Abstract
Obstructive sleep apnea (OSA) is a common form of sleep disordered breathing and has a relatively high prevalence in the general population. The frequency and severity of OSA is associated with age, male sex, and obesity, and OSA has been linked to cardiovascular complications and death. Importantly, OSA has a strong association with both prevalent and incidental hypertension and has a particularly high prevalence in patients with resistant hypertension. In these patients, CPAP and other OSA-directed treatments have been proposed as therapy to help control blood pressure (BP), especially in patients who have not attained optimal BP control despite maximum pharmacological therapy. OSA has also been associated with alterations in cardiac structure and function, although most studies are small and highly limited in study design. Existing data suggest an association between OSA greater left ventricle (LV) mass and hypertrophy that appears independent of confounders including hypertension and obesity. Although less clear and more controversial, OSA severity has been linked to LV systolic and diastolic function, pulmonary hypertension, and right ventricular hypertrophy. Further studies are needed to confirm the potential causal role of OSA in these observed associations with cardiac abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep-disordered breathing (SDB) refers to a broad range of syndromes of respiratory disturbance while sleeping, the most common of which are obstructive sleep apnea (OSA) and central sleep apnea (CSA). Although several questionnaires exist to screen for the presence of OSA, overnight polysomnography, either in-hospital or at home, is the gold standard for diagnosing SDB [1]. Polysomnography allows for the quantification of apneas (complete cessation of the airflow) and hypopneas (significant reduction in the airflow) lasting more than 10 s and accompanied by some degree of oxygen desaturation per hour of sleep. The number of apneas and hypopneas occurring per hour of sleep is used to generate the apnea-hypopnea index (AHI), which defines clinical categories of SDB as follows: none (AHI ≤ 5), mild (AHI >5 to ≤15), moderate (AHI >15 to ≤30), and severe (AHI > 30). Polysomnography can also determine whether the apneas-hypopneas are secondary to anatomical collapse and obstruction of the airway, as is the case in OSA, or by a reduction of central drive, as is the case in CSA.
Among middle-aged white cohorts, OSA is thought to have a prevalence of approximately 9 % in women and 17 % in men [2] but is likely underdiagnosed [3]. Although a similar prevalence of OSA has been described in white Europeans and Asians, there is little data regarding the prevalence among Hispanics and African-Americans. In addition to the well-recognized association with obesity, several additional patient characteristics influence the prevalence of OSA. The prevalence of OSA is higher with greater age. In addition, a higher prevalence is observed among men compared to women in younger cohorts, although among women, the prevalence of OSA increases dramatically after menopause [4]. The high prevalence of obesity and the aging population are expected to contribute to a rising prevalence of OSA. Importantly, OSA is associated with cardiovascular morbidity and death [5, 6, 7•], which has led to significant interest in OSA diagnosis and treatment benefits.
OSA and Hypertension
The overlap between OSA and hypertension (HTN) is well-recognized, with up to 50 % of patients with OSA having concomitant HTN [8••] while an estimated 37 % [9] and 56 % [10] of patients with HTN have OSA. Although several animal studies support a causal role of OSA and hypoxemia in incident HTN [11, 12], it has been difficult to demonstrate a causal relationship in humans. This is largely due to the association of OSA with multiple other clinical characteristics and comorbidities which may confound the observed association between OSA and HTN, including age, sex, BMI, metabolic syndrome, and diabetes mellitus among others. Obesity is a particularly important potential confounder. The Wisconsin Sleep Cohort study demonstrated an association between OSA and prevalent HTN that was independent of other confounder factors, including BMI [2, 3]. Later, both the Wisconsin study [3, 13••] and the Sleep Heart Health Study (SHHS) confirmed a relationship between OSA and incident HTN that was independent of potential confounders. It has also been suggested that some potential confounders, such as age and sex, may also modify the association between OSA and HTN [4, 14••].
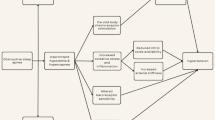
Several different mechanisms have been described as potential pathways to explain the association between OSA and HTN and other cardiovascular complications (Fig. 1). First, nocturnal cyclic hypoxemias and hypercapnias have been shown to trigger an increase in sympathetic activity as shown by an increase in noradrenaline plasma levels, elevated catecholamines in urine [5, 6, 7•, 15] and elevated muscle sympathetic activity [8••, 16]. Second, cyclic shifts in intrathoracic pressures due to respiratory effort against an occluded airway may produce significant changes in ventricular loading conditions [9, 17], increase intracardiac transmural pressures [10, 18], and associated secondary changes in autonomic activity [11, 12, 19]. Third, hypoxemia can lead to formation of free oxygen radicals [20], which in turn may lead to activation of inflammatory pathways, as reflected in circulating C-reactive protein levels [21], and to cytokine liberation—processes that have been implicated in the atherosclerosis [22].
OSA and Resistant Hypertension
Resistant hypertension is defined as a blood pressure (BP) above goal despite the appropriate use of three antihypertensive medications, including at least one diuretic, or controlled BP using more than three antihypertensive medications [23]. The prevalence of resistant hypertension is thought to be approximately 20–30 % of hypertensive patients [23] and has been associated with higher risks of cardiovascular complications and stroke [24]. OSA is the most prevalent comorbidity in patients with resistant hypertension [25]. In a cross-sectional study of 437 subjects, 82 % of patients with resistant hypertension also had some degree of OSA and 56 % of these patients had moderate or severe OSA [26]. Among the patients with OSA, those with resistant hypertension had more severe OSA than those without resistant hypertension [26]. Patients with resistant hypertension tended to be older, more frequently male, more obese, and had a higher prevalence of diabetes [26], features which have also been associated with OSA.
In addition to the previously discussed mechanisms that may mediate the relationship between OSA and hypertension, hyperactivation of the renin-angiotensin-aldosterone system has been proposed as an additional mechanism by which OSA may predispose to resistant hypertension. Several studies have found an association between OSA and hyperaldosteronism in patients with resistant hypertension [27, 28] although this finding has not been universal [29]. Whether resistant hypertension is directly related to OSA, as opposed to shared comorbidities, remains unclear.
Treatment with CPAP and Hypertension
Continuous positive airway pressure (CPAP) is the most effective and frequently prescribed treatment for OSA and is recommended for patients with severe OSA or with less severe OSA but associated diurnal hypersomnolence [30•]. CPAP is associated with reductions in BP, with the greatest reduction noted among patients with HTN on antihypertensive medications [31] or with uncontrolled HTN [32, 33]. However, the benefits of CPAP treatment are dependent on patient’s OSA severity and, more importantly, on CPAP’s adherence [34]. Adherence rates have been reported as low as 46 % [35], although strategies that enhance patient education have achieved adherence rates above 80 % [36]. Two mechanisms have been hypothesized to explain the BP reduction associated with CPAP. First, CPAP may stop the cyclic hypoxemias secondary to apneas. However, in a study of 381 patients with OSA, CPAP significantly reduced BP while the combination sham-CPAP with nocturnal oxygen was not associated with a reduction in BP, supporting the hypothesis that improvement in BP with CPAP is not only mediated through amelioration of intermittent hypoxemia [37••]. Second, CPAP may decrease BP by decreasing sympathetic tone [16]. However, here too, results have not been consistent [32, 38]. More generally, the magnitude of effect of CPAP on BP and the existence of patient subgroups particularly responsive to CPAP remain unclear. A recent meta-analysis evaluated 7 randomized clinical trials of patients with OSA diagnosed by PSG, and concomitant HTN included a total of 794 patients, the majority with moderate/severe OSA [31]. CPAP was associated with a significant reduction in 24-h ambulatory blood pressure, but not with a significant reduction in systolic blood pressure. The observed BP reduction was largely related to in a reduction of overnight BP [31]. Subjects with resistant hypertension showed the greatest benefit from CPAP treatment. However, as with a previous meta-analysis [34], this study was limited by the small sample sizes of included studies (only two of them surpassed 100 patients) and lack in uniformity when selecting the placebo group (sham-CPAP vs no treatment) and, in general, short follow-up [31]. It is suspected that greater reductions in ambulatory BP measures with CPAP are associated with higher initial AHI and with greater adherence to CPAP [34].
Although weight loss is difficult to achieve and to maintain, the combination of weight loss and CPAP may be the best strategy for BP reduction [39]. Weight loss is an important predictor of improvement in both OSA, with a reduction of 5 AHI for each decrease in 10 kg [40•], and HTN, with a reduction of 6 mmHg of SBP and 4.6 mmHg of DBP for each 10 kg weight loss [41]. Finally, recent attention has also focused on the potential use of renal denervation as a treatment for resistant hypertension in patients with OSA, although data are limited [42].
OSA and Cardiac Morphology and Function
OSA is associated with a higher risk of incident HF [43••]. Indeed, in the Sleep Heart Health Study, patients with moderate to severe OSA had a 2.38-fold higher risk of having HF compared to those without OSA, independent of potential clinical confounders [44]. Much attention has focused on potential alterations in cardiac structure and function associated with OSA as in intermediate phenotype preceding overt HF. However, most existing studies are limited by relatively small sample size and variability in control groups making between study comparisons difficult.
Left Ventricle Hypertrophy
Left ventricular hypertrophy (LVH) is a common consequence of hypertension and a potent risk factor for HF. As discussed above, OSA is clearly associated with hypertension. Similarly, obesity is highly prevalent in OSA and is also associated with LVH [45]. Several studies have observed an association between OSA and LVH [46, 47••, 48], but it has been difficult to demonstrate an association between OSA and higher left ventricular mass index (LVMI) or LVH independent of obesity and hypertension (Table 1) [46]. However, in 2058 middle-age subjects (58 % women) from the SHHS who underwent echocardiography, severe OSA (AHI ≥ 30) was associated with a higher odds of having concomitant LVH (OR of 1.78 [95 % CI 1.14–2.79]) independent of age, sex, BMI, hypertension, and prevalent cardiovascular disease [47••]. The association was stronger between LVH and nocturnal hypoxemia, especially among women, in whom the association between LVMI and AHI was not independent of potential confounders. Similarly, a recent study of 80 subjects free of HTN, diabetes, or known cardiovascular disease observed greater LVH among subjects with moderate or severe OSA compared to those with no or mild OSA [48]. These studies suggest that OSA is associated with LVH even in the absence of concomitant HTN.
Both concentric and eccentric LV remodeling are common in OSA, although significant variability in prevalence estimates exists between studies [47••, 49, 50]. In a recent study of 121 patients (mean age 35.9 ± 10.1, 80 % men) with newly diagnosed essential HTN, moderate to severe OSA was associated with greater concentric remodeling (based on the relative wall thickness) compared to those with no or mild OSA [50]. Similarly, in Resist-POL, which included 155 patients with resistant hypertension, concentric hypertrophy was independently associated with both OSA severity and night-time SBP [49]. However, the SHHS observed a higher prevalence of both concentric and eccentric hypertrophy in subjects with OSA compared to those without OSA, although only eccentric hypertrophy was independently associated with OSA severity (OR for eccentric hypertrophy of 1.84 [95 % CI 1.12–3.03] for severe OSA compared to no OSA) [47••].
As BP lowering in hypertensive persons is associated with reduction in LV mass [51] and CPAP is associated with BP reduction in OSA, it has been hypothesized that CPAP treatment may reduce LV mass. Indeed Cloward et al. described that CPAP treatment was able to reverse LVH in 25 patients newly diagnosed of severe OSA with very significant nocturnal hypoxemia after 6 months of treatment with CPAP [52]. However, this finding of an association between CPAP treatment and reduction in LV mass has not being confirmed in bigger trials.
Left Ventricular Diastolic Function
OSA has also commonly been associated with diastolic dysfunction. Using three-dimensional echocardiography, Oliveira et al. found that, compared to controls without OSA, patient with OSA had larger left atrial (LA) size and worse LA function and that LA size was proportional to the severity of OSA [53]. In addition, subjects with OSA had mild diastolic dysfunction compared to controls, based on transmitral inflow velocities and mitral tissue Doppler velocities. However, multivariable adjustment for relevant potential confounders—including BMI and SBP—was not performed [53]. In a subsequent analysis of 30 patients with moderate or severe OSA (AHI ≥20), the same group found that treatment with CPAP was associated with improvement in diastolic function and in measures of LA function compared to a control sham CPAP group. The treatment effect on BP was not reported but could have informed whether changes in BP explained the observed changes in diastolic function [54]. Additional studies have demonstrated abnormalities of diastolic function, based on transmitral flow patterns and LA enlargement, in OSA [55, 56], although this finding has not been universal (Table 2). Indeed, some have argued that observed association of OSA with diastolic measures is primarily due to OSA-associated comorbidities and as opposed to OSA itself [46]. In addition, while improvement in diastolic measurements has been observed with OSA treatment, it is unclear if this is primarily related to improvement in OSA itself, or to concomitant improvement in hypertension [54].
Of note, the association of OSA with LA enlargement and LA functional impairment [57] is concordant with the observed association of OSA with a higher risk of incident atrial fibrillation (AF) [58], in addition to recurrent atrial fibrillation after electrical ablation [59]. Indeed patients with OSA who were CPAP users demonstrate lower rates of recurrence than CPAP nonusers following electrical ablation for AF [60].
Left Ventricular Systolic Function
Despite the association of OSA with incident HF, studies have failed to show any association between OSA severity and LVEF [47••, 61]. More recently, global longitudinal strain (GLS) has been proposed as a more sensitive measurement of LV systolic function [62]. OSA does appear to be associated with impaired GLS despite preserved LVEF [63]. In a study of 60 patients, comparing patients with OSA to obese controls, GLS was impaired in OSA. Intriguingly, improvement in GLS following surgical correction of OSA was highly correlated with the reduction in AHI [64].
Although OSA has not been associated with LVEF, among HFrEF patients in particular, CPAP has been proposed as an intervention to improve LVEF [65•]. In a recent meta-analysis including a total of 259 patients from 10 studies, CPAP was associated with a significant improvement in LVEF, an effect that was most prominent among patients with concomitant OSA and HF [66]. Notably, this study was limited by the inclusion of very small studies (none included more than 50 patients) and significant heterogeneity in definition of both OSA cases and controls [66].
Pulmonary Hypertension and Right Ventricular Hypertrophy
OSA has been listed as a cause of pulmonary hypertension secondary to hypoxemia [67]. However, the evidence that links OSA and pulmonary hypertension is not strong and published studies are in general small. In a study of 27 subjects with OSA free of coexistent cardiac or pulmonary disease, one third had mild pulmonary hypertension [68]. Comparing those with versus without pulmonary hypertension, no differences in pulmonary function or BMI were noted that could explain the differences in pulmonary pressures, although no significant difference in OSA severity (assessed by the AHI) was noted either. The authors suggested that individual susceptibility to nocturnal hypoxemia secondary to OSA could be the cause for observed pulmonary hypertension [68]. Indeed, most studies have shown that pulmonary hypertension is less linked to AHI and more to daytime hypoxemia [69], obstructive ventilatory pattern [70], obesity and secondary hypoventilation syndrome [71]. Therefore, given our current knowledge, it is hard to determine whether OSA is a cause of pulmonary hypertension or if it is only a contributory factor in patients with hypoxemia due to other conditions [72]. Despite this controversy, in at least one study of 20 subjects with pulmonary hypertension and OSA, pulmonary pressures and pulmonary vascular resistance were significantly reduced after 4 months of treatment with CPAP [73].
Limited data exists regarding the relationship between OSA and right ventricular structure and function. From individuals participating in both the Framingham Heart Study and SHHS, a case-control analysis of 90 subjects with severe OSA and controls with low AHI matched for age, sex, and BMI demonstrated thicker right ventricular free walls—consistent with right ventricular hypertrophy—among the severe OSA cases [74•]. This finding is potentially consistent with higher pulmonary pressure and right ventricular afterload in severe OSA. No other abnormalities in structure or function were noted [74•] and similar findings have not been described in other studies, possibly due to the infrequency with which RV wall thickness is assessed.
Patent Foramen Ovale
The prevalence of a permanent foramen ovale (PFO) in the general population is approximately 25 % [75]. However, the prevalence in series of OSA patients has been significantly higher, between 47 % [76] and 69 % [77]. Additionally, a high prevalence of right-to-left shunt has been observed in OSA [78]. Both findings may help explain the association between OSA and stroke [79], supporting the hypothesis of potential paradoxical embolization. However, these studies were small and subject of significant limitations. Further studies are needed to confirm these observations and inform the potential of benefit with PFO closure in this population [80].
Conclusions
OSA is a cause of HTN and can contribute to the development of uncontrollable HTN. Treatment of OSA with CPAP appears to improve BP in subjects with OSA, although further study is required to define the magnitude of treatment benefit. OSA is associated with left ventricular hypertrophy, diastolic dysfunction, and subtle impairments of systolic function. OSA has also been associated with pulmonary hypertension and right ventricular hypertrophy. Importantly, however, our current understanding of the impact of OSA on cardiac structure and function is limited due to the small size of most studies, inter-study variability in selecting OSA cases and defining controls, and inadequate adjustment for potential confounders. Disentangling the cardiovascular effects of OSA from the confounding effects of shared risk factors is a major challenge. Further prospective studies in large, diverse populations are necessary to clarify the impact of OSA on cardiac structure and function independent of common comorbidities.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21(7):749–57.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14.
Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–52.
Lin C, Davidson T, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12:481–96.
Martinez-Garcia M-A, Campos-Rodriguez F, Catalán-Serra P, Soler-Cataluña J-J, Almeida-Gonzalez C, De la Cruz Morón I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly. Role of long-term CPAP treatment: a prospective observational trial. Am J Respir Crit Care Med. 2012;186(9):909–16.
Quan S, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future. Circulation. 2004;109:951–7.
Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. 2009;6(8):e1000132. Based on the Sleep Heart Health Study, the biggest and most consistent community study with a prospective study of the mortality risk associated with OSA severity.
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in collaboration With the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). JACC. 2008;52(8):686–717. A consistent and extensive review based on the cardiovascular complications of OSA. Done by a committee of experts for the AHA/ACC.
Sjöström C, Lindberg E, Elmasry A, Hägg A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002;57(7):602–7.
Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105(8):1135–9.
Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99(1):106–9.
Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90(4):1600–5.
Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. A prospective study in 709 subjects from the Wisconsin Sleep Cohort that found a dose response association between apnea index and incidental hypertension after 4 years of follow-up.
O'Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, et al. Prospective study of sleep-disordered breathing and hypertension. Am J Respir Crit Care Med. 2009;179(12):1159–64. A prospective study of 2,470 subjects from the Sleep Heart Health Study studying the predictive value of AHI for hypertension and the impact of obesity in this association.
Vardhan V, Shanmuganandan K. Hypertension and catecholamine levels in sleep apnoea. Med J Armed Forces India. 2012;68(1):33–8.
Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131(5):1406–13.
Orban M, Bruce CJ, Pressman GS, Leinveber P, Romero-Corral A, Korinek J, et al. Dynamic changes of left ventricular performance and left atrial volume induced by the Mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol. 2008;102(11):1557–61.
Buda AJ, Pinsky MR, Ingels NB, Daughters GT, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301(9):453–9.
Somers VK, Dyken ME, Skinner JL. Autonomic and hemodynamic responses and interactions during the Mueller maneuver in humans. J Auton Nerv Syst. 1993;44(2-3):253–9.
Park A-M, Suzuki YJ. Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in mice. J Appl Physiol. 2007;102(5):1806–14.
Punjabi N, Beamer B. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30(1):29–34.
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9.
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–19.
de la Sierra A, Banegas JR, Oliveras A, Gorostidi M, Segura J, de la Cruz JJ, et al. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J Hypertens. 2012;30(6):1211–6.
Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LKG, Amaro ACS, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–7.
Muxfeldt ES, Margallo VS, Guimarães GM, Salles GF. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27(8):1069–78.
Gonzaga CC, Gaddam K, Ahmed MI, Pimenta E, Thomas SJ, Harding SH, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6(4):363.
Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26(5):281–7.
Dobrowolski P, Klisiewicz A, Florczak E, Prejbisz A, Bieleń P, Sliwiński P, et al. Independent association of obstructive sleep apnea with left ventricular geometry and systolic function in resistant hypertension: the RESIST-POL study. Sleep Med. 2014;15(11):1302–8.
Epstein LJ, Kristo D, Strollo Jr PJ, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):1–14. A guideline designed to assist in obstructive sleep apnea treatment including information for outcomes and long-term follow up for each treatment option.
Hu X, Fan J, Chen S, Yin Y, Zrenner B. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2015;17(3):215–22.
Muxfeldt ES, Margallo V, Costa LMS, Guimarães G, Cavalcante AH, Azevedo JCM, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65(4):736–42.
Lozano L, Tovar JL, Sampol G, Romero O, Jurado MJ, Segarra A, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28(10):2161–8.
Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167(8):757–64.
Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–8.
Jurado-Gamez B, Bardwell WA, Cordova-Pacheco LJ, García-Amores M, Feu-Collado N, Buela-Casal G. A basic intervention improves CPAP adherence in sleep apnoea patients: a controlled trial. Sleep Breath. 2015;19(2):509–14.
Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–85. A randomized, controlled clinical trial including 281 subjects with significant cardiovascular disease comparing the effects of overnight CPAP versus oxygen treatment in ambulatory systolic blood pressure.
Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, Merino-Sanchez M, Gonzalez-Benitez MA, Beltran-Robles M, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129(6):1459–67.
Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–75.
Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, et al. Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169(17):1619–26. An study in 264 subjects with the type 2 diabetes mellitus and obesity of the impact of weight loss in obstructive sleep apnea severity.
Aucott L, Poobalan A, Smith WCS, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45(6):1035–41.
Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58(4):559–65.
Gottlieb D, Yenokyan G, Newman A, O'Connor G, Punjabi N, Quan S, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure. The Sleep Heart Health Study. Circulation. 2010;122(4):352–60. A prospective study of the sex-specific associations between obstructive sleep apnea and cardiovascular complications (heart failure and coronary heart disease) after more 8 years of follow-up in 4,422 subjects from the Sleep Heart Health Study.
Shahar E, Whitney C, Redline S, Lee E, Newman A, Nieto F, et al. Sleep-disordered breathing and cardiovascular disease. Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19.
Abel E, Litwin S. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419.
Niroumand M, Kuperstein R, Sasson Z, Hanly PJ. Impact of obstructive sleep apnea on left ventricular mass and diastolic function. Am J Respir Crit Care Med. 2001;163(7):1632.
Chami HA, Devereux RB, Gottdiener JS, Mehra R, Roman MJ, Benjamin EJ, et al. Left ventricular morphology and systolic function in sleep-disordered breathing: the Sleep Heart Health Study. Circulation. 2008;117(20):2599–607. A cross-sectional study in 2,058 subjects from the Sleep Heart Health Study describing the association between left ventricular structure and function assessed by echocardiography and sleep apnea severity.
Aslan K, Deniz A, Cayli M, Bozdemir H, Sarica Y, Seydaoglu G. Early left ventricular functional alterations in patients with obstructive sleep apnea syndrome. Cardiol J. 2013;20(5):519–25.
Dobrowolski P, Prejbisz A, Klisiewicz A, Florczak E, Rybicka J, Januszewicz A, Hoffman P. Determinants of concentric left ventricular hypertrophy in patients with resistant hypertension: RESIST-POL study. Hypertens Res. 2015.
Prejbisz A, Florczak E, Pręgowska-Chwała B, Klisiewicz A, Kuśmierczyk- Droszcz B, Zieliński T, et al. Relationship between obstructive sleep apnea and markers of cardiovascular alterations in never-treated hypertensive patients. Hypertens Res. 2014;37(6):573–9.
Larstorp ACK, Okin PM, Devereux RB, Olsen MH, Ibsen H, Dahlöf B, et al. Regression of ECG-LVH is associated with lower risk of new-onset heart failure and mortality in patients with isolated systolic hypertension; The LIFE study. Am J Hypertens. 2012;25(10):1101–9.
Cloward TV. Left ventricular hypertrophy is a common echocardiographic abnormality in severe obstructive sleep apnea and reverses with nasal continuous positive airway pressure. Chest. 2003;124(2):594–601.
Oliveira W, Campos O, Bezerra Lira-Filho E, Cintra FD, Vieira M, Ponchirolli A, et al. Left atrial volume and function in patients with obstructive sleep apnea assessed by real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21(12):1355–61.
Oliveira W, Campos O, Cintra F, Matos L, Vieira MLC, Rollim B, et al. Impact of continuous positive airway pressure treatment on left atrial volume and function in patients with obstructive sleep apnoea assessed by real-time three-dimensional echocardiography. Heart. 2009;95(22):1872–8.
Kepez A, Niksarlioglu EYO, Hazirolan T, Ranci O, Kabul HK, Demir AU, et al. Early myocardial functional alterations in patients with obstructive sleep apnea syndrome. Echocardiography. 2009;26(4):388–96.
Usui Y, Takata Y, Inoue Y, Tomiyama H, Kurohane S, Hashimura Y, et al. Severe obstructive sleep apnea impairs left ventricular diastolic function in non-obese men. Sleep Med. 2013;14(2):1–5.
Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9(3):321–7.
Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing. Am J Respir Crit Care Med. 2006;173:910–6.
Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011;108(1):47–51.
Fein AS, Shvilkin A, Shah D, Haffajee CI, Das S, Kumar K, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62(4):300–5.
Hanly P. Ventricular function in snorers and patients with obstructive sleep apnea. Chest. 1992;102(1):100.
Shah AM, Solomon SD. Myocardial deformation imaging. Circulation. 2012;125(2):244–8.
Haruki N, Takeuchi M, Nakai H, Kanazawa Y, Tsubota N, Shintome R, et al. Overnight sleeping induced daily repetitive left ventricular systolic and diastolic dysfunction in obstructive sleep apnoea: quantitative assessment using tissue Doppler imaging. Eur J Echocardiogr. 2009;10(6):769–75.
Cho KI, Kwon JH, Kim SM, Park TJ, Lee HG, Kim TI. Impact of obstructive sleep apnea on the global myocardial performance beyond obesity. Echocardiography. 2012;29(9):1071–80.
Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–41. An study in patients with heart failure with reduced ejection fraction and severe sleep apnea in whom treatment with CPAP for 1-month improved left ventricular function and systolic blood pressure.
Sun H, Shi J, Li M, Chen X. Impact of continuous positive airway pressure treatment on left ventricular ejection fraction in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. PLoS ONE. 2013;8(5), e62298.
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2011;32(4):385–92.
Sajkov D, Cowie RJ, Thornton AT, Espinoza HA, McEvoy RD. Pulmonary hypertension and hypoxemia in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1994;149(2):416–22.
Apprill M, Weitzenblum E, Krieger J, Oswald M, Kurtz D. Frequency and mechanism of daytime pulmonary hypertension in patients with obstructive sleep apnoea syndrome. Cor Vasa. 1991;33(1):42–9.
Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest. 1996;109(2):380–6.
Bady E, Achkar A, Pascal S, Orvoen-Frija E, Laaban JP. Pulmonary arterial hypertension in patients with sleep apnoea syndrome. Thorax. 2000;55(11):934–9.
Krieger J, Sforza E, Apprill M, Lampert E, Weitzenblum E, Ratomaharo J. Pulmonary hypertension, hypoxemia, and hypercapnia in obstructive sleep apnea patients. Chest. 1989;96(4):729–37.
Sakov D, Wang T, Saunders N, Bune A, Douglas MR. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(2):152.
Guidry UC, Mendes LA, Evans JC, Levy D, O'Connor GT, Larson MG, et al. Echocardiographic features of the right heart in sleep-disordered breathing: the Framingham Heart Study. Am J Respir Crit Care Med. 2001;164(6):933–8. From the Framingham Study it included 90 subjects with severe OSA and compared with 90 non-OSA subjects. They found significant right ventricular hypertrophy in severe OSA subjects.
Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59(1):17–20.
Lau EMT, Jaijee SK, Melehan KL, Wong KK, Yee BJ, Grunstein RR, et al. Prevalence of patent foramen ovale and its impact on oxygen desaturation in obstructive sleep apnea. Int J Cardiol. 2013;165(1):35–40.
Shanoudy H, Soliman A, Raggi P, Liu JW, Russell DC, Jarmukli NF. Prevalence of patent foramen ovale and its contribution to hypoxemia in patients with obstructive sleep apnea. Chest. 1998;113(1):91–6.
Beelke M, Angeli S, Del Sette M, De Carli F, Canovaro P, Nobili L, et al. Obstructive sleep apnea can be provocative for right-to-left shunting through a patent foramen ovale. Sleep. 2002;25(8):856–62.
Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–77.
Zanchetta M, Pedon L, Maiolino P. Obstructive sleep apnea and patent foramen ovale. Circulation. 2004;109(7):e69–author reply e69.
Otto ME, Belohlavek M, Romero-Corral A, Gami AS, Gilman G, Svatikova A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99(9):1298–302.
Drager LF, Bortolotto LA, Figueiredo AC, Caldin Silva B, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131(5):1379.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Shah declares research support from Novartis, Gilead, and Actelion. Dr. Querejeta Roca declares no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding Sources
Dr. Shah is supported in part by K08-HL-116792 and a grant from the American Heart Association (14CRP20380422).
Additional information
This article is part of the Topical Collection on Hypertension and the Heart
Rights and permissions
About this article
Cite this article
Querejeta Roca, G., Shah, A.M. Sleep Disordered Breathing: Hypertension and Cardiac Structure and Function. Curr Hypertens Rep 17, 91 (2015). https://doi.org/10.1007/s11906-015-0604-7
Published:
DOI: https://doi.org/10.1007/s11906-015-0604-7