the endpoint was significantly higher for E/e’ at baseline measurements and TRPG, TVI, TRPG/TVI, and TAPSE at under-Tx measurements. Of those parameters, E/e’ at baseline measurements and TRPG/TVI and TAPSE at under-Tx measurements were examined by multivariate analysis for independent associations with the composite endpoint. As shown in Table 4, age, female gender and TAPSE at under-TX measurements were independently associated with adverse events. TRPG/TVI at under-Tx measurements was not selected as an independent predictor of the endpoint due to the modest but significant correlation between the two indices (y = − 1.01x + 23.06, R = − 0.493, p = 0.001) as shown in Fig. 2. TRPG/TVI at under-Tx measurements was an independent predictor of adverse events when TAPSE at under-Tx measurements was not included as a dependent factor in multivariate analysis.
Linear regression analysis between TRPG/TVI and TAPSE at under-Tx measurements. There was the modest but significant correlation between TRPG/TVI and TAPSE at under-Tx measurements (y = − 1.01x + 23.06, R = − 0.493, p = 0.001). Under-Tx measurements during PAH-specific treatment, TAPSE tricuspid annular plane systolic excursion, TRPG peak tricuspid regurgitation pressure gradient, TVI time velocity integral of right ventricular outflow tract
Classification of adverse event risk by TRPG/TVI and TAPSE
Since the results of multivariate analysis (Table 4) suggested that TRPG/TVI and TAPSE during PAH-specific therapies are predictors of adverse events in PAH patients, ROC analysis was performed to determine optimal cutoff values of the two indices for identifying a high-risk group. As shown in Fig. 3, 3.89 for TRPG/TVI at under-Tx measurements and 18.9 mm for TAPSE at under-Tx measurements were selected as cutoff values with the maximum area under the curve. Figure 4 shows the relationships between TRPG/TVI and TAPSE at under-Tx measurements and the distributions of patients with and those without adverse events. By use of the cutoff values, patients could be divided into four subsets: a group with TRPG/TVI < 3.89 and TAPSE > 18.9 mm (n = 16, subset 1), a group with TRPG/TVI ≥ 3.89 and TAPSE > 18.9 mm (n = 4, subset 2), a group with TRPG/TVI < 3.89 and TAPSE ≤ 18.9 mm (n = 7, subset 3), and a group with TRPG/TVI ≥ 3.89 and TAPSE ≤ 18.9 mm (n = 14, subset 4).
Receiver operating characteristic analysis to determine optimal cutoff values for identifying subjects at risk for the composite endpoint. Cutoff values with maximum area under the curve were 3.89 for TRPG/TVI at under-Tx measurements (a) and 18.9 mm for TAPSE (b) at under-Tx measurements. Under-Tx measurements during PAH-specific treatment, TAPSE tricuspid annular plane systolic excursion, TRPG peak tricuspid regurgitation pressure gradient, TVI time velocity integral of right ventricular outflow tract
Relationships between TRPG/TVI and TAPSE at under-Tx measurements. Patients with PAH were divided into four subsets based on cutoff levels of TRPG/TVI and TAPSE at under-Tx measurements (dotted line showing each cutoff level). Patients with adverse events and those without adverse events are shown by solid circles and open circles, respectively. Under-Tx measurements during PAH-specific treatment, TAPSE tricuspid annular plane systolic excursion, TRPG peak tricuspid regurgitation pressure gradient, TVI time velocity integral of right ventricular outflow tract
The clinical characteristics in patients at under-Tx measurements in each of the four subsets are shown in Table 5. Patients in the subset 4 had smaller body mass index than those in the subsets 1 and 3 and had higher brain natriuretic peptide level than that in subset 1 and 2, though the differences were not statistically significant. Additionally, there were no significant differences in the prevalence of idiopathic/heritable PAH, epoprostenol use and triple combination therapy among the four subsets.
Figure 5 shows results of Kaplan–Meier analysis of event-free survival in the four subsets. There was a statistically significant difference between the four event-free curves (log-rank test, χ2 = 18.8, p < 0.001). Multiple comparison using Bonferroni correction (p values < 0.017 being considered statistically significant) indicated that the curve of subset 1 was significantly different from that of subset 4 (p < 0.001). As shown in Table 6, multivariate analyses by Cox's proportional hazards model adjusted for age, sex and body mass index indicated that subset 4 had a significantly higher event risk than did subset 1 (HR = 25.49, 95% CI 4.70–476.97, p < 0.0001).
Discussion
The results of this study indicated that increased TRPG/TVI and reduced TAPSE in PAH patients who underwent specific PAH-targeted medical treatments for a sufficient period of time are associated with significantly increased risk of cardiac death and unscheduled hospitalization. The findings support the notion that combined assessment of the noninvasive measures, TRPG/TVI and TAPSE, enables risk classification and provides useful information for treatment of PAH.
The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) expert consensus document on pulmonary hypertension recommends PVR as a robust diagnostic criterion for PAH [12]. The fundamental pathophysiological abnormality underlying PAH is progressive vascular remodeling and obliteration of the peripheral pulmonary arterial vasculature. Even in the pre-symptomatic stage of PAH (WHO functional class 1), PVR is substantially above normal through latent pulmonary vascular remodeling. In patients who have a slight limitation of activity (WHO functional class 2), increases in PVR do not affect cardiac output because RV function is compensated by hypertrophy. However, severely increased PVR and the simultaneous fall in pulmonary arterial pressure and cardiac output due to decompensation of RV function are observed in PAH patients at an advanced stage (WHO functional class 3 or 4) [13, 14]. Earlier studies [15, 16], mostly using invasive measures to assess RV function, showed that impaired RV wall contractility in PAH is an independent predictor of survival. Recently there are several reports regarding the efficacy of RV free wall strain for predicting long-term outcome in PAH patients [17, 18]. However, measurement of RV free wall strain still requires sufficient quality of images using high performance equipment, and thus it is less feasible than TAPSE in routine clinical use. Although TAPSE is influenced by several factors including overall motion of the heart, LV systolic function, and RV loading conditions [18], it is simple, practical, reproducible and more suitable for repetitive assessment for RV function for long-term follow-up. TRPG/TVI and TAPSE were reported to be correlated well with invasive PVR [8] and with angiographic or magnetic resonance imaging-derived RV ejection fraction [4], respectively. Based on these findings, we selected TRPG/TVI and TAPSE for combined assessment of PVR and RV function in patients with PAH. Noninvasive evaluation using echocardiography has the potential advantage of having excellent feasibility for serial assessment of disease progression compared with invasive measures.
Previous studies [19] have shown that the inverse correlation between RV function and PVR is significant but modest, as was indicated by data shown in Fig. 2. The weak correlation between TRPG/VTI and TAPSE can be explained by the variability of RV adaptation to chronic pressure overload [13, 14], especially according to causative conditions. Patients with systemic sclerosis-associated PAH exhibited lower RV contractility than that in idiopathic PAH patients, whereas PVR was not significantly different [15]. Experimental and clinical investigations have demonstrated that impaired RV wall contractility is associated with multiple processes, including chronic volume overload [20], fibrosis of the RV wall [21], capillary rarefaction [22], RV remodeling [23], and RV–LV interdependence [24, 25], as well as chronic pressure overload. These findings indicate that not only increased afterload but also other factors are involved in adaptation and decompensation of RV function in PAH.
Since increased PVR and reduced RV functions have been suggested to have distinct roles in the natural history of PAH [13, 14, 19], we postulated that the prognosis-related hemodynamic status of PAH can be classified into four subsets by use of cutoff values of TRPG/TVI and TAPSE, like the Forrester classification of acute heart failure. This notion was supported by the results shown in Figs. 4 and 5. The most interesting characteristic shown in Fig. 4 is that patients with low TAPSE and high TRPG/TVI (subset 4) are at the highest risk for adverse events. It seems that a reduction in RV contractile performance is closely associated with poor prognosis even if it was independent of decompensation in response to chronic pressure overload. Possibly, additional information by a surrogate marker of RV wall contractility can supplement what PVR-based prediction cannot provide. The clinical characteristics of patient in the subset 4 were not significantly different from those in the other subsets (Table 5), indicating importance of TRPG/TVI and TAPSE assessment for detection of this high-risk group of patients. Subset 3 (low TAPSE and low TRPG/TVI) had moderate risk of cardiac death and unscheduled hospitalization in spite of normal or slightly elevated PVR. This finding suggests that the prognostic value of subset 3 is determined by a variability of RV adaptation to chronic pressure overload. In fact, RV dysfunction has been shown to occasionally progress even when increase of PVR is preserved within tolerate range [7, 14]. Although individual discriminative capacity of TRPG/TVI or TAPSE for a single parameter was moderate (AUCs = 0.821 and 0.863, respectively) (Fig. 3), a point we tried to make is that a combination of the not very strong predictors is useful for characterization of hemodynamic abnormalities (Fig. 4) and for assessment of prognosis (Fig. 5). Classification of PAH into four subsets appears to be useful for both risk classification and selection of a therapeutic strategy in PAH.
The results of the present study show that abnormal values for indices of PVR and RV systolic function are predictors of adverse events during long-term follow-up only when they are assessed after PAH-specific therapies have been performed for a sufficient period (Table 3). The pathogenesis of PAH is multifactorial, involving reversible functional changes and irreversible structural abnormalities. The progressive increase in PVR is attributed to a combination of reversible conditions such as vasoconstriction and unchangeable alterations such as the development of plexiform lesions. Similarly, the key determinant of RV dysfunction is complex interaction between reactive adaptation for excessive afterload and irreversible myocardial degeneration. Since the clinical efficiency of PAH-specific therapies depends on the reversibility of various components that comprise the increasing severity of PAH, it is important for accurate determination of the prognosis to assess the response to PAH-specific therapies at appropriate timing. Recent guidelines recommend that judgments regarding the severity of pulmonary hypertension and the treatment response be made on the basis of regular follow-up evaluations every 3–6 months [2]. To accurately assess the severity and prognosis of PAH, a single clinical index assessed once soon after the start of therapies is insufficient. Rather, it is important to observe the changes over time during the course while providing appropriate combination therapies for a sufficiently long period, and noninvasive echocardiography may contribute greatly to the observation of changes over time.
Despite significant therapeutic advances and identification of reasonable goals of therapies, outcomes of PAH patients are still suboptimal. Physician reluctance to proceed to the most aggressive therapies seems to be a contributing factor [2]. Follow-up of pathophysiological abnormality is essential for assessing response to treatment and achieving the goals with optimal application of current therapies. Serial monitoring of RV function and PVR by echocardiography is of crucial importance for evaluating the severity of hemodynamic function and deciding the therapeutic strategy for PAH from early to advanced stages.
Study limitations
There are limitations in the present study. First, since the size of the study population was small and the observation period was relatively short, statistical power might have been insufficient for characterizing all echocardiographic parameters. Second, a substantial limitation of this study is the population heterogeneity based on inclusion of patients with various types of PAH. The specific event rates for individual groups according to various causative conditions or different natural histories were not assessed because of the small population size. Nevertheless, there are few published data indicating that prognosis predictors differ in different PAH subgroups, and thus it is difficult to address this issue, as described in the guidelines [2]. On the other hand, inclusion of patients with various prognoses was required to determine the predictive power of combined assessment of TRPG/TVI and TAPSE. In other words, inclusion of heterogeneous subject groups is advantageous for testing whether analysis of the TRPG/TVI-TAPSE relationship is applicable for all patients with PAH. Third, treatments during the follow-up period after echocardiographic assessment differed in the patients with various underlying causes. After collection of data for under-Tx measurements in this study, some of the patients additionally received treatments according to a goal-oriented therapies strategy, including triple combination therapies and optimal dosage of epoprostenol. Thus, the possibility that inter-group differences in PAH-specific medications modified event rates in the four subsets with different patterns of TRPG/VTI and TAPSE values cannot be excluded. Further investigation will be needed to validate specific cutoff values of TAPSE and TRPG/TVI for prognostic predictors in various types of PAH both before and during treatment.
Conclusion
The results of the present study indicate that changes in PVR and RV function in PAH take different patterns depending on patients and that both increased PVR and reduced RV function contribute to adverse events in PAH patients who have undergone specific PAH-targeted medical treatments for a sufficient period of time. Serial monitoring of RV function and PVR by echocardiographic methods is a feasible and reasonable measure for monitoring disease progression and assessing prognosis in PAH patients. Whether risk classification by a pattern of the TRPG/TVI-TAPSE relationship is valid in all PAH patients remains to be further investigated.
References
Ogawa A, Satoh T, Tamura Y, Fukuda K, Matsubara H (2017) Survival of Japanese patients with idiopathic/heritable pulmonary arterial hypertension. Am J Cardiol 119:1479–1484
Galiè N, Humbert M, Vachiery JL, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk-Noordegraaf A, Beghetti M, Ghofrani A, Gomez-Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology [ESC] and the European Respiratory Society [ERS]. Eur Heart J 37:67–119
Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD (2012) The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 141:354–362
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography, endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 23:685–713
Farber HW, Foreman AJ, Miller DP, McGoon MD (2011) REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 17:56–63
Wright LM, Dwyer N, Celermajer D, Kritharides L, Marwick TH (2016) Follow-up of pulmonary hypertension with echocardiography. JACC Cardiovasc Imaging 9:733–746
Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM (2013) Right heart adaptation to pulmonary arterial hypertension. Physiology and pathobiology. J Am Coll Cardiol 62:D22–33
Kouzu H, Nakatani S, Kyotani S, Kanzaki H, Nakanishi N, Kitakaze M (2009) Noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. Am J Cardiol. 103:872–876
Mazurek JA, Vaidya A, Mathai SC, Roberts JD, Forfia PR (2017) Follow-up tricuspid annular plane systolic excursion predicts survival in pulmonary arterial hypertension. Pulm Circ 7:361–371
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM (2006) Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 174:1034–1041
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–271
McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53:1573–1619
Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J (2012) RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovas Imaging 5:378–387
van de Veerdonk MC, Marcus JT, Westerhof N, de Man FS, Boostra A, Heymans MW, Bogaard HJ, Vonk-Noordegraaf A (2015) Signs of right ventricular deterioration in clinically stable patient with pulmonary arterial hypertension. Chest 147:1063–1071
Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, Boyce D, Kelemen BW, Bacher AC, Shah AA, Hummers LK, Wigley FM, Russell SD, Saggar R, Maughan WL, Hassoun PM, Kass DA (2013) Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Failure 6:953–963
Campo A, Mathai SC, Pavec JL, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, Girgis RE, Hassoun PM (2010) Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 182:252–260
da Costa-Junior AA, Ota-Arakaki JS, Ramos RP, Uellendahl M, Mancuso FJN, Gil MA, Fischer CH, Moises VA, de Camargo-Carvalho AC, Campos O (2017) Diagnostic and prognostic value of right ventricular strain in patients with pulmonary arterial hypertension and relatively preserved functional capacity studied with echocardiography and magnetic resonance. Int J Cardiovasc Imaging 33:39–46
van Kessel M, Seaton D, Chan J, Yamada A, Kermeen F, Butler T, Sabapathy S, Morris N (2016) Prognostic value of right ventricular free wall strain in pulmonary hypertension patients with pseudo-normalized tricuspid annular plane systolic excursion values. Int J Cardiovasc Imaging 32:905–912
Kawut SM, Al-Naamani N, Agerstrand C, Rosenzweig EB, Rowan C, Barst RJ, Bergmann S, Horn EM (2009) Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest 135:752–759
Szabó G, Soós P, Bährle S, Radovits T, Weigang E, Kekesi V, Merkely B, Hagi S (2006) Adaptation of the right ventricle to an increased afterload in the chronically volume overloaded heart. Ann Thorac Surg 82:989–995
Shehata ML, Lossnitzer D, Skrok J, Boyce D, Lechtzin N, Mathai SC, Girgis RE, Osman N, Lima JA, Bluemke DA, Hassoun PM, Vogel-Claussen J (2011) Myocardial delayed enhancement in pulmonary hypertension: pulmonary hemodynamics, right ventricular function, and remodeling. Am J Roentgenol 196:87–94
Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF (2009) Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 120:1951–1960
Grapsa J, Gibbs JS, Dawson D, Watson G, Patni R, Athanasiou T, Punjabi PP, Howard LS, Nihonyannopoulos P (2012) Morphologic and functional remodeling of the right ventricle in pulmonary hypertension by real time three dimensional echocardiography. Am J Cardiol 109:906–913
Ito M, Kodama M, Kashimura T, Obata H, Mitsuma W, Hirono S, Tomita M, Ohno Y, Tanabe N, Aizawa Y (2012) Comparison of patients with pulmonary arterial hypertension with versus without right-sided mechanical alternans. Am J Cardiol 109:428–431
Amano H, Toyoda S, Arikawa T, Inami S, Otani N, Nishi Y, Kitagawa Y, Taguchi I, Abe S, Inoue T (2013) Left ventricular function in pulmonary hypertension. Heart Vessels 28:505–509
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawamukai, M., Hashimoto, A., Koyama, M. et al. Risk classification of pulmonary arterial hypertension by echocardiographic combined assessment of pulmonary vascular resistance and right ventricular function. Heart Vessels 34, 1789–1800 (2019). https://doi.org/10.1007/s00380-019-01429-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01429-7