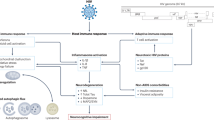
Abstract
The implementation of combination antiretroviral therapy (cART) has changed HIV infection into a chronic illness, conveying extensive benefits, including greater longevity and advantages for the central nervous system (CNS). However, studies increasingly confirm that the CNS gains are incomplete, with reports of persistent immune activation affecting the CNS despite suppression of plasma HIV RNA. The rate of cognitive impairment is unchanged, although severity is generally milder than in the pre-cART era. In this review, we discuss cognitive outcomes from recently published clinical HIV studies, review observations on HIV biomarkers for cognitive change, and emphasize longitudinal imaging findings. Additionally, we summarize recent studies on CNS viral invasion, CD8 encephalitis, and how CNS involvement during the earliest stages of infection may set the stage for later cognitive manifestations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combination antiretroviral therapy (cART) has transformed the HIV epidemic into a chronic yet complex illness that is largely manageable for people with access to life-saving therapies [1]. Combination therapies are now deliverable in as few as one to two pills daily; with adherence and longitudinal follow-up, they are highly successful in suppressing plasma HIV RNA to levels undetectable by standard assays. Yet, cognitive impairment remains frequent, has been quantified in study participants despite plasma HIV RNA suppression, and is associated with markers of ongoing immune activation [2, 3].
Multiple lines of evidence confirm that cART is inadequate to ameliorate central nervous system (CNS) injury for all patients. In research studies, among participants with suppression of plasma HIV RNA, CNS microglial activation is apparent by positron emission tomography (PET, [11C]-PK11195 tracer) and the extent of microglial activation is associated with worse cognitive performance on executive functioning tests [4•]. Diffusion tensor imaging (DTI), a method that evaluates the integrity of deep brain fibers, uncovers CNS injury among suppressed participants, and the degree of compromised CNS fiber integrity correlates to worse cognitive performance [5–7]. Persistent blood monocyte activation measured by the monocyte/macrophage scavenger receptor, CD163, is apparent in participants with suppressed plasma HIV RNA who have HIV-associated neurocognitive disorder (HAND) [3]. Even markers of brain injury have been identified in some individuals with chronic infection, as measured by cerebrospinal fluid (CSF) neurofilament (NFL) elevations in 8 % of asymptomatic suppressed HIV-infected participants compared to only 2 % of controls [8].
While many studies indicate HIV-specific mechanisms, particularly that related to inflammation, there is a similar bulk of data to emphasize the role of comorbidities in current era cognitive impairment. The CNS Anti-Retroviral Therapy Effects Research (CHARTER) study conducted at five academic centers in the USA and published in 2010 noted the greatest probability of cognitive impairment among individuals with comorbidities, including cerebrovascular disease, head injuries, substance use, and depression. More recently, in a brain autopsy series of 144 HIV-infected individuals, about 50 % had moderate or severe cerebral small vessel disease (CVD), with the extent of CVD associating with previous exposure to protease inhibitors [9•]. This study also noted correlations between HAND and mild CVD [OR (confidence intervals) 4.8 (1.1–21.2)]. Although this important study provides unequivocal evidence of high rates of CVD in HIV, the enrollment period (1999–2011) may expose cohort biases related to the antiretroviral medications used during that era that are no longer broadly employed.
A recent review itemized the likely increased risk of clinical stroke in the HIV-infected population, a finding that may be particularly important for women [10]. HIV may also contribute to young age of stroke, not only among treatment naïve individuals but also those during early phase of treatment [11]. Vasculopathies have been associated with HIV, including vessel remodeling by atherosclerosis, elongation and distension of vessels [12, 13], and alterations in cerebral vasoreactivity [14].
More recently, cognitive impairment in the setting of HIV has been linked to multimorbidity, defined as the presence of two or more independent disease processes that can augment each other and achieve worse outcomes. The Veterans Aging Cohort Study (VACS) index of multimorbidity combines age, traditional HIV clinical measures (e.g., plasma HIV RNA and current CD4+ count), and non-HIV disease markers from more than one organ system (e.g., renal and liver function, anemia, and hepatitis C co-infection) [15]. The possible multisystem contributions to HAND is suggested in a recent Women’s Interagency HIV Study (WIHS) publication linking liver fibrosis to cognitive performance in models that were adjusted for both hepatitis C and HIV [16]. Both studies, however, raise concern that the relationship is not mechanistic but rather an indication of common underlying pathophysiological processes, most probably chronic inflammation.
The diagnostic challenge associated with risk for other neurodegenerative disorders, such as Alzheimer’s disease (AD), as etiology to cognitive impairment in older HIV-infected patients is an emerging issue. A recent publication employed the more general criteria of mild cognitive impairment (MCI), nosology typically used in HIV-uninfected studies and often prodromal of AD. Participants were virally suppressed and over age 50 years and had a seven-times higher risk of meeting MCI criteria compared to seronegative control (n = 75) [17]. With treatment earlier in the course of HIV, HAND is increasingly found to be independent of clinical markers of immune compromise (e.g., CD4+ counts). Among young treatment-naïve participants (age 27–42 years, n = 608) with CD4+ counts >500 cells/μL, 20 % had at least mild impairment in neuropsychological testing and 3 % had moderate-to-severe impairment [18•]. While worse performance was associated with duration of HIV, it was also linked to diagnosis of diabetes and the presence of cardiovascular risk factors that compromise the Framingham risk score.
Longitudinal studies support the risk for persistent cognitive impairment, and large cohort studies in the USA uncover challenges with broad uptake of therapy. The Multicenter AIDS Cohort Study (MACS) completed a longitudinal substudy of 197 HIV-infected men who have sex with men (MSM) sampled to be free of major cognitive confounding factors [19]. Across a 3-year period, the frequency of HAND increased from 25 to 31 % (p = 0.048), and during this period, 77 % remained stable, 13 % deteriorated, and 10 % improved in their diagnostic HAND staging. In this sample, 75 % of participants were on cART at baseline and the mean CD4+ count was 589 cells/mm3. Rates of persistent impairment, deterioration, and improvement among successfully suppressed participants were not reported, although progression was linked to poorer treatment outcome variables (e.g., viral load). Similarly, the CHARTER study reported the 3-year incidence and predictors of cognitive change in 436 enrollees of whom 46 % were impaired at baseline and noted that 61 % remained stable, 17 % improved, and 23 % declined [20]. Predictors of cognitive change included treatment-related variables, disease severity indicators, baseline demographics, and premorbid intelligence quotient.
These two major US cohort studies highlight an urgent gap in implementing treatment. Although rates of cognitive impairment exceeded 50 % in the CHARTER cohort, less than half had suppressed plasma HIV RNA and 44 % of those on cART were not suppressed. In the WIHS (cross-sectional) conducted in the US and the MACS (longitudinal) reports, only 53 and 70 % of participants achieved undetectable HIV RNA during study visits, respectively. Given that suppression of plasma virus is a universally accepted early step in treating cognitive symptoms and that treatment-related factors were related to cognition, these studies uncover a crucial opportunity for treatment of persistent impairment.
Substantial controversy remains regarding the research categorization of asymptomatic neurocognitive impairment (ANI) within the HAND nosology [21]. The ANI designation may be used in as many as 70 % of research participants who are impaired on cognitive testing, but the absence of functional symptoms is often determined only by self-report [22]. Several studies buttress the likelihood that the “asymptomatic” terminology is misleading and that these individuals remain at risk for outcomes associated with impairment. Compared to unimpaired HIV-infected participants, ANI individuals more frequently convert to symptomatic disease [23]. In a study of mixed asymptomatic and symptomatic participants with HAND, researchers uncovered impairment in the objective skills needed to manage health care literacy [24]. In a separate study, individuals with ANI were found to perform similarly to participants with mild symptomatic impairment (mild neurocognitive disorder (MND)) on both neuropsychological tests and objective tests of everyday function, yet ANI participants discrepantly reported normal or above normal perceived performance [25]. Symptoms, such as irritability and communication difficulty, are more readily identified when collateral information can be acquired, but proxy input is not commonly included in research studies [26]. These studies highlight important contributions to the asymptomatic designation driven by impaired insight and less proximal objective informants than are typical in non-HIV neurodegenerative disorder studies.
Some researchers have questioned whether HAND terminology appropriately attributes cognitive deficits to HIV alone. In the Cognitive Impairment in People with HIV in the European Region (CIPHER) study of 248 healthy HIV-infected participants (mean CD4+ count of 550 cells, 88 % on cART), investigators found no difference in the frequency of cognitive impairment compared to demographically similar HIV-uninfected controls [27]. These data should be interpreted with caution given such a limited sample of controls (n = 45) of which nearly one third met criteria for cognitive impairment and the sample selection from regular clinic attendees. The authors noted important differences in rates of moderate to severe depression in this study, affecting 29 % of HIV-infected participants compared to only 8 % of HIV-uninfected controls (p < 0.001). Given knowledge that HIV is linked to motor, behavioral, and cognitive manifestations, such a high discrepancy in rates of depression highlights continued concern for HIV-related CNS morbidity.
Biomarkers for HAND
To date, markers of immune activation and neuronal injury show greatest promise as potential biomarkers of HAND, although no marker has emerged to have sufficient performance characteristics for clinical use [3]. CSF monocyte/macrophage inflammatory markers MCP-1 and sCD14 correlate to MR spectroscopy (MRS) neuronal damage and inflammatory abnormalities among treated participants, 70 % of which achieved CSF and plasma viral suppression [28]. CSF sCD14 was also found to associate with CSF NFL, a marker of neuronal injury, in another cross-sectional analysis studying a mixed group of participants that were either treatment-naïve or off treatment (n = 48) [29]. These findings demonstrate the importance of understanding the role of monocytes/macrophages in HAND, particularly noting the associations of these markers to HAND despite virologic suppression. This is also bolstered by a recent study using a myeloid only mouse model, demonstrating that macrophages from humans can sustain and transmit infection [30].
Some data have shown that T-lymphocyte activation is linked to HAND. In a study of 86 HIV-infected participants with about 70 % achieving viral suppression, the upregulation of HLA-DR, a marker for T-lymphocyte activation, was detected on CD4+ and CD8+ T-lymphocytes in both blood and CSF samples compared with healthy controls [31]. Decreasing CD4/CD8 ratios in the CSF and the increased frequencies of HLA-DR expressing CD4+ and CD8+ T cells in the CSF also were found to associate with HAND severity and T2 MRI signal abnormalities in the periventricular white matter and basal ganglia [31].
Potential relationships between persistent intracellular HIV DNA and cognitive impairment were explored in a retrospective study of 44 HIV-infected participants with plasma and CSF viral suppression. Here, higher HIV DNA levels were associated with cognitive impairment severity in the subset of older participants (age 50–71, n = 26) [32]. These associations were not detected cross-sectionally in a separate but similar study (n = 80, CSF and blood viral suppression = 97 %); however, change in HIV DNA did link to change in cognitive performance in some domains [33].
CSF NFL is a structural component of myelinated axons and can be detected with active neuronal damage. NFL was shown to be sensitive for detecting HIV-associated dementia (HAD), the most severe form of HAND, and was superior to other CSF markers, including total and phosphorylated tau (t- and p-tau), soluble amyloid precursor protein (sAPP), and amyloid beta fragments [34]. Regardless of CD4+ counts, individuals with HAD also displayed the most distorted overall CSF biomarker profiles (e.g., CSF NFL, sAPP, t-tau), white blood cell count (WBC), and blood-brain barrier (BBB) integrity by albumin ratio. These findings suggest a more extensive inflammatory response and presence of active neuronal injury in HAD compared to neuroasymptomatic disease. In a longitudinal study of treatment effect (n = 78), CSF NFL levels decreased in 63 % of participants after cART initiation, although levels remained higher than those in healthy controls after cART [8]. Recently, elevated NFL levels in plasma, independent of CSF, have been associated with the presence of HAD in participants not taking cART, implying that NFL in blood could be useful as a minimally invasive biomarker for active neuronal injury in demented patients [35]. Markers of ongoing injury are still needed for patients with suppressed plasma HIV RNA, and blood markers would be optimal.
CNS Imaging in HIV
Imaging studies that investigate mixed treatment populations (e.g., both with and without plasma viral suppression) continue to limit our understanding of disease severity among optimally adherent and suppressed patients. A longitudinal volumetric study of 51 asymptomatic HIV-infected participants (mean plasma HIV RNA = 9608 copies/ml, about 20 % not on cART) demonstrated faster atrophy rates in HIV in regions that included the neocortex (from frontal lobes to parietal lobes) and the thalamus compared to 65 HIV-uninfected controls [36•]. The authors did not report the proportion of HIV-infected participants suppressed on therapy. Those with higher CD4+ counts had slower expansion of Sylvian fissure and slower atrophy rates in the insulae, hippocampi, and frontal and temporoparietal cortices, a finding that could be interpreted as a window of neuroprotection linked to early treatment initiation [36•]. Another preliminary longitudinal study reports that, despite 24 months of cART that was started within days of infection and with demonstrated plasma viral suppression, volumetric decreases of 2 % were found in caudate (p = 0.002), putamen (p<0.001), and pallidum (p = 0.034), with a 1 % decrease in total subcortical gray matter (p = 0.002) (n = 38, median age 29 years, CD4+ count 386 cells/mm3). These preliminary data lacked an HIV-uninfected comparison group to assure that these rates exceeded that of healthy controls; however, given the age of these participants, such atrophy would be unusual [37].
Magnetic resonance spectroscopy (MRS) measures the concentration of key metabolites in the brain and can provide insight into neuronal health, cellular metabolism, and inflammation. Several longitudinal studies suggest that virologic suppression does not achieve complete normalization of MRS abnormalities, such as normalization of N-acetyl aspartate (NAA) reflecting neuronal integrity, or choline (CHO), a marker of inflammation [38, 39]. These persistent MRS abnormalities were seen in HIV regardless of cognitive status. Nadir CD4+ count, duration of HIV infection, and older age correlate with persistent abnormalities in markers of neuronal health (NAA/Cr, n = 260 on stable cART, 75 % with plasma HIV RNA suppression) [40]. In a study highlighted earlier in this review, the ability to detect potential microglial activation linked to cognitive performance among suppressed patients using PET imaging may represent one of the greatest breakthroughs in understanding ongoing brain injury despite effective cART from a study published recently; however, larger studies are needed [4•].
A study of white matter hyperintensities (WMHs) used a combination of DTI and fluid-attenuated inversion recovery (FLAIR) images in over 80 HIV-infected participants with a broad age range. Authors found that older age was associated with an increased frequency of WMH in HIV, but not in controls [41]. Investigations of DTI also uncovered impaired microstructural integrity in the internal capsule, cerebral peduncle, and corona radiata associated with age and HCV co-infection. Alterations in white matter integrity, as measured by DTI, correlated with cognitive performance in older HIV-infected individuals (mean age = 64, 90 % with plasma HIV RNA < 400 copies/ml) compared to age-matched controls [6]. Diffuse white matter alterations were detected in another DTI study comparing HIV-infected participants on suppressive therapy (n = 100) with matched controls (n = 70); however, the contributions from residual pre-cART damage cannot be excluded in any of these studies and, in the later study, the DTI abnormalities correlated with the number of years spent with a CD4+ cell count below 500 cells/μl [7].
The neuroanatomic structures involved in HIV infection are largely consistent across the various imaging modalities, with the most common affected brain regions being the frontal white matter (FWM) [38, 39], basal ganglia (BG) [38, 39, 42, 43], and the thalami [42]. The effect of HIV infection on frontostriatal circuitry was similarly reported by a meta-analysis combining six task-based functional MRI (fMRI) studies (n = 105 HIV-infected and n = 102 HIV-uninfected controls) where frontostriatal dysfunction correlated to degree of cognitive impairment, disease progression, and treatment effect [44].
Insights from Early HIV Infection
HIV RNA has been found in the CSF as early as 8 days following estimated infection [45], and through blood and CSF viral sequencing, independent replication in the CNS has been observed within the first year of HIV infection [46•]. We do not yet understand when brain changes that underlie irreversible, long-term cognitive consequences begin and whether there is a window period for early intervention that can be neuroprotective. The growing body of early HIV infection studies may bridge this gap.
In primary HIV infection (PHI), generally defined as within the first year of infection, a previous report suggested normal performance on neuropsychological testing in most of the participants [47]. Nonetheless, the Chicago Early HIV Infection cohort (CEHI, n = 15, estimated duration of HIV < 100 days, Fiebig III to V) identified worse performance on tasks of psychomotor speed and visual recall [48]. Refining this to the window of acute HIV infection (AHI), a recent study from Thailand (n = 36, 64 % in Fiebig stage I or II) found that as many as 25 % were at least one standard deviation below mean performance on two or more cognitive tests, and this group of cases had higher CSF HIV RNA [49]. At 3 and 6 months post cART, a subset of individuals did not improve.
In cross-sectional DTI studies from the CEHI cohort [48], PHI participants had early impairments in white matter tract integrity at diagnosis. Brain volumetric analyses further revealed reduced parenchyma volumes in PHI compared to controls, suggesting neuronal loss [48]. In contrast, a cross-sectional analysis from the Primary Infection Stage CNS Events Study (PISCES) did not find DTI abnormalities at baseline (median 4 months post infection) compared to controls [50]. A conference proceeding noted earlier in this review identified volumetric reductions over time in deep gray matter structures despite 24 months of suppressive cART [37].
In the PISCES study, BBB integrity was modestly altered during PHI, demonstrated by elevated CSF/plasma albumin ratios, CSF protein levels, and DTI alterations [50, 51]. CSF and plasma concentrations of matrix metalloproteinases (MMPs), a group of extracellular proteases involved in BBB permeability, were compared between 52 PHI and 21 controls in the CEHI study. Here, authors demonstrated reduced plasma MMP-2 in PHI and that elevation of CSF MMP-2 correlated to reduced white matter integrity, basal ganglia volume, and motor speed [52]. Longitudinal studies note that neuroinflammation in PHI can escalate with time in the absence of treatment. A longitudinal MRS study in treatment-naïve PHI reported increases in inflammatory markers (CHO/Cr and MI/Cr) in the frontal white matter and parietal gray matter over a median of 6 months [53]. Sequential analysis of CSF neopterin concentrations and percentages of activated CD4+ and CD8+ T cells in CSF similarly demonstrated a steady, rising trend in most of these 44 treatment-naïve PISCES participants [54].
Elevated NFL concentration was seen in 44 % of 92 PHI PISCES participants and varied with infection duration [51]. These elevations correlated with reductions in neuronal integrity (NAA/Cr) on MRS, but not with neuropsychological performance, suggesting a pre-clinical brain injury [51]. Treatment led to an attenuation, but not normalization of levels of inflammatory markers in frontal white matter and parietal grey matter. In AHI participants from Thailand, normal CSF NFL levels were seen in all but one participant (n = 32) and remained within normal levels after 24 weeks of cART [55]. Together, these studies highlight CNS HIV infection and immune activation being established very early after initial HIV infection and reasonable support for the conjecture that there may be a window period for protection from neuronal injury after early CNS invasion.
CNS HIV Escape and CD8 Encephalitis
CNS HIV escape is a rare but biologically important phenomenon, defined as either a detectable CSF HIV RNA level despite undetectable plasma HIV RNA level or a CSF HIV RNA level at least one log10 above a fairly well-controlled level in plasma [56, 57]. Patients with symptomatic CNS HIV escape can present with symptoms ranging from mild headache or sensory disturbance to encephalopathy or coma and typically respond to cART adjustments according to CSF HIV genotyping or increasing the CNS penetration effectiveness (CPE) [56, 57]. Drug resistance has been reported in the CSF in case series of symptomatic CNS escape participants treated with long-term suppressive cART, including one after 9 years of a three drug PI-based regimen [58] and another after 6 years of two-drug PI-based regimen [59]. There are currently few published data to guide clinicians on when CNS escape is likely and this is a major gap in the field, since symptomatic CNS escape is likely occurring in the minority of cases of cognitive impairment seen in clinic populations.
A recent cross-sectional study (n = 69) found that 10 % of asymptomatic participants with undetectable plasma HIV RNA had low but detectable levels of CSF HIV RNA [60]. Higher occurrence rates of 15–19 % of participants with detectable CSF HIV RNA despite plasma viral suppression have been reported in cART simplification studies [61, 62]. Among participants with long-term plasma HIV RNA suppression (n = 45, <40 copies/ml), 17 % of CSF samples (12/70) had detectable CSF HIV RNA using a single copy assay with detection limit 0.3 copies/ml [63••]. Despite the exceptionally low level of CSF HIV replication, CSF neopterin was higher in the detectable group compared with the undetectable group, suggesting that macrophage activation may foster low-level HIV persistence or vice versa. This is consistent with other asymptomatic CSF viral escape reports that employ standard assays for HIV RNA quantitation [60, 64]. As these studies were cross-sectional, it remains less clear if the presence of actively replicating virus in the CNS is transient or persistent and what role asymptomatic CNS escape may plan in CNS compartmentalization or symptomatic escape. Deep sequencing of four symptomatic CNS HIV escape cases revealed the presence of minority variants in CSF, supporting the concept of local CNS replication and differential evolution of HIV in the CNS [65].
CD8 encephalitis, a diagnosis pathologically defined by extensive perivascular and parenchymal infiltration of CD8+ T-lymphocytes [66•], has been reported in treated individuals and further broadens the possible CNS manifestations in treated HIV [66•, 67]. In a case series of CD8 encephalitis, 8 out of 14 individuals had been on stable cART for at least 2 years, with undetectable plasma HIV RNA and either CD4+ count >350 cells/mm3 or CD4/CD8 ratio >0.7 [67]. All presented with unexpected, acute or subacute brain dysfunction (e.g., dizziness, headache, memory disorders, confusion, status epilepticus), and all had elevated CSF lymphocyte counts with a disproportionately elevated CD8/CD4 ratio (CD8+ 65–87 %), in the absence of blood CD8+ lymphocytosis in all but one person. The improvement of CD8 encephalitis after steroid treatment suggests a shared feature with autoimmune conditions; total recovery was reported in 5/14 patients with parallel resolution of MRI brain abnormalities. Both CNS HIV escape and CD8 encephalitis expand our understanding of cognitive impairment in people living with HIV on suppressive cART, as they are proof of concept that CNS viral replication and widespread neuroinflammation can persist and associate with dramatic neurologic symptomatology.
Summary and Recommendations
Conclusions
The persistence of HAND in the era of cART is likely due to multiple etiologies. While cerebrovascular risk factors grow in importance with an aging HIV-infected population, the issue of persistent CNS immune activation despite suppressive cART should not be disregarded, as its presence has been linked with neurocognitive impairment, neuroimaging abnormalities, and neuronal damage markers. The observations from CNS HIV escape offer a potential source of persistent immune activation, and the occurrence of CD8 encephalitis highlights that a partly recovered yet dysfunctional host immune system may be of importance in affecting clinical outcomes.
While there is a persistent risk for developing cognitive impairment despite treatment, hopeful intervention trials are developing. In a recent, small, randomized control trial targeting HIV-infected participants with cognitive symptoms despite suppressive cART in both plasma and CSF (n = 9; HIV RNA < 50 copies/ml), adding a CCR5 inhibitor (maraviroc) to a backbone cART regimen was associated with medium to large effect sizes, favoring improved global cognitive performance at 6 and 12 months [68]. In another single arm, open-labeled study of maraviroc intensification, cognitive improvement was noted, as was a reduction in intracellular HIV DNA and decreased CD38+ T-lymphocytes, providing mechanistic links to this improvement [69]. With the expanding narrative about usefulness of CCR5 blockade for neuroinflammatory disease [70], CCR5 inhibitors merit larger randomized control trials. Notably, these findings contrast with a study of neuroasymptomatic participants where CCR5 inhibitors were added to first-line therapy (i.e., cART naïve). Authors identified no benefit to augmentation, highlighting the likelihood that the opportunity for augmentation is best guided to those with symptomatic impairment despite cART [71].
Another potential candidate for intervention is paroxetine, which was shown to be beneficial for HIV-infected participants with cognitive impairment in a preliminary conference report [72]. It continues to be reasonable to recommend physical exercise [73] and potentially cognitive rehabilitation [74] in an armamentarium that includes vigilance in management of comorbidities in this complex and multietiology cognitive impairment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance,•• Of major importance
Antiretroviral Therapy Cohort C. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9. doi:10.1016/S0140-6736(08)61113-7.
Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45(2):174–82. doi:10.1097/QAI.0b013e318042e1ee.
Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27(9):1387–95. doi:10.1097/QAD.0b013e32836010bd.
Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28(1):67–72. doi:10.1097/01.aids.0000432467.54003.f7. Demonstration of incomplete efficacy of cART in settling intracerebral immune activation despite virologic suppression.
Tate DF, Conley J, Paul RH, Coop K, Zhang S, Zhou W, et al. Quantitative diffusion tensor imaging tractography metrics are associated with cognitive performance among HIV-infected patients. Brain Imaging Behav. 2010;4(1):68–79. doi:10.1007/s11682-009-9086-z.
Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, et al. Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp. 2014;35(3):975–92. doi:10.1002/hbm.22228.
Su T, Caan MW, Wit FW, Schouten J, Geurtsen GJ, Cole JH, et al. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. AIDS. 2016;30(2):311–22. doi:10.1097/QAD.0000000000000945.
Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One. 2014;9(2):e88591. doi:10.1371/journal.pone.0088591.
Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28(9):1297–306. doi:10.1097/QAD.0000000000000262. Tackling cerebral small vessel disease may be one of major challenge in aging people living with HIV.
Chow FC. HIV infection, vascular disease, and stroke. Semin Neurol. 2014;34(1):35–46. doi:10.1055/s-0034-1372341.
Benjamin LA, Corbett EL, Connor MD, Mzinganjira H, Kampondeni S, Choko A, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology. 2016;86(4):324–33. doi:10.1212/WNL.0000000000002278.
Edwards NJ, Grill MF, Choi HA, Ko NU. Frequency and risk factors for cerebral arterial disease in a HIV/AIDS neuroimaging cohort. Cerebrovasc Dis. 2016;41(3-4):170–6. doi:10.1159/000442755.
Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology. 2015;85(13):1139–45. doi:10.1212/WNL.0000000000001976.
Chow FC, Boscardin WJ, Mills C, Ko N, Carroll C, Price RW, et al. Cerebral vasoreactivity is impaired in treated, virally suppressed HIV-infected individuals. AIDS. 2016;30(1):45–55. doi:10.1097/QAD.0000000000000875.
Marquine MJ, Umlauf A, Rooney AS, Fazeli PL, Gouaux BD, Paul Woods S, et al. The veterans aging cohort study index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr. 2014;65(2):190–7. doi:10.1097/QAI.0000000000000008.
Valcour VG, Rubin LH, Obasi MU, Maki PM, Peters MG, Levin S, et al. Liver fibrosis linked to cognitive performance in HIV and hepatitis C. J Acquir Immune Defic Syndr. 2016. doi:10.1097/QAI.0000000000000957.
Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, et al. Elevated rates of mild cognitive impairment in HIV disease. J Neurovirol. 2015;21(5):576–84. doi:10.1007/s13365-015-0366-7.
Wright EJ, Grund B, Cysique LA, Robertson KR, Brew BJ, Collins G, et al. Factors associated with neurocognitive test performance at baseline: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16 Suppl 1:97–108. doi:10.1111/hiv.12238. International cohort points out that development of cognitive impairment could be relatively early after infection before significant immune compromise.
Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86(4):334–40. doi:10.1212/WNL.0000000000002277.
Heaton RK, Franklin Jr DR, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60(3):473–80. doi:10.1093/cid/ciu862.
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi:10.1212/01.WNL.0000287431.88658.8b.
Heaton RK, Clifford DB, Franklin Jr DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi:10.1212/WNL.0b013e318200d727.
Grant I, Franklin Jr DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–62. doi:10.1212/WNL.0000000000000492.
Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I, et al. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22(1):110–7. doi:10.1037/0894-4105.22.1.110.
Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, et al. Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Res Hum Retroviruses. 2013;29(6):949–56. doi:10.1089/AID.2012.0229.
Murray KJ, Cummins D, Batterham M, Trotter G, Healey L, O’Connor CC. Does the informal caregiver notice HIV associated mild cognitive impairment in people living with HIV? AIDS Care. 2016;28(2):221–7. doi:10.1080/09540121.2015.1084989.
McDonnell J, Haddow L, Daskalopoulou M, Lampe F, Speakman A, Gilson R, et al. Minimal cognitive impairment in UK HIV-positive men who have sex with men: effect of case definitions and comparison with the general population and HIV-negative men. J Acquir Immune Defic Syndr. 2014;67(2):120–7. doi:10.1097/QAI.0000000000000273.
Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, et al. Plasma and cerebrospinal fluid biomarkers predict cerebral injury in hiv-infected individuals on stable combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;69(1):29–35. doi:10.1097/QAI.0000000000000532.
McGuire JL, Gill AJ, Douglas SD, Kolson DL, Group CHA-RTER. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol. 2015;21(4):439–48. doi:10.1007/s13365-015-0333-3.
Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, et al. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016. doi:10.1172/JCI84456.
Grauer OM, Reichelt D, Gruneberg U, Lohmann H, Schneider-Hohendorf T, Schulte-Mecklenbeck A, et al. Neurocognitive decline in HIV patients is associated with ongoing T-cell activation in the cerebrospinal fluid. Ann Clin Transl Neurol. 2015;2(9):906–19. doi:10.1002/acn3.227.
de Oliveira MF, Murrel B, Perez-Santiago J, Vargas M, Ellis RJ, Letendre S, et al. Circulating HIV DNA correlates with neurocognitive impairment in older HIV-infected adults on suppressive ART. Sci Rep. 2015;5:17094. doi:10.1038/srep17094.
Cysique LA, Hey-Cunningham WJ, Dermody N, Chan P, Brew BJ, Koelsch KK. Peripheral blood mononuclear cells HIV DNA levels impact intermittently on neurocognition. PLoS One. 2015;10(4):e0120488. doi:10.1371/journal.pone.0120488.
Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One. 2014;9(12):e116081. doi:10.1371/journal.pone.0116081.
Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–40. doi:10.1016/j.ebiom.2015.11.036.
Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2014;35(7):1755–68. doi:10.1016/j.neurobiolaging.2014.01.008. The accelerated rate of atrophy highlights structural changes or neuronal damage can take place during preclinical state.
Kallianpur KJ, Colby D, Jahanshad N, Fletcher JL, Ananworanich J, Clifford K et al. Brain volumetric changes after 2 years of ART initiated during acute HIV infection. Abstract CROI 2016.
Sailasuta N, Ananworanich J, Lerdlum S, Sithinamsuwan P, Fletcher JL, Tipsuk S, et al. Neuronal-glia markers by magnetic resonance spectroscopy in HIV before and after combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2015. doi:10.1097/QAI.0000000000000779.
Gongvatana A, Harezlak J, Buchthal S, Daar E, Schifitto G, Campbell T, et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol. 2013;19(3):209–18. doi:10.1007/s13365-013-0162-1.
Harezlak J, Cohen R, Gongvatana A, Taylor M, Buchthal S, Schifitto G, et al. Predictors of CNS injury as measured by proton magnetic resonance spectroscopy in the setting of chronic HIV infection and CART. J Neurovirol. 2014;20(3):294–303. doi:10.1007/s13365-014-0246-6.
Seider TR, Gongvatana A, Woods AJ, Chen H, Porges EC, Cummings T, et al. Age exacerbates HIV-associated white matter abnormalities. J Neurovirol. 2015. doi:10.1007/s13365-015-0386-3.
Wade BS, Valcour VG, Wendelken-Riegelhaupt L, Esmaeili-Firidouni P, Joshi SH, Gutman BA, et al. Mapping abnormal subcortical brain morphometry in an elderly HIV + cohort. Neuroimage Clin. 2015;9:564–73. doi:10.1016/j.nicl.2015.10.006.
Wade BS, Valcour V, Busovaca E, Esmaeili-Firidouni P, Joshi SH, Wang Y et al. Subcortical shape and volume abnormalities in an elderly HIV+ cohort. Proc SPIE Int Soc Opt Eng. 2015;9417. doi:10.1117/12.2082241.
Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS. 2014;28(6):803–11. doi:10.1097/QAD.0000000000000151.
Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82. doi:10.1093/infdis/jis326.
Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11(3):e1004720. doi:10.1371/journal.ppat.1004720. A demonstration of CNS as a potential reservoir for viral replication, contributing to additive difficulty in eradication strategy.
Moore DJ, Letendre SL, Morris S, Umlauf A, Deutsch R, Smith DM, et al. Neurocognitive functioning in acute or early HIV infection. J Neurovirol. 2011;17(1):50–7. doi:10.1007/s13365-010-0009-y.
Ragin AB, Wu Y, Gao Y, Keating S, Du H, Sammet C, et al. Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol. 2015;2(1):12–21. doi:10.1002/acn3.136.
Kore I, Ananworanich J, Valcour V, Fletcher JL, Chalermchai T, Paul R, et al. Neuropsychological impairment in acute HIV and the effect of immediate antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70(4):393–9. doi:10.1097/QAI.0000000000000746.
Wright PW, Vaida FF, Fernandez RJ, Rutlin J, Price RW, Lee E, et al. Cerebral white matter integrity during primary HIV infection. AIDS. 2015;29(4):433–42. doi:10.1097/QAD.0000000000000560.
Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207(11):1703–12. doi:10.1093/infdis/jit088.
Li S, Wu Y, Keating SM, Du H, Sammet CL, Zadikoff C, et al. Matrix metalloproteinase levels in early HIV infection and relation to in vivo brain status. J Neurovirol. 2013;19(5):452–60. doi:10.1007/s13365-013-0197-3.
Young AC, Yiannoutsos CT, Hegde M, Lee E, Peterson J, Walter R, et al. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology. 2014;83(18):1592–600. doi:10.1212/WNL.0000000000000932.
Suh J, Sinclair E, Peterson J, Lee E, Kyriakides TC, Li FY, et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J Neuroinflammation. 2014;11:199. doi:10.1186/s12974-014-0199-y.
Peluso MJ, Valcour V, Ananworanich J, Sithinamsuwan P, Chalermchai T, Fletcher JL, et al. Absence of cerebrospinal fluid signs of neuronal injury before and after immediate antiretroviral therapy in acute HIV infection. J Infect Dis. 2015;212(11):1759–67. doi:10.1093/infdis/jiv296.
Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–74. doi:10.1097/QAD.0b013e328355e6b2.
Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8. doi:10.1086/650538.
Beguelin C, Vazquez M, Bertschi M, Yerly S, de Jong D, Rauch A, et al. Viral escape in the CNS with multidrug-resistant HIV-1. J Int AIDS Soc. 2014;17(4 Suppl 3):19745. doi:10.7448/IAS.17.4.19745.
Mangioni D, Muscatello A, Sabbatini F, Soria A, Rossi M, Bisi L, et al. A case of cerebrospinal fluid viral escape on a dual antiretroviral regimen: worth the risk? Clin Infect Dis. 2014;59(11):1655–6. doi:10.1093/cid/ciu679.
Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–25. doi:10.1086/657342.
Gutmann C, Cusini A, Gunthard HF, Fux C, Hirschel B, Decosterd LA, et al. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS. 2010;24(15):2347–54. doi:10.1097/QAD.0b013e32833db9a1.
Vernazza P, Daneel S, Schiffer V, Decosterd L, Fierz W, Klimkait T, et al. The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) Trial. AIDS. 2007;21(10):1309–15. doi:10.1097/QAD.0b013e32814e6b1c.
Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28(15):2251–8. doi:10.1097/QAD.0000000000000400. Ongoing low level viral replication was present in a sizable portion of PLWH on long term suppressive cART, which was associated with significant elevation of immune marker.
Calcagno A, Atzori C, Romito A, Ecclesia S, Imperiale D, Audagnotto S, et al. Cerebrospinal fluid biomarkers in patients with plasma HIV RNA below 20 copies/mL. J Int AIDS Soc. 2014;17(4 Suppl 3):19719. doi:10.7448/IAS.17.4.19719.
Tong CY, Costelloe S, Hubb J, Mullen J, O’Shea S, Marta M, et al. Deep sequencing of HIV-1 in cerebrospinal fluid. Clin Infect Dis. 2015;61(6):1022–5. doi:10.1093/cid/civ417.
Gray F, Lescure FX, Adle-Biassette H, Polivka M, Gallien S, Pialoux G, et al. Encephalitis with infiltration by CD8+ lymphocytes in HIV patients receiving combination antiretroviral treatment. Brain Pathol. 2013;23(5):525–33. doi:10.1111/bpa.12038. The emergence of CD8 encephalitis expands the consideration in managing patients present with neurologic symptoms in cART era with a totally different treatment strategy.
Lescure FX, Moulignier A, Savatovsky J, Amiel C, Carcelain G, Molina JM, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis. 2013;57(1):101–8. doi:10.1093/cid/cit175.
Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS. 2016;30(4):591–600. doi:10.1097/QAD.0000000000000951.
Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20(6):571–82. doi:10.1007/s13365-014-0279-x.
Martin-Blondel G, Brassat D, Bauer J, Lassmann H, Liblau RS. CCR5 blockade for neuroinflammatory diseases—beyond control of HIV. Nat Rev Neurol. 2016;12(2):95–105. doi:10.1038/nrneurol.2015.248.
Winston A, Bouliotis G, Kulasegaram R, Clarke A, Post5 FA, Nelson M et al. A randomised controlled trial of maraviroc-intensified bPI ART on cognitive function. Abstract CROI 2016.
Sacktor N, Skolasky RL, Haughey N, Munro C, Moxley R, Steiner J et al. Paroxetine and fluconazole therapy for HAND: a double-blind, placebo-controlled trial. Abstract CROI 2016.
Dufour CA, Marquine MJ, Fazeli PL, Henry BL, Ellis RJ, Grant I, et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J Neurovirol. 2013;19(5):410–7. doi:10.1007/s13365-013-0184-8.
Livelli A, Orofino GC, Calcagno A, Farenga M, Penoncelli D, Guastavigna M, et al. Evaluation of a cognitive rehabilitation protocol in HIV patients with associated neurocognitive disorders: efficacy and stability over time. Front Behav Neurosci. 2015;9:306. doi:10.3389/fnbeh.2015.00306.
Acknowledgments
This work is supported by K24-MH098759 (VV) and R01MH095613 (VV and SS). Dr. Chan is supported by funds from the National Institutes of Mental Health, US NIH T32AG023481.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Phillip Chan reports grants from National Institute of Health (NIH): National Institute of Mental Health (NIMH) T32AG023481.
Joanna Hellmuth declares she has no conflict of interest.
Serena Spudich reports grants from National Institute of Health (NIH): National Institute of Mental Health (NIMH) and National Institute of Neurological Disorders and Stroke (NINDS).
Victor Valcour reports grants from National Institute of Health (NIH): National Institute of Mental Health (NIMH) and National Institute of Neurological Disorders and Stroke (NINDS) and consultant fees from ViiV Healthcare and Merck.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Complications of Antiretroviral Therapy
Rights and permissions
About this article
Cite this article
Chan, P., Hellmuth, J., Spudich, S. et al. Cognitive Impairment and Persistent CNS Injury in Treated HIV. Curr HIV/AIDS Rep 13, 209–217 (2016). https://doi.org/10.1007/s11904-016-0319-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-016-0319-7