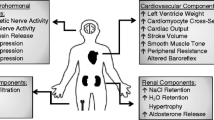
Abstract
Purpose of Review
We review the known mechanisms of sodium-sensitive hypertension in the metabolic syndrome with a focus on preclinical models, differences between these models, and methodological limitations. We also identify future directions for a better understanding and treatment of this common condition.
Recent Findings
Rigorous methodologies to measure blood pressure in preclinical models may clarify some of the inconsistencies in the literature. Renal, neural, hormonal, and cardiovascular systems are dysregulated and contribute to elevated blood pressure. Local renin-angiotensin systems enhance systemic hormone signaling to increase blood pressure.
Summary
Since the original description of metabolic syndrome, investigators from many fields have contributed to an increasingly complex and mechanistic understanding of this common condition. These systems integrate to regulate sodium transport in the kidney leading to hypertension and enhanced sodium sensitivity. An array of non-uniform preclinical models are used and support clinical studies to inform which models are pathophysiologically relevant for further mechanistic studies to guide targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
What is the Metabolic Syndrome?
The metabolic syndrome, as defined by the International Diabetes Federation, is a group of factors that includes visceral adiposity, dyslipidemia, and hypertension [1]. The term, metabolic syndrome, was first coined by Haller [2] in 1977, though a clustering of conditions predisposing to atherosclerosis had been noted in the 1920s [3]. In 1988, Reaven [4] posited insulin resistance as the central etiology of the metabolic syndrome, or syndrome X. Today, the metabolic syndrome affects up to one-third of Americans [5].
What is Sodium Sensitivity?
The magnitude of rise or fall in blood pressure with increases or decreases of sodium intake is termed sodium sensitivity and is caused by a primary increase in renal sodium reabsorption. In both humans and animal models, sodium sensitivity is widely variable across individuals. Kawasaki and Bartter demonstrated this wide variation in the first description of sodium sensitivity in humans [6]. Despite this variability, individuals with the metabolic syndrome are more sodium-sensitive than healthy controls.
Epidemiology of Sodium Sensitivity Hypertension in the Metabolic Syndrome
Among factors in the metabolic syndrome, obesity and insulin resistance are independently associated with hypertension. In the GenSalt study, the metabolic syndrome was associated with a ~40% increase in sodium sensitivity compared with controls after multivariate adjustment [7]. In the INTERSALT study [8], which observed epidemiologic trends in blood pressure and sodium intake across communities throughout the world, each 10 kg increase in body weight associated with an average of 3.0 and 2.2 mmHg increase in systolic and diastolic blood pressure, respectively. The Framingham study [9] estimated obesity, or adiposity, as a major predictive factor for almost 75% of individuals with hypertension, and the Trials of Hypertension Prevention study [10] demonstrated dramatic reductions in blood pressure with weight loss. Approximately one-half of patients with essential hypertension have insulin resistance [11]. In turn, obesity and insulin resistance-associated hypertension is more sodium-sensitive than in hypertensive controls without obesity [12].
Sodium-Sensitive Hypertension in the Metabolic Syndrome via Renal Sodium Transport
Based on Guyton’s theory, the final common pathway for all forms of hypertension is decreased renal sodium excretion [13], which expands the blood volume, and increases systemic blood pressure to excrete excess sodium to achieve steady-state. While increased vascular smooth muscle tone may acutely increase blood pressure under many physiologic scenarios, including the metabolic syndrome, compensatory sodium excretion must be impaired to permit this increased blood pressure to persist (i.e. hypertension). Evidence of many endocrine and metabolic pathophysiologic processes have been described in the metabolic syndrome including enhanced general and renal sympathetic nervous system activity [14, 15]; oxidative stress [16, 17] and inflammation [18] in the kidney; insulin activation of renal tubular epithelial cells [19,20,21,22]; and/or activation of the systemic, or local renin-angiotensin systems including enhanced mineralocorticoid action [23,24,25]. The final common pathway for these mechanisms is impaired glomerular filtration rate, or increased tubular sodium reabsorption via expression or post-translational modification of sodium transporters and/or their regulatory components [26].
Despite what we have learned regarding the mechanisms of sodium-sensitive hypertension, major guidelines for blood pressure management do not define specific classes of antihypertensive medications for individuals with the metabolic syndrome [27]. In this review, we will outline the advantages and disadvantages of commonly used preclinical models, highlight the known mechanisms of the sodium-sensitive hypertension associated with parameters of the metabolic syndrome, and discuss current gaps in the literature and future directions in this important field of research.
Preclinical Models of Obesity, Insulin Resistance, and the Metabolic Syndrome
It is important to distinguish obesity and insulin resistance from the metabolic syndrome, although all three are strongly associated with hypertension [8, 28, 29]. The metabolic syndrome represents a specific subset of individuals with insulin resistance, a high, but not universal, prevalence of obesity [30], and dyslipidemia. In the existing literature of preclinical models, these distinct disease states are often used interchangeably. For example, a high fat diet in mice is referred to as a mouse model of the metabolic syndrome and leads to obesity, insulin resistance, sodium-sensitive hypertension, but not the dyslipidemia (elevated triglycerides) seen in humans with the metabolic syndrome per se [31]. While this may be inaccurate, the mechanisms of sodium-sensitive hypertension in obesity, insulin resistance, and the metabolic syndrome are likely shared based on common pathophysiologic features. However, several of the pathways we will discuss may be particularly relevant for a subset of these conditions.
Preclinical Models of Sodium-Sensitive Hypertension in the Metabolic Syndrome
There is little uniformity across species and models (e.g. a high fat-fed dogs vs. high fat-fed C57Bl/6 mice vs. db/db mice) of identified mechanisms of hypertension in the metabolic syndrome. We characterized changes in body weight, plasma insulin, plasma lipid profile, urinary sodium excretion, and blood pressure in C57BL/6 mice fed high fat and/or high fructose diets previously associated with obesity and insulin resistance [32, 33]. While high fat-feeding faithfully recapitulated many features of the metabolic syndrome, high fructose feeding in mice, unlike rats, does not always induce obesity and insulin resistance. Among mouse models of the metabolic syndrome, NZBWF1, and KKAy/a strains develop obesity, insulin resistance, and elevated blood pressure, but the mechanisms are unknown. Leptin receptor-deficient db/db mice develop obesity and insulin resistance, but hypertension is variable [34]. Of note, these mice also develop diabetes with glycosuria and osmotic diuresis [35] that can stimulate compensatory sodium reabsorption, thereby confounding the assessment of sodium excretion and the renin-angiotensin-aldosterone system, a commonly ascribed pathway in hypertension.
Although the majority of transgenic mice are produced on a C57BL/6 background, these mice are well known to be resistant to elevations in blood pressure compared with other mouse strains or with rats [36]. Therefore, small changes in blood pressure may translate to a clinically significant difference in humans. For example, patients with hypertension due to Liddle’s syndrome, i.e., gain of function mutations in subunits of the epithelial sodium channel (ENaC), have an average mean arterial pressure 43 mmHg higher than controls [37]. Transgenic mice on a C57Bl/6 background carrying similar mutations do not have an elevated blood pressure on a normal sodium diet and only a ~10 mmHg increase in mean arterial pressure on a high sodium diet [38]. Similarly, some patients with Gordon’s syndrome, another form of sodium-sensitive hypertension, have systolic blood pressures >200 mmHg [39], but transgenic mice with mutant, inactive with no lysine kinase 4 (WNK4), recapitulating this condition, have a systolic blood pressure <10 mmHg higher than controls, and only 13 mmHg higher than mice overexpressing wild-type WNK4 [40].
We have observed a consistent, reproducible, small increase in blood pressure in high fat-fed mice [31], as well as increased sodium sensitivity [31, 41]. However, as reviewed by Kennedy et al. [42], there are multiple, inconsistent studies of hypertension in mouse models of the metabolic syndrome. Table 1 summarizes experiments comparing high fat or fructose-fed C57BL/6 mice to controls. In chronically high fat-fed mice, blood pressure varied widely from −13 to +37 mmHg [33, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] compared to controls. Blood pressure measurement in mouse models of the metabolic syndrome are confounded by technical considerations such as dietary sodium, phytoestrogen content, and feeding patterns [61].
Feeding patterns will be different between high fat-fed and control groups due to the dramatic difference in the caloric content and, presumably, taste of diets. Differences in food, and therefore sodium, intake can significantly confound the interpretation of blood pressure data. Similarly, blood pressure is acutely regulated by fasting, another potential confounder if diets are particularly unpalatable [62]. Mouse models based on fructose-spiked drinking water also dramatically decrease sodium intake, as the mice will also eat less food.
Multiple groups have validated [63, 64] or invalidated [65, 66] tail-cuff plethysmography. Its low cost and relative simplicity may perpetuate its use, though radiotelemetry systems are generally accepted as the gold standard. A thorough discussion of protocols for blood pressure measurement is beyond the scope of this review, but Van Vliet [61] provides an elegant summary on the justification and protocols for radiotelemetric blood pressure measurement. One limitation of plethysmography in studying blood pressure in models of the metabolic syndrome is the inability to measure pressure in active, unrestrained mice. Obese mice are often less active, and we observed that changes in blood pressure with locomotion are a significant contributor to differences in blood pressure between control and high fat-fed mice. Thus, mouse models, and certainly other species, require standardization to study the mechanisms of hypertension in the metabolic syndrome. Published results should detail dietary constituents, locomotion, and methods of blood pressure measurement.
Mechanisms of Sodium-Sensitive Hypertension in the Metabolic Syndrome
Renal Sodium Reabsorption in the Metabolic Syndrome
Within the kidney, several tubular sodium transporters are potential mediators of the observed impaired sodium excretion. Huang et al. [41] demonstrated that deletion of serum and glucocorticoid kinase 1, a kinase that activates the ENaC, prevented high fat diet-induced hypertension, but this kinase can also modulate sodium transporters proximal to the aldosterone-sensitive distal nephron [67,68,69]. Using microperfusion, we examined sodium transport in isolated cortical collecting ducts, and found no difference in sodium flux between low fat and high fat-fed mice, excluding the ENaC, thiazide-sensitive sodium-driven chloride bicarbonate exchanger, and pendrin as potential mediators. We also excluded ENaC activity as a mechanism for impaired natriuresis and increased blood pressure of high fat feeding as benzamil, a pharmacologic inhibitor of ENaC, had no differential effect on sodium excretion or blood pressure in low fat- vs. high fat-fed mice [31]. Based on these data, high fat feeding likely increases sodium transport in upstream segments of the nephron. In mice, insulin-mediated activation of insulin receptor substrate 2 in proximal tubule cells stimulates sodium reabsorption [70], although these experiments have not been performed in a model of insulin resistance. Davies et al. [71] showed that high fat feeding of mice increases sodium-potassium-2 chloride cotransporter (NKCC2) phosphorylation and decreases AMP kinase activity. Sodium-chloride cotransporter (NCC) expression is increased in obese Zucker rats [48], and its activity is stimulated by insulin infusion in Sprague Dawley rats [72]. These findings provide a basis for future experiments using genetic knockouts or pharmacotherapies to measure the contribution of upstream transporters in mediating the effect of high fat diet on the kidney tubule.
Role of the Sympathetic Nervous System in Sodium-Sensitive Hypertension of the Metabolic Syndrome
Several investigators have demonstrated a role for the systemic and renal sympathetic nervous system in hypertension of the metabolic syndrome. Data from obese individuals [73, 74] and animal models, including carbohydrate- and high fat-fed mice [75], rats [76], and dogs [77, 78] demonstrate increased production of catecholamines and blood pressure sensitivity to vasopressors and alpha adrenergic blockade. Hyperleptinemia, associated with obesity and the metabolic syndrome, permissively stimulates the sympathetic nervous system and increases blood pressure [79, 80], despite selective resistance to its anorexigenic effects. Melanocortin 4 receptors (MC4R) likely mediate leptin-dependent effects on blood pressure. Using a combination of leptin receptor deficient mice and MC4R knockout mice, Rahamouni et al. [81] elegantly demonstrated that both leptin and insulin centrally activate renal sympathetic nerve activity via MC4R. Concordant observations were made using MC4R agonists in mice and humans [81,82,83] and antagonists in mice [84, 85] and spontaneously hypertensive rats [86]. In contrast, melanocortin 4 receptor knockout mice and humans with inactivating mutations develop obesity, hyperinsulinemia, and hyperleptinemia, but not hypertension [87,88,89].
Granger et al. were the first to demonstrate a role for renal nerves in this form of hypertension. For this study, authors demonstrated that high fat feeding in mongrel dogs decreased sodium excretion and increased blood pressure, but that bilateral renal denervation abrogated these effects [90]. The development of clinical renal denervation technology has made possible the targeting of renal nerves. for the treatment of hypertension. In the first reported phase 3 clinical trial, SYMPLICITY III, there was a trend toward improvement in blood pressure in obese vs. non-obese individuals, but further studies are needed to determine if this therapy vs. sham control is more efficacious in individuals with metabolic syndrome-related hypertension [91].
The mechanisms by which obesity and/or insulin resistance-mediated renal sympathetic nerve activity increases renal sodium transport will be an interesting area for future research. Classic studies have demonstrated that renal nerves regulate sodium-hydrogen exchanger 3 [92, 93] and NKCC2 [93, 94] via norepinephrine, renin, angiotensin II, and nitric oxide [95,96,97]. More recently, Ellison and colleagues have shown that norepinephrine activates the NCC [98]. The contribution of sympathetic nerves to activity of specific renal transporters within the metabolic syndrome will be the next frontier in this field.
Role of Oxidative Stress and Inflammation in Sodium-Sensitive Hypertension of the Metabolic Syndrome
Obesity stimulates systemic and renal oxidative stress in mice [17] and rats [16]. By inhibiting nitric oxide signaling, oxidative stress results in endothelial dysfunction, resistance vessel constriction, and increased sodium reabsorption. The sodium-potassium-2 chloride cotransporter [99] is disinhibited by declining nitric oxide, as is oxidative stress-response kinase-1, indirectly activating sodium-potassium-2 chloride cotransporter [100]. Both hypertension and inflammation are reduced with infusion of tempol, a free radical scavenger, in high fat-fed rats [18].
Recent studies by Harrison et al. [101, 102] have implicated TH1 and TH17 cell infiltration in the renal parenchyma as a direct mechanism to enhance both proximal and distal sodium transport. Moreover, Obesity is known to modulate these same lymphocyte subtypes [103]. It remains to be explored to what degree these inflammatory mechanisms contribute to hypertension in the metabolic syndrome.
Role of Insulin in Sodium-Sensitive Hypertension of the Metabolic Syndrome
A compelling case has been made that insulin resistance promotes hypertension in the metabolic syndrome through compensatory hyperinsulinemia. Physiologic concentrations of insulin increase renal sodium reabsorption in rats [104], dogs [105], and humans [106]. Yet, insulin infusion does not increase blood pressure in mice or humans [107, 108]. In addition, genetic causes of obesity, e.g., deletions or mutations in MC4R or leptin, are not associated with hypertension despite insulin resistance and concomitant hyperinsulinemia [34].
Given the robust and long-standing association of insulin and blood pressure, absence of evidence is not evidence of absence. There are several potential reasons for an association between insulin levels and blood pressure without tangible proof of causality. Several investigators have hypothesized that insulin increases renal sodium reabsorption either directly or indirectly due to vasodilation [109]. A natural experiment that could answer this question is the measurement of renal sodium reabsorption with the use of diet-induced insulin resistance (e.g., high fat diet) in vascular endothelial specific insulin receptor knockout mice. However, to our knowledge, this experiment has not been reported.
Another important area of knowledge is the impact of insulin resistance (and the resultant metabolic milieu) on insulin-mediated blood pressure regulation. Deletion of renal tubular insulin receptors have demonstrated a paradoxical increase in blood pressure and decreased nitric oxide production in otherwise insulin-sensitive mice [110, 111], though constitutive deletion that may have alter tubule development in utero [112]. We are currently studying renal tubular insulin receptor knockout mice to address the contribution of insulin signaling in the kidney to hypertension of the metabolic syndrome. Brands and colleagues published compelling data on the role of insulin to stimulate renal sodium transport under conditions of type 1 diabetes mellitus, i.e., hypoinsulinemic hyperglycemia [105]. Whether a putative role for insulin in hypertension requires overt diabetes rather than the metabolic syndrome is currently unknown.
Role of Systemic and Local Renin-Angiotensin Systems in Sodium-Sensitive Hypertension of the Metabolic Syndrome
The renin-angiotensin-aldosterone system plays a pathophysiologic role in hypertension of the metabolic syndrome both directly, by increasing of renal sodium reabsorption, and indirectly, via many of the pathways previously described. In obese mice [113, 114] and humans [115], adipocytes increase their production of angiotensinogen. Genetic deletion of angiotensinogen in adipocytes lowers plasma angiotensin II and systolic blood pressure in mice, and weight loss lowers angiotensin II in humans [116].
Aldosterone, via the mineralocorticoid receptor, may play a critical role in the magnitude of hypertension in the metabolic syndrome. Aldosterone levels correlate with obesity in dogs [117] and humans [118], possibly via adipocyte-derived angiotensinogen or adipocyte-derived mineralocorticoid releasing factors [119]. Drugs inhibiting the mineralocorticoid receptor are effective antihypertensive agents in individuals with the metabolic syndrome [56] independent of the reduction of insulin resistance associated with mineralocorticoid receptor blockade [120]. Moreover, high fat-fed mice do not exhibit elevated aldosterone, and have only mildly elevated blood pressure despite obesity and insulin resistance [31]. Whether mineralocorticoids are needed to more accurately model hypertension of the metabolic syndrome in mice is unknown.
Angiotensin II and aldosterone also influence the vasculature to induce or maintain hypertension, possibly by reducing nitric oxide bioavailability [121]. Activation of the mineralocorticoid receptor in vascular endothelial cells can raise blood pressure prior to a detectable increase in sodium reabsorption [122], and vascular smooth muscle cell-specific mineralocorticoid receptor deletion lowers blood pressure [123].
Local renin-angiotensin systems may play a distinct role in hypertension of the metabolic syndrome. The intrarenal renin-angiotensin system appears to amplify the effect of systemic angiotensin II [124] on sodium transport and blood pressure. Whether ablation of this intrarenal axis is sufficient to ameliorate the hypertension seen in diet-induced or genetic models of the metabolic syndrome is unknown. The local renin-angiotensin system in adipocytes can stimulate the sympathetic nervous system via leptin, or production of aldosterone-releasing factors [119]. Adipocyte-specific knockout of angiotensinogen protects mice from elevated systemic angiotensin II and high blood pressure in a diet-induced model of the metabolic syndrome [113]. Massiera et al. [114] demonstrated that adipocyte-derived angiotensinogen contributes to growth of adipose tissue, and increase of circulating angiotensinogen and systemic blood pressure. Moreover, renin-angiotensin system inhibitors decrease obesity and hyperinsulinemia in male NZO/BL6 F1 rats, a genetic model of insulin resistance [125].
While this evidence provides a rationale for renin-angiotensin system blockade in patients with the metabolic syndrome, few studies have specifically addressed the use of these agents in obese individuals. One notable study is the TReatment in Obese Patients with HYpertension (TROPHY) trial [126], which compared the efficacy of an ACE inhibitor, lisinopril, or a diuretic, hydrochlorothiazide, given at various doses to obese and hypertensive individuals. The number of individuals who responded to this antihypertensive regimen was greater with lisinopril (40% versus 33%, P < 0.05), although plasma glucose improved in the lisinopril group and worsened in the hydrochlorothiazide group [126].
Conclusion
Obesity, insulin resistance, and the metabolic syndrome engender hypertension through multiple pathways in preclinical animal models, and very likely in humans as well. Renal, neural, hormonal, and cardiovascular systems integrate information to maintain blood pressure despite the substantial stressors that occur in daily life (e.g., eating, fasting, breathing, and locomotion). This integration and interdependence greatly complicate the study of hypertension associated with the metabolic syndrome. Yet, important work from an equally diverse group of investigators has progressively mapped out the mechanisms of this condition. Increased standardization within species would improve the reproducibility of data. In addition, differences in the dominant pathophysiologic pathway within and across species limit the translational power of any one pathway in elucidating hypertension in humans with the metabolic syndrome. Thus, knowledge gleaned from human studies or human samples will be critical in directing future investigation of mechanisms within preclinical models. Despite these challenges, hypertension in the metabolic syndrome has a profound impact on global human health, highlighting the need for further study.
References
Alberti KG, Zimmet P, Shaw J, Group IDFETFC. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366(9491):1059–62.
Haller H. Epidermiology and associated risk factors of hyperlipoproteinemia. Z Gesamte Inn Med. 1977;32(8):124–8.
Joslin E. The prevention of diabetes mellitus. JAMA. 1921;76:79–84.
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607.
Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. Adults. Diabetes Care. 2004;27(10):2444–9.
Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64(2):193–8.
Chen J. Sodium sensitivity of blood pressure in Chinese populations. Curr Hypertens Rep. 2010;12(2):127–34.
Dyer AR, Elliott P, Shipley M. Body mass index versus height and weight in relation to blood pressure. Findings for the 10,079 persons in the INTERSALT Study. Am J Epidemiol. 1990;131(4):589–96.
Garrison RJ, Kannel WB, Stokes J 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16(2):235–51.
Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134(1):1–11.
Zavaroni I, Mazza S, Dall’Aglio E, Gasparini P, Passeri M, Reaven GM. Prevalence of hyperinsulinaemia in patients with high blood pressure. J Intern Med 1992;231(3):235–240.
Dyer AR, Elliott P, Shipley M, Stamler R, Stamler J. Body mass index and associations of sodium and potassium with blood pressure in INTERSALT. Hypertension. 1994;23(6 Pt 1):729–36.
Guyton AC. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension. 1992;19(1 Suppl):I2–8.
Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25(5):909–20.
Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14(6 Pt 2):103S–15S.
Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension. 2001;37(2 Pt 2):554–60.
Quigley JE, Elmarakby AA, Knight SF, Manhiani MM, Stepp DW, Olearzcyk JJ, et al. Obesity induced renal oxidative stress contributes to renal injury in salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2009;36(7):724–8.
Chung S, Park CW, Shin SJ, Lim JH, Chung HW, Youn DY, et al. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant. 2010;25(2):389–99.
Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003;64(6):2163–71.
Endre T, Mattiasson I, Berglund G, Hulthn UL. Insulin and renal sodium retention in hypertension-prone men. Hypertension. 1994;23(3):313–9.
Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317(6):350–7.
Skott P, Vaag A, Bruun NE, Hother-Nielsen O, Gall MA, Beck-Nielsen H, et al. Effect of insulin on renal sodium handling in hyperinsulinaemic type 2 (non-insulin-dependent) diabetic patients with peripheral insulin resistance. Diabetologia. 1991;34(4):275–81.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100(24):14,211–6.
Kenyon CJ. Mineralocorticoid-induced hypertension in obese Zucker rats. J Hypertens. 2002;20(11):2151–2.
Tirosh A, Garg R, Adler GK. Mineralocorticoid receptor antagonists and the metabolic syndrome. Curr Hypertens Rep. 2010;12(4):252–7.
Nishida H, Sohara E, Nomura N, Chiga M, Alessi DR, Rai T, et al. Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension. 2012;60(4):981–90.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–94.
Lima NK, Abbasi F, Lamendola C, Reaven GM. Prevalence of insulin resistance and related risk factors for cardiovascular disease in patients with essential hypertension. Am J Hypertens. 2009;22(1):106–11.
Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47(5):699–713.
Nizar JM, Dong W, McClellan RB, Labarca M, Zhou Y, Wong J, et al. Sodium-sensitive elevation in blood pressure is ENaC independent in diet-induced obesity and insulin resistance. Am J Physiol Renal Physiol. 2016;310(9):F812-20.
Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats. J Nutr. 1982;112(8):1546–54.
Mills E, Kuhn CM, Feinglos MN, Surwit R. Hypertension in CB57BL/6J mouse model of non-insulin-dependent diabetes mellitus. Am J Physiol. 1993;264(1 Pt 2):R73–8.
Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17(12 Pt 2):1949–53.
Mozaffari MS, Abdelsayed R, Liu JY, Zakhary I, Baban B. Renal distal tubule proliferation and increased aquaporin 2 level but decreased urine osmolality in db/db mouse: treatment with chromium picolinate. Exp Mol Pathol. 2012;92(1):54–8.
Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transpl. 2003;18(10):1999–2004.
Matsushita T, Miyahara Y, Matsushita M, Yakabe K, Yamaguchi K, Furukawa K, et al. Liddle’s syndrome in an elderly woman. Intern Med. 1998;37(4):391–5.
Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, et al. A mouse model for Liddle’s syndrome. J Am Soc Nephrol: JASN. 1999;10(12):2527–33.
Achard JM, Disse-Nicodeme S, Fiquet-Kempf B, Jeunemaitre X. Phenotypic and genetic heterogeneity of familial hyperkalaemic hypertension (Gordon syndrome). Clin Exp Pharmacol Physiol. 2001;28(12):1048–52.
Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38(10):1124–32.
Huang DY, Boini KM, Osswald H, Friedrich B, Artunc F, Ullrich S, et al. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am J Physiol Renal Physiol. 2006;291(6):F1264–73.
Kennedy AJ, Ellacott KL, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3(3–4):156–66.
Deji N, Kume S, Araki S, Isshiki K, Araki H, Chin-Kanasaki M, et al. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun. 2012;418(3):559–64.
Deji N, Kume S, Araki S, Soumura M, Sugimoto T, Isshiki K, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol. 2009;296(1):F118–26.
Lautz J, Kessler H, Blaney JM, Scheek RM, Van Gunsteren WF. Calculating three-dimensional molecular structure from atom-atom distance information: cyclosporin A. Int J Pept Protein Res. 1989;33(4):281–8.
Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104(9):1085–94.
Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17(7):883–7.
Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, et al. Enhanced phosphorylation of Na(+)-Cl- co-transporter in experimental metabolic syndrome: role of insulin. Clin Sci (Lond). 2012;123(11):635–47.
Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30(10):769–78.
Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54(7):2012–8.
Huang DY, Boini KM, Friedrich B, Metzger M, Just L, Osswald H, et al. Blunted hypertensive effect of combined fructose and high-salt diet in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase SGK1. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R935–44.
Roncon-Albuquerque R, Jr., Moreira-Rodrigues M, Faria B, Ferreira AP, Cerqueira C, Lourenco AP, et al. Attenuation of the cardiovascular and metabolic complications of obesity in CD14 knockout mice. Life Sci. 2008;83(13–14):502–10.
Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R781–8.
Rong X, Li Y, Ebihara K, Zhao M, Naowaboot J, Kusakabe T, et al. Angiotensin II type 1 receptor-independent beneficial effects of telmisartan on dietary-induced obesity, insulin resistance and fatty liver in mice. Diabetologia. 2010;53(8):1727–31.
Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58(2):271–9.
Costa MV, Fernandes-Santos C, Faria Tda S, Aguila MB, Mandarim-de-Lacerda CA. Diets rich in saturated fat and/or salt differentially modulate atrial natriuretic peptide and renin expression in C57BL/6 mice. Eur J Nutr. 2012;51(1):89–96.
Mingorance C, Duluc L, Chalopin M, Simard G, Ducluzeau PH, Herrera MD, et al. Propionyl-L-carnitine corrects metabolic and cardiovascular alterations in diet-induced obese mice and improves liver respiratory chain activity. PloS One. 2012;7(3):e34268.
Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, et al. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension. 2013;61(1):246–52.
Inoue E, Ichiki T, Takeda K, Matsuura H, Hashimoto T, Ikeda J, et al. Beraprost sodium, a stable prostacyclin analogue, improves insulin resistance in high-fat diet-induced obese mice. J Endocrinol. 2012;213(3):285–91.
Gamliel-Lazarovich A, Raz-Pasteur A, Coleman R, Keidar S. The effects of aldosterone on diet-induced fatty liver formation in male C57BL/6 mice: comparison of adrenalectomy and mineralocorticoid receptor blocker. Eur J Gastroenterol Hepatol. 2013;25(9):1086–92.
Van Vliet BN, McGuire J, Chafe L, Leonard A, Joshi A, Montani JP. Phenotyping the level of blood pressure by telemetry in mice. Clin Exp Pharmacol Physiol. 2006;33(11):1007–15.
Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol. 2003;549(Pt 1):313–25.
Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens 2008;21(12):1288–1291.
Kubota Y, Umegaki K, Kagota S, Tanaka N, Nakamura K, Kunitomo M, et al. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol Pharm Bull. 2006;29(8):1756–8.
Plehm R, Barbosa ME, Bader M. Animal models for hypertension/blood pressure recording. Methods Mol Med. 2006;129:115–26.
Zhao X, Ho D, Gao S, Hong C, Vatner DE, Vatner SF. Arterial pressure monitoring in mice. Curr Protoc Mouse Biol. 2011;1:105–22.
Yun CC. Concerted roles of SGK1 and the Na+/H+ exchanger regulatory factor 2 (NHERF2) in regulation of NHE3. Cell Physiol Biochem. 2003;13(1):29–40.
Faresse N, Lagnaz D, Debonneville A, Ismailji A, Maillard M, Fejes-Toth G, et al. Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Ren Physiol. 2012;302(8):F977–85.
Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86(4):1151–78.
Zheng Y, Yamada H, Sakamoto K, Horita S, Kunimi M, Endo Y, et al. Roles of insulin receptor substrates in insulin-induced stimulation of renal proximal bicarbonate absorption. J Am Soc Nephrol. 2005;16(8):2288–95.
Davies M, Fraser SA, Galic S, Choy SW, Katerelos M, Gleich K, et al. Novel mechanisms of Na + retention in obesity: phosphorylation of NKCC2 and regulation of SPAK/OSR1 by AMPK. Am J Physiol Ren Physiol. 2014;307(1):F96–F106.
Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Ren Physiol. 2006;290(5):F1055–64.
Sowers JR, Whitfield LA, Catania RA, Stern N, Tuck ML, Dornfeld L, et al. Role of the sympathetic nervous system in blood pressure maintenance in obesity. J Clin Endocrinol Metab. 1982;54(6):1181–6.
Wofford MR, Anderson DC Jr, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14(7 Pt 1):694–8.
Asirvatham-Jeyaraj N, Fiege JK, Han R, Foss J, Banek CT, Burbach BJ, et al. Renal denervation normalizes arterial pressure with no effect on glucose metabolism or renal inflammation in obese hypertensive mice. Hypertension. 2016;68(4):929–36.
Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28(6):1047–54.
Rocchini AP, Moorehead CP, DeRemer S, Bondie D. Pathogenesis of weight-related changes in blood pressure in dogs. Hypertension. 1989;13(6 Pt 2):922–8.
Rocchini AP, Mao HZ, Babu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension. 1999;33(1 Pt 2):548–53.
Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens. 2002;20(7):1245–50.
do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57(5):918–26.
Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23(14):5998–6004.
Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148(11):5339–47.
Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52.
Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33(1 Pt 2):542–7.
da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43(6):1312–7.
da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension. 2008;51(4):884–90.
Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46(2):326–32.
Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–41.
Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348(12):1085–1095.
Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25(4):893–7.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370(15):1393–1401.
Healy V, Thompson C, Johns EJ. The adrenergic regulation of proximal tubular Na(+)/H(+) exchanger 3 in the rat. Acta Physiol (Oxf). 2014;210(3):678–89.
DiBona GF, Sawin LL. Effect of renal nerve stimulation on NaCl and H2O transport in Henle’s loop of the rat. Am J Physiol. 1982;243(6):F576–80.
Chan YL. Adrenergic control of bicarbonate absorption in the proximal convoluted tubule of the rat kidney. Pflugers Arch. 1980;388(2):159–64.
DiBona GF. Neural regulation of renal tubular sodium reabsorption and renin secretion. Fed Proc. 1985;44(13):2816–22.
Thomson SC, Vallon V. Alpha 2-adrenoceptors determine the response to nitric oxide inhibition in the rat glomerulus and proximal tubule. J Am Soc Nephrol. 1995;6(5):1482–90.
Quan A, Baum M. The renal nerve is required for regulation of proximal tubule transport by intraluminally produced ANG II. Am J Physiol Ren Physiol. 2001;280(3):F524–9.
Terker AS, Yang CL, McCormick JA, Meermeier NP, Rogers SL, Grossmann S, et al. Sympathetic stimulation of thiazide-sensitive sodium chloride cotransport in the generation of salt-sensitive hypertension. Hypertension. 2014;64(1):178–84.
Ramseyer VD, Ortiz PA, Carretero OA, Garvin JL. Angiotensin II-mediated hypertension impairs nitric oxide-induced NKCC2 inhibition in thick ascending limbs. Am J Physiol Ren Physiol. 2016;310(8):F748–F54.
Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signaling pathway. J Cell Sci. 2008;121(Pt 20):3293–304.
Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am. 2017;101(1):169–93.
Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol. 2016;27(3):677–86.
Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37(5):365–71.
Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA. Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am J Physiol Ren Physiol. 2001;281(4):F639–48.
Manhiani MM, Cormican MT, Brands MW. Chronic sodium-retaining action of insulin in diabetic dogs. Am J Physiol Renal Physiol. 2011;300(4):F957–65.
DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Inv. 1975;55(4):845–55.
Airaksinen J, Lahtela JT, Ikaheimo MJ, Sotaniemi EA, Takkunen JT. Intravenous insulin has no effect on myocardial contractility or heart rate in healthy subjects. Diabetologia. 1985;28(9):649–52.
Gotzsche PC, Kelbaek H, Vissing SF, Nielsen SL, Munck O, Christensen NJ, et al. Acute cardiovascular effects of insulin in hyperglycaemic type I diabetics. Scand J Clin Lab Invest. 1991;51(1):93–7.
Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Inv. 1991;87(6):2246–52.
Li L, Garikepati RM, Tsukerman S, Tiwari S, Ecelbarger CM. Salt sensitivity of nitric oxide generation and blood pressure in mice with targeted knockout of the insulin receptor from the renal tubule. Am J Physiol Regul Integr Comp Physiol. 2012;303(5):R505–12.
Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, et al. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci U S A. 2008;105(17):6469–74.
Traykova-Brauch M, Schonig K, Greiner O, Miloud T, Jauch A, Bode M, et al. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med. 2008;14(9):979–84.
Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, et al. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60(6):1524–30.
Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15(14):2727–9.
Harte A, McTernan P, Chetty R, Coppack S, Katz J, Smith S, et al. Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation. 2005;111(15):1954–61.
Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45(3):356–62.
de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43(1):41–7.
Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92(11):4472–5.
Schinner S, Willenberg HS, Krause D, Schott M, Lamounier-Zepter V, Krug AW, et al. Adipocyte-derived products induce the transcription of the StAR promoter and stimulate aldosterone and cortisol secretion from adrenocortical cells through the Wnt-signaling pathway. Int J Obes. 2007;31(5):864–70.
Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62(2):313–9.
Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102(25):9056–61.
Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24(7):2454–63.
McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18(9):1429–33.
Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, et al. The absence of intrarenal ACE protects against hypertension. J Clin Invest. 2013;123(5):2011–23.
Ortlepp JR, Breuer J, Eitner F, Kluge K, Kluge R, Floege J, et al. Inhibition of the renin-angiotensin system ameliorates genetically determined hyperinsulinemia. Eur J Pharmacol. 2002;436(1–2):145–50.
Reisin E, Weir MR, Falkner B, Hutchinson HG, Anzalone DA, Tuck ML. Lisinopril versus hydrochlorothiazide in obese hypertensive patients: a multicenter placebo-controlled trial. Treatment in Obese Patients With Hypertension (TROPHY) Study Group. Hypertension. 1997;30(1 Pt 1):140–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
JMN is supported by an American Heart Association Postdoctoral Fellowship on behalf of the Grossman Family (16POST27770003). VB is supported by the National Institutes of Health (1R01 DK091565).
Additional information
This article is part of the Topical Collection on Hypertension and Metabolic Syndrome
Rights and permissions
About this article
Cite this article
Nizar, J.M., Bhalla, V. Molecular Mechanisms of Sodium-Sensitive Hypertension in the Metabolic Syndrome. Curr Hypertens Rep 19, 60 (2017). https://doi.org/10.1007/s11906-017-0759-5
Published:
DOI: https://doi.org/10.1007/s11906-017-0759-5