Abstract
Despite over 30 years of research, the contribution of type I interferons (IFN-Is) to both the control of HIV replication and initiation of immunologic damage remains debated. In acute infection, IFN-Is, likely from plasmacytoid dendritic cells (pDCs), activate NK cells and upregulate restriction factors targeting virtually the entire HIV life cycle. In chronic infection, IFN-Is may also contribute to CD4 T cell loss and immune exhaustion. pDCs subsequently infiltrate lymphoid and mucosal tissues, and their circulating populations wane in chronic infection; IFN-I may be produced by other cells. Data from nonhuman primates indicate prompt IFN-I signaling is critical in acute infection. Whereas some studies showed IFN-I administration without combination antiretroviral therapy (cART) is beneficial, others suggest that stimulating or blocking IFN-I signaling in chronic ART-suppressed HIV infection has had positive results. Here, we describe the history of HIV and IFN-I, IFN-I’s sources, IFN-I’s effects on HIV control and host defense, and recent interventional studies in SIV and HIV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The association of type I interferon (IFN-I) with AIDS predates the discovery of HIV itself. Serum IFN-I activity was first described as being enriched in “homosexual men with Kaposi’s sarcoma and lymphadenopathy” in 1982 [1]. Shortly thereafter, Buimovici-Klein and colleagues published a letter in The Lancet entitled “Is presence of interferon predictive for AIDS?” [2]. Although these studies proposed using IFN-I activity as a prognostic indicator of an eventual AIDS diagnosis in lieu of a known etiologic agent, they were the first to suggest that IFN-I may be part of the disease process. In the subsequent 30 years, the link between HIV and IFN-I has been intensely studied. Elevated plasma levels of IFN-I and interferon-stimulated genes (ISGs) in HIV-infected patients and SIV-infected monkeys have been reported many times over. These associative studies have demonstrated unequivocally that untreated infection induces widespread induction of the IFN-I system without any measurable effect on viral load. Administration of IFN-α as monotherapy or as an adjunct to antiretroviral therapy has been intensely studied (reviewed in detail below)—and the results have varied widely. Conversely, the administration of a vaccine against endogenous IFN-α to end-stage AIDS patients stabilized CD4 counts [3, 4].
More recently, evidence for a link between IFN-I and HIV pathogenesis was provided by several groups studying SIV infection in natural host monkey species that do not develop AIDS [5, 6]. After acute SIV infection, these species rapidly mute their IFN-I responses whereas disease-susceptible macaque species maintain IFN-I signaling indefinitely. These species have several other notable differences from pathogenic species, including lack of microbial translocation, limited infection of memory cells, and absence of emergent gastrointestinal viruses [7, 8]. It is unclear whether the ability of natural hosts to shut off the IFN-I system is directly related to pathogenesis or a secondary effect. Nevertheless, the consistent observations across multiple natural host species helped invigorate interest in IFN-I as a mediator of HIV immunopathogenesis.
Cumulatively, the HIV/IFN-I field has definitively demonstrated that (i) at the host level, IFN-I production and responses are not evaded or suppressed by HIV but are insufficient to clear or control HIV and (ii) IFN-I as monotherapy is ineffective to control HIV. In the sections below, we will focus on novel data that addresses (i) IFN-I induction of antiviral responses, (ii) IFN-I driving HIV-related disease progression, and (iii) lessons from IFN-I modulation studies about how the innate system could be manipulated for treatment and prevention.
What Are the Sources of IFN-I in HIV Infection?
Antagonism of the IFN-I system remains of significant clinical interest for the treatment of HIV-related immune activation. However, sustained in vivo inhibition of the IFN-I system can be difficult to achieve. An alternative strategy to modulate the IFN-I system may be the depletion of IFN-I-producing cells. However, which immune subsets are most important to IFN-I production in HIV infection is debated.
Plasmacytoid DCs in HIV/SIV Infection
Classical Studies of Plasmacytoid DCs
Among cell types capable of making IFN-I in response to HIV, plasmacytoid dendritic cells (pDCs) are most intensely studied. Shortly after the discovery that pDCs and the elusive “natural interferon-producing cell” were, in fact, the same cellular subset, several studies documented their kinetics and activity in HIV infection (Fig. 1a) (reviewed in [9]). pDCs are found at reduced levels in chronic HIV infection compared to uninfected controls; similarly, nonhuman primate (NHP) studies have shown that the depletion of circulating pDCs occurs as early as 3 days post-infection and never completely normalizes. Numerous studies from the early 2000s reported that pDCs from HIV-infected patients have an attenuated capacity to produce IFN-α. However, recent data show that in response to some stimuli, pDCs from HIV-infected persons are hyperresponsive [10, 11].
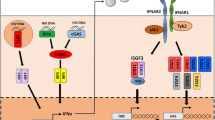
Sources and effects of type I interferons in HIV infection. A HIV or HIV-infected CD4 T cells activate plasmacytoid dendritic cells (pDCs) to produce type I interferon (IFN-I). B In chronic HIV infection, pDCs are depleted from the peripheral circulation and accumulate in the rectal mucosa, where they may be activated by microbial products such as lipopolysaccharide (LPS) and other pathogen-associated molecular patterns (PAMPs) and/or create an inflammatory environment that contributes to ongoing intestinal barrier dysfunction and microbial translocation. C Activated pDCs produce IFN-I in acute HIV infection, but whether pDCs or other cells are the predominant IFN-I producers in chronic HIV infection remains debated. D IFN-I signaling stimulates CD8 T cells to upregulate MHC molecules that bind NK cell inhibitory receptors, rendering CD8 T cells resistant to NK-cell-mediated cytotoxicity. E IFN-I signaling facilitates the proliferation and survival of NK cells and activates NK cell cytotoxicity, which may contribute to the eradication of HIV-infected CD4 T cells. F IFN-I suppresses CD4 T cell effector function and inhibits CD4 T cell proliferation but also induces restriction factors that limit HIV replication at many steps of the replication cycle, including HIV entry, reverse transcription, nuclear entry/integration, transcription, and budding
pDCs Are Depleted from the Blood but Accumulate in Lymph Nodes and Mucosa in HIV/SIV Infection
The NHP model has been highly instructive in understanding pDC biology. pDCs accumulate in draining lymph nodes (LNs) rapidly after SIV infection, where most undergo apoptosis, contributing to the loss of circulating pDCs [12]. Similarly, subsequent groups reported a massive accumulation of pDCs in the rectal and vaginal mucosae after acute SIV infection and in chronic HIV infection (Fig. 1b) [9, 13–15], although it is unknown whether mucosal pDCs undergo apoptosis as they do in LNs. Mucosal retention of pDCs has not been observed in SIV-infected natural host species, which also avoid the persistent IFN-I signaling seen in pathogenic hosts, suggesting that altered localization of pDCs may account for continued IFN-I production in pathogenic infection [5, 6, 16•]. Overall, data from several groups have formed a consolidated model in which pDC depletion from the blood occurs due to relocation and seemingly permanent retention in lymphoid tissues.
Do pDCs Make IFN-I in Chronic HIV/SIV Infection?
pDCs have been assumed to be the primary producer of IFN-Is in HIV infection, as they secrete massive amounts of IFN-α after in vitro HIV stimulation compared to other cell types (Fig. 1c) (reviewed in [17]). Their ultimate contribution to host IFN-I production has been harder to establish in vivo. While there is a general consensus that pDCs are likely responsible for the bulk of IFN-I production in the acute phase (1–2 weeks) of infection, the role of pDCs in chronic infection is less clear. ISGs are easily detected in chronic HIV/SIV infection, but plasma IFN-α and IFNA messenger RNA (mRNA) are found at extremely low levels after acute infection, if at all [6]. This finding may be attributed to the low numbers of circulating pDCs with local IFN-α production inducing ISG expression in target cells within LNs and mucosae [16•]. A second potential explanation is that pDCs are required only in the early phase of infection to “jump-start” systemic IFN-I responses with their constitutive expression of interferon regulatory factor 7 (IRF7). Non-pDCs then upregulate IRF7 levels, which drive ISG induction via IFN-β [18, 19]. Several studies have addressed this issue in vitro recently, and the data are conflicting:
Evidence Against a Role for pDCs in Chronic IFN-I Production
Inhibition of pDC activation using TLR7/9 antagonists in SIV-infected cynomolgus macaques has minimal impact on ISG production [19]. Indeed, based on flow cytometric analysis of unstimulated pDCs taken directly ex vivo, IFN-α is detectable during acute SIV infection of cynomolgus macaques but wanes quickly. This is consistent with the finding that pDC-depleted mice have intact antiviral responses to local herpes simplex virus-1 infections, but they are impeded in their IFN-I responses to systemic herpesvirus infections [20].
Evidence Supporting pDCs Making Chronic IFN-I
In vivo imaging studies have shown that virtually all IFNα-expressing cells in SIV-infected monkeys also express the pDC-marker CD123 [21], and depletion of pDCs in humanized mice abolishes IFN-I and ISG responses to HIV infection [22]. We have observed in rhesus macaques that virtually all detectable IFNα made in response to in vitro stimulation with AT-2-treated SIV originated from pDCs [23]. Similar data has been seen for African green monkeys, where in vitro depletion of pDCs completely abolishes IFN-I activity [24]; however, pDC depletion only partially abrogates IFN-I production to HSV stimulation.
Given the current in vivo evidence available, it is plausible that pDCs may indeed be the primary cell type producing IFN-I in chronic infection, but their scarcity and distribution in tissues render them difficult to assess ex vivo. However, another possibility is that pDCs may only be responsible for IFN-I during the peak of viral replication, after which alternative cells are responsible. This “switch” may occur due to the emergence of new potential stimuli after acute infection, particularly microbial products [7] and novel gastrointestinal viruses [8] that are found after pathogenic infection. Antagonism of the IFN-I system remains of clinical interest in treating HIV-related residual inflammation. Thus, identifying the primary sources of IFN production has high potential for interventional strategies.
Effects of IFN on Antiviral Gene Expression
ISGs with Anti-HIV/SIV Activity
In the past decade, several ISGs that target HIV throughout its life cycle, i.e., restriction factors, have been identified (Fig. 1f). The best characterized restriction factors are TRIM5α, which interferes with HIV-1 uncoating [25]; apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3), which induces cytidine to uridine editing of HIV cDNA, resulting in hypermutation of the plus-strand DNA [26]; and Tetherin/BST2, which is expressed on infected cell surfaces, where it binds virions and prevents their release [27–29].
Several novel IFN-I-inducible anti-HIV factors have recently been described. SAM domain and HD domain-containing protein 1 (SAMHD1) was shown to inhibit HIV reverse transcription in resting T cells [30] by removing the triphosphate from deoxynucleoside triphosphates (dNTPs), decreasing deoxynucleotide precursors [31]. MX2 prevents HIV integration into the host DNA, possibly by blocking gag capsid-dependent nuclear entry [32••, 33••]. Many highly exposed seronegative subjects have polymorphisms conferring increased levels of MX2 expression [34]. Similarly, elite controllers upregulate schlafen 11 (SLFN11) in CD4 T cells, especially central memory CD4 T cells, compared to viremic noncontrollers or patients on antiretroviral therapy (ART) [35•]. SLFN11 binds transfer RNAs (tRNAs) to prevent HIV’s alteration of tRNA composition, which facilitates its protein production [36•]. IFN-stimulated gene 15 (ISG15) subsequently prevents HIV release from budding cells [37]. More recently, an elaborate live-cell imaging system was used to identify >50 novel factors that impact HIV replication [38••], although the majority of these remain to be confirmed by in-depth study. In sum, IFN-stimulated antiviral genes target virtually every step of the HIV life cycle.
Whether all or only some of these restriction factors are critical for controlling HIV infection in vivo remains unknown. In addition, the degree to which these restriction factors contribute to virus suppression during combination antiretroviral therapy (cART), or conversely, the extent to which their absence may contribute to low-level viremia, remains to be determined. Furthermore, the expression of restriction factors in the diverse tissues that HIV infects has not been rigorously studied but may illuminate more details about the pathogenesis of reservoir establishment.
Several ISGs Act to Amplify the IFN-I Response
More than 300 ISGs have been identified. Whereas some ISGs restrict virus replication, others enhance pathogen-associated molecular pattern (PAMP) detection and IFN signalling and amplify the IFN response. The best characterized of these ISGs is the IRF7 transcription factor [39••]. IRF7, “the master regulator” of IFN-I expression [40], is activated by innate signaling from both the TLR and RLR family of pathogen recognition receptors. In response to viral PAMPs, pDCs rapidly produce IFN-α/β that induce IRF7 transcription in neighboring cells, amplifying systemic IFN-I production. Indeed, IRF7 polymorphisms have been associated with decreased IFN-α production by pDCs in response to inactivated HIV-1 [41]. In addition to being a restriction factor, TRIM5α acts as a pattern recognition receptor for the HIV capsid and induces innate signaling [42]. The ISG cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) synthase (cGAS) stimulates IFN-I production in response to cytosolic DNA [38••, 43••]. cGAMP activates the protein “stimulator of IFN genes” (STING) to activate IκB kinase and TANK-binding kinase 1, which activate NFκB and IFN regulatory factor 3 (IRF3), culminating in IFN-I production [44••, 45••]. cGAS also triggers an IFN-I-independent antiviral gene repertoire [43••]. Another DNA binding protein, IFN-γ-inducible protein 16 (IFI16) [46], also activates STING, resulting in IFN-I production [47]. Consistent with their roles in DNA detection, cGAS and IFI16 mRNA levels are higher in viremic than suppressed HIV-infected patients [48]. IFI16 may also contribute to low CD4 T cell counts and increased T cell activation by inducing inflammasome activation and pyroptosis in quiescent CD4 T cells [49••, 50•]. Thus, numerous ISGs, once induced, act to perpetuate IFN-I production. This feed forward cycle of IFN-I induction accelerates innate responses to combat acute viral infections. However, in chronic, nonclearing infections such as HIV, the persistence of the innate response may have detrimental cytopathic effects.
Effect of IFN on Antiviral Cellular Immunity
Type I IFNs support the proliferation and survival of NK cells [51], stimulate their activation, and enhance their cytotoxic activity (Fig. 1e) [52]. Although IFN-Is may activate NK cells directly through the IFN-α receptor (IFNAR), recent data suggest IFN-I also induces NK cells and DCs to upregulate IL-15Rα, which binds and cis- or trans-presents IL-15 to NK cells, respectively [53, 54]. NK cells mediate antiviral responses primarily through the release of cytotoxic granules. However, activated NK cells modulate T cell responses by regulating antigen presentation—NK cell depletion prior to LCMV infection can actually enhance antigen-specific CD4 and CD8 T cell function [55, 56]. Intriguingly, IFN-I also renders CD8 T cells resistant to NK-cell-mediated cytotoxicity by stimulating their upregulation of MHC molecules that bind NK cell inhibitory receptors (Fig. 1d) [57•]. In the absence of IFN-I signaling, activated CD8 T cells upregulate NK cell activating ligands that render them susceptible to NK-cell-mediated cytotoxicity [58•]. Thus, in acute infections, type I IFN signaling may simultaneously activate NK cell cytotoxicity and render activated CD8 T cells resistant to NK cell attack.
Recent data from the LCMV mouse model indicate that chronic type I IFN signaling may be detrimental to antiviral CD4 T cell responses (Fig. 1). Infection with the clone 13 strain of LCMV results in a chronic infection with persistent, uncontrolled viremia, resembling HIV infection, that can model the deleterious effects of persistent TLR signaling on the immune system. Indeed, this model was used to characterize the exhaustion marker PD1 on dysfunctional CD8 T cells in unresolved LCMV infection [59]. ISGs are transiently upregulated in infection with the self-limited LCMV Armstrong (Arm) strain but persistently upregulated with the chronic LCMV clone 13 (Cl13) infection [60•], and these ISGs were upregulated in PD1+ CD4 and CD8 T cells [61]. Blocking IFN-I signaling during acute LCMV-Arm infection decreased the number and function of CD8 T cells and delayed virus clearance [60•, 62••]. In contrast, IFN receptor blockade before and after establishment of chronic LCMV infection with the LCMV-Cl13 strain resulted in increased early viremia but improved virus control during chronic infection. CD4 T cell function was restored in conjunction with decreased PD-L1 and IL-10 expression on DCs [60•, 62••]. Accordingly, PD1 signaling blockade has also been shown to reduce viremia in LCMV [59], SIV [63], and HIV infection in humanized mice [64]. Collectively, these data suggest early IFN-I signaling may enhance viral control during acute infection but contribute to persistent viremia during chronic infection by inhibiting cellular immunity.
Manipulation of the IFN-I System In Vivo
In chronic infections such as HIV, it is possible that the effect of IFN-I on NK cell cytotoxicity or CD4 and CD8 T cell responses may be more important than induction of ISGs and restriction factors on host survival. Significant progress has been made in establishing humanized murine models of HIV infection so that gene-targeting studies can begin to systematically unravel the interplay of these factors. Given the complexity of the IFN-I system, in vivo manipulation is critical to understanding the relative contributions of its effects on HIV pathogenesis.
HIV infection can be envisioned as three different biological conditions: acute infection—a dynamic state with an evolving cytokine storm, upregulation of antiviral factors, establishment of the reservoir, followed by a decrease in viremia to the virus set point; chronic untreated HIV infection—with steady cytokine production, constant yet low-level ISG expression, stable viremia, and gradual CD4 T cell depletion; and chronic HIV infection with virologic suppression—with even lower cytokine production, undetectable viremia, and CD4 T cell stability or recovery. Manipulation of IFN signaling in these three stages may have differential impacts. In the sections below, we will summarize the clinical information that has been gained from in vivo manipulation of IFN-I signaling in the setting of lentivirus infection, with special emphasis on recent interventional studies in NHP/SIV models.
Early IFN-α Monotherapy Clinical Studies in HIV Infection
The first study of type I IFN administration in HIV-infected patients was published just 3 years after the initial clinical description of AIDS [65]. Subcutaneous (SQ) IFN-α administration to patients with Kaposi’s sarcoma (KS) [65, 66] resulted in lower HIV burden and higher CD4:CD8 T cell ratios in some treated subjects compared to placebo (see Table 1). Subsequently, several studies evaluated SQ or intramuscular (IM) IFN-α in subjects who were asymptomatic and/or with minimal immunosuppression [67–70]. Overall, IFN-α-treated groups had a less severe CD4 decline (and in some instances an increase), lower HIV burden, fewer opportunistic infections, and slower disease progression, despite increased frequency of activated CD8 T cells.
Optimism arose from several studies of oral IFN-α reported administration in the early 1990s. In one influential study of 32 critically ill AIDS patients, 16/16 control patients died whereas 14/16 IFN-α-treated patients survived to hospital discharge [71]. A larger study from the same group reported increased Karnofsky scores and fewer symptoms in the treated subjects [72]. Whereas some studies reported an attenuation of CD4 depletion (or even an increase) with oral IFN-α [73, 74], several large, randomized, placebo-controlled, double-blind studies failed to show a statistically significant benefit [75–77]. With the unarguable efficacy of cART evident by the late 1990s, particularly in patients with lower CD4 T cell counts, and the preponderance of negative data from these rigorous studies, oral IFN-α monotherapy as an HIV treatment was abandoned.
IFN-α as an Adjunct to Antiretroviral Therapy
The approval of zidovudine and, eventually, many other antiretrovirals, renewed interest in parenteral IFN-α as an adjunct therapy. The combination of zidovudine with IM or SQ IFN-α in KS patients conferred decreased HIV burden in most studies, although the IFN-α and zidovudine doses and study duration varied widely (see Table 2) [78–80]. Subsequently, over a dozen studies have been performed in subjects on one, two, or three (or more) antiretrovirals with diverse CD4 T cell counts and HIV burdens. The majority of studies were performed in participants with detectable p24 antigen or HIV RNA and showed a decline in virus burden when IFN-α + ART was compared to ART alone [81–85]. More recently, a study of patients with hepatitis C and suppressed HIV RNA levels who received IFN-α with ribavirin showed a decrease in CD4 T cell-associated total and integrated HIV DNA that persisted after stopping IFN-α and ribavirin [86]. However, none of these studies showed a statistically significant, sustained improvement in CD4 T cell recovery with the addition of IFN-α to ART, and rates of progression were unaffected by 52 weeks of IFN-α in one study with 15 years of follow-up [85]. While IFN-α administration can decrease virus rebound in the setting of a structured treatment interruption, lower CD4 T cell counts ensue [87, 88]. The net result from these clinical studies is that, despite a suppressive effect on HIV burden, adding IFN-α to an effective ART regimen does not significantly improve CD4 T cell reconstitution or clinical outcome.
IFN-α Administration in Natural Host Species of SIV
As noted above, SIV infection of disease-susceptible macaque species (rhesus, cynomolgus, pigtailed) results in an early surge of ISG expression that persists indefinitely. In contrast, SIV infection of natural host species (sooty mangabeys, African green monkeys) results in a nonpathogenic infection—and despite early expression, ISGs quickly normalize [5, 6]. To test the possibility that the resolution of ISGs was responsible for protection from disease, two studies tested the effect of IFN-α administration in natural host species. The administration of high doses of recombinant IFN-α during days 9–24 of acute SIVagm infection in African green monkeys did not upregulate ISGs, decrease viremia, or affect T cell activation or CD4 T cell decline [89]. In contrast, SIV levels decreased in the one African green monkey treated during chronic infection, without changes in T cell activation [89]. Similarly, administration of recombinant IFN-α to chronically infected sooty mangabeys resulted in a modest ISG upregulation and decreased viremia, yet had no effect on cell-associated virus, CD4 T cell activation, or CD4 T cell counts [90•]. A modest, but transient, reduction of CD8 immune activation was observed. Thus, exogenous IFN-α in nonpathogenic SIV infection decreases viremia during chronic but not acute infection with minimal impact on immune activation, viremia, or disease progression.
In Vivo Inhibition of TLR Signaling in HIV/SIV Infection
As noted above, pDCs are key producers of type I IFNs. Administration of chloroquine, which inhibits TLR7 and TLR9 signaling by preventing endosomal acidification, during acute SIVmac251 infection unexpectedly increased ISG expression and decreased CD4 T cell recovery [91]. Similarly, administration of hydroxychloroquine to ART-naïve patients resulted in lower CD4 T cell counts, higher HIV RNA levels, and possibly decreased CD8 T cell activation [92, 93], whereas administration to immunologic nonresponders (lack of CD4 T cell reconstitution after ART) reduced T cell activation and inflammation and improved CD4 T cell frequency [94]. However, in another study of immunologic nonresponders, the addition of chloroquine to ART for 24 weeks increased IFN-α2 levels but had no impact on CD4 T cell counts, T cell activation, or circulating inflammatory markers [95]. Thus, indirect attempts to block IFN-I signaling thus far have had mixed success but appear to require ART suppression to exert any beneficial effect.
In Vivo Blockade of IFNAR in SIV Infection
We recently performed a comprehensive in vivo study in which we manipulated IFN-I signaling during SIV challenge and acute infection in rhesus macaques (RMs) [96••]. In one group of RMs, an antagonist of the IFNAR (IFN-1ant) was administered during acute SIVmac251 infection; pegylated IFN-α2a was given to a second cohort of RMs during acute infection, starting 1 week prior to rectal inoculation; and a third group of RMs received placebo saline during acute infection [96••]. Interfering with IFN-I signaling during acute SIV infection proved to have a profound impact on disease progression and survival. Administration of IFN-1ant from day 0 through day 28 of acute SIV infection delayed the upregulation of ISGs, including many of the aforementioned antiviral mediators, and many pattern recognition receptors by several days, but ultimately, they had comparable expression levels to placebo RMs. Nevertheless, this delay of IFN-I signaling during the first 10 days of infection proved to have a dramatic effect on the chronic phase of infection, as higher viremia, increased CD4 T cell depletion, and accelerated progression to AIDS and death ensued. In contrast, administration of pegylated IFN-α2a starting 1 week prior to rectal challenge conferred protection against systemic infection, as all RMs needed repeat exposures to become systemically infected with the high inoculum dose. This protective effect was dependent upon ISG upregulation. An IFN-I-tolerant state was established, potentially mediated by FOXO3a, ultimately resulting in delayed ISG expression during acute infection and a concomitant increase in cell-associated SIV and CCR5+ CD4 T cell depletion [96••]. Together, these data indicate that the precise timing of antiviral gene expression during acute SIV infection in the pathogenic host can profoundly influence disease outcome. The finding that IFN-α2a prevented infection, at least temporarily, contrasts with the results of a previous study targeting transmission and acute SIV infection. Administration of IFN-α2b or the IFN-αB/D chimera to rhesus macaques starting 1 day before intravenous challenge with SIV DeltaB670 and continued through 90 days post-infection decreased peak antigenemia but did not impact disease progression [97]. The differential impact on acquisition may be attributed to the pathogenicity of SIV DeltaB670 or to the contribution of the rectal mucosal barrier, both structurally and as a consequence of local ISG upregulation and its consequences. ISGs were not evaluated in the intravenous challenge study, so whether they were persistently upregulated or whether tolerance developed is unknown. In contrast, 14 weeks of pegylated IFN-α2a administration starting during chronic SIV infection resulted in transient ISG upregulation but no impact on plasma SIV RNA levels in rhesus macaques [98]. Taken together, no NHP interventional study has demonstrated that IFN-I administration can slow progression.
Vaccination Against IFN-α in HIV-Infected Patients
In the 1990s, several clinical trials of an adjuvanted vaccine targeting endogenous IFN-α2a were pursued (see Table 1). Vaccination induced anti-IFN-α antibody production, but there was no detectable effect on CD4 T cell counts or HIV burden. However, vaccine responders had fewer HIV-related events compared to nonresponders or people who received the placebo [3, 4, 99], but the responders also had higher CD4 T cell counts, and some subjects were taking ART, rendering the data difficult to interpret.
Conclusion
Based on the combined human and animal model data, administering IFN-I during intravenous challenge, acute infection, or viremic or ART-suppressed chronic infection yielded no benefit, whereas it protected against rectal acquisition as long as IFN-I signaling was upregulated, suggesting that manipulation of the IFN system may have some potential for HIV prevention but a mucosal interface may be necessary. It is worth noting that a similar experiment has not been tested with the vaginal challenge model. Although it is an anatomical barrier, the vaginal mucosa does not contain the large population of potential target cells for HIV or SIV that are resident in the rectum. Whether a similar IFN-I-induced protective effect would be observed with a vaginal challenge is unknown, as several studies have shown that increased local inflammation may instead facilitate infection by recruiting target cells that are already present in the rectal mucosa. Indeed, increased vaginal inflammation is associated with increased susceptibility to HIV infection [100, 101]. Thus, the net result of simultaneous target cell recruitment to the vaginal mucosa and antiviral gene induction in response to IFN-I administration remains unknown, and the mode of acquisition may be necessary to consider with future vaccine platforms. In addition, the creation of an IFN-I-tolerant state with repeated IFN-α administration raises concern about vaccines that elicit ISGs. A vaccine that prevented HIV temporarily but ultimately disarmed the innate immune system and precipitated faster disease progression would be counterproductive. Elucidating the triggers of an IFN-I-tolerant state, and ultimately preventing it, will be critical when considering adjuvanted vaccines for HIV prevention. Alternatively, inducing higher but transient ISG expression during acute infection may be beneficial, but this has not been clearly demonstrated with interventions used thus far. In addition, the timing of when ISGs are beneficial, unnecessary, or detrimental in HIV infection, particularly with effective cART, remains largely unknown.
Outstanding Questions
-
1.
Can IFN-I signaling be further increased during acute infection, and if so, would this increase virus control or increase detrimental immune activation?
-
2.
Would adding IFN-I to cART during acute infection reduce the reservoir and slow progression more than cART alone?
-
3.
Which cells are producing IFN-I in chronic infection?
-
4.
Could supplementation of IFN-I to cART treatment during chronic infection reduce the viral reservoir?
-
5.
Would blocking IFN-I signaling while administering cART during acute infection be detrimental because of the decreased restriction factor expression or advantageous because of decreased target cell recruitment and virus spread?
-
6.
Would blocking IFN-I signaling while administering cART during chronic infection increase the reservoir because of the decreased restriction factor expression, and could it be used as a tool to reverse latency?
-
7.
Conversely, could blockade of IFN-I in cART-suppressed HIV infection decrease end-organ disease and the reservoir because of decreased immune activation and inflammation and increased T cell responses?
Despite over 30 years of research, the exact role of IFN-I in HIV disease progression remains unclear. Numerous questions remain, and whether augmenting or blocking IFN signaling is the best strategy for prevention, treatment, and cure remains a pivotal area for HIV research.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
DeStefano E, Friedman RM, Friedman-Kien AE, Goedert JJ, Henriksen D, Preble OT, et al. Acid-labile human leukocyte interferon in homosexual men with Kaposi’s sarcoma and lymphadenopathy. J Infect Dis. 1982;146(4):451–9.
Buimovici-Klein E, Lange M, Klein RJ, Cooper LZ, Grieco MH. Is presence of interferon predictive for AIDS? Lancet. 1983;2(8345):344.
Gringeri A, Musicco M, Hermans P, Bentwich Z, Cusini M, Bergamasco A, et al. Active anti-interferon-alpha immunization: a European-Israeli, randomized, double-blind, placebo-controlled clinical trial in 242 HIV-1-infected patients (the EURIS study). J Acquir Immune Defic Syndr Hum Retrovirol Off Publ Int Retrovirol Assoc. 1999;20(4):358–70.
Gringeri A, Santagostino E, Cusini M, Muca-Perja M, Marinoni A, Mannucci PM, et al. Absence of clinical, virological, and immunological signs of progression in HIV-1-infected patients receiving active anti-interferon-alpha immunization: a 30-month follow-up report. J Acquir Immune Defic Syndr Hum Retrovirol Off Publ Int Retrovirol Assoc. 1996;13(1):55–67.
Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119(12):3544–55. doi:10.1172/jci40093.
Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119(12):3556–72. doi:10.1172/jci40115.
Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–66. doi:10.1038/nrmicro2848.
Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151(2):253–66.
Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87(4):609–20. doi:10.1189/jlb.0909635.
Sabado RL, O'Brien M, Subedi A, Qin L, Hu N, Taylor E, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116(19):3839–52. doi:10.1182/blood-2010-03-273763.
O'Brien M, Manches O, Sabado RL, Baranda SJ, Wang Y, Marie I, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121(3):1088–101. doi:10.1172/jci44960.
Barratt-Boyes SM, Wijewardana V, Brown KN. In acute pathogenic SIV infection plasmacytoid dendritic cells are depleted from blood and lymph nodes despite mobilization. J Med Primatol. 2010;39(4):235–42. doi:10.1111/j.1600-0684.2010.00428.x.
Reeves RK, Evans TI, Gillis J, Wong FE, Kang G, Li Q, et al. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J Infect Dis. 2012;206(9):1462–8. doi:10.1093/infdis/jis408.
Li H, Gillis J, Johnson RP, Reeves RK. Multi-functional plasmacytoid dendritic cells redistribute to gut tissues during simian immunodeficiency virus infection. Immunology. 2013;140(2):244–9. doi:10.1111/imm.12132.
Lehmann C, Jung N, Forster K, Koch N, Leifeld L, Fischer J, et al. Longitudinal analysis of distribution and function of plasmacytoid dendritic cells in peripheral blood and gut mucosa of HIV infected patients. J Infect Dis. 2014;209(6):940–9. doi:10.1093/infdis/jit612.
Kwa S, Kannanganat S, Nigam P, Siddiqui M, Shetty RD, Armstrong W, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2011;118(10):2763–73. doi:10.1182/blood-2011-02-339515. This study demonstrates that pDCs rapidly migrate to the intestinal and lymphoid tissues after acute SIV infection and are decreased in the peripheral blood. This may result in local inflammation with IFN-I production, suggesting targeting pDCs may attenuate HIV disease.
O'Brien M, Manches O, Bhardwaj N. Plasmacytoid dendritic cells in HIV infection. Adv Exp Med Biol. 2013;762:71–107. doi:10.1007/978-1-4614-4433-6_3.
Bruel T, Dupuy S, Demoulins T, Rogez-Kreuz C, Dutrieux J, Corneau A, et al. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog. 2014;10(1):e1003915. doi:10.1371/journal.ppat.1003915.
Kader M, Smith AP, Guiducci C, Wonderlich ER, Normolle D, Watkins SC, et al. Blocking TLR7- and TLR9-mediated IFN-alpha production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog. 2013;9(7):e1003530. doi:10.1371/journal.ppat.1003530.
Swiecki M, Wang Y, Gilfillan S, Colonna M. Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections. PLoS Pathog. 2013;9(10):e1003728. doi:10.1371/journal.ppat.1003728.
Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84(15):7886–91. doi:10.1128/jvi. 02612-09.
Li G, Cheng M, Nunoya J, Cheng L, Guo H, Yu H, et al. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog. 2014;10(7):e1004291. doi:10.1371/journal.ppat.1004291.
Bosinger SE, Johnson ZP, Folkner KA, Patel N, Hashempour T, Jochems SP, et al. Intact type I interferon production and IRF7 function in sooty mangabeys. PLoS Pathog. 2013;9(8):e1003597. doi:10.1371/journal.ppat.1003597.
Jochems SP, Petitjean G, Kunkel D, Liovat AS, Ploquin MJ, Barre-Sinoussi F et al. Modulation of type I interferon-associated viral sensing during acute simian immunodefiency virus (SIV) infection in African green monkeys. J Virol. 2014
Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427(6977):848–53. doi:10.1038/nature02343.
Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418(6898):646–50. doi:10.1038/nature00939.
Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–30.
Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139(3):499–511. doi:10.1016/j.cell.2009.08.039.
Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3(4):245–52. doi:10.1016/j.chom.2008.03.001.
Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, et al. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med. 2012;18(11):1682–7. doi:10.1038/nm.2964.
Zhang R, Bloch N, Nguyen LA, Kim B, Landau NR. SAMHD1 restricts HIV-1 replication and regulates interferon production in mouse myeloid cells. PLoS ONE. 2014;9(2):e89558. doi:10.1371/journal.pone.0089558.
Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, et al. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature. 2013;502(7472):559–62. doi:10.1038/nature12542. In this study, MX2 was demonstrated to suppress HIV and SIV infection, likely by preventing cDNA integration, suggesting its induction may facilitate virus control.
Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, et al. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature. 2013;502(7472):563–6. doi:10.1038/nature12653. This study demonstrates that MX2 is an IFN-inducible antiviral factor that works by inhibiting nuclear import of HIV, thereby preventing cDNA integration. This study furthers the data indicating that MX2 is a pivotal antiviral gene.
Sironi M, Biasin M, Cagliani R, Gnudi F, Saulle I, Ibba S, et al. Evolutionary analysis identifies an MX2 haplotype associated with natural resistance to HIV-1 infection. Mol Biol Evol. 2014;31(9):2402–14. doi:10.1093/molbev/msu193.
Abdel-Mohsen M, Raposo RA, Deng X, Li M, Liegler T, Sinclair E, et al. Expression profile of host restriction factors in HIV-1 elite controllers. Retrovirology. 2013;10:106. doi:10.1186/1742-4690-10-106. In this study, genes differentiating elite controllers, untreated non-controllers, non-controllers on ART, and uninfected subjects were identified. A score including an array of antiviral genes was derived to assess IFN-I expression, and increased SLFN11 expression distinguished elite controllers.
Li M, Kao E, Gao X, Sandig H, Limmer K, Pavon-Eternod M, et al. Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature. 2012;491(7422):125–8. doi:10.1038/nature11433. This study identifies SLFN11 as a gene that prevents the altered transfer RNA pool induced by HIV and prevents protein translation.
Pincetic A, Kuang Z, Seo EJ, Leis J. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J Virol. 2010;84(9):4725–36. doi:10.1128/JVI. 02478-09.
Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–5. In this study, over 380 ISGs were screened to determine the antiviral factors that contribute to control of numerous viral infections, demonstrating that IRF1, cGAS, RIG-I act on many viruses and other genes such as MX2 and IFITM3 are more targeted to HIV.
Litvak V, Ratushny AV, Lampano AE, Schmitz F, Huang AC, Raman A, et al. A FOXO3-IRF7 gene regulatory circuit limits inflammatory sequelae of antiviral responses. Nature. 2012;490(7420):421–5. In this study, FOXO3 was shown to downregulate multiple ISGs, including HIV restriction factors, by decreasing expression of IRF7, the master regulator of IFN-I signaling. This may be relevant to the study of vaccines that stimulate IFN-I production.
Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–7. doi:10.1038/nature03464.
Chang J, Lindsay RJ, Kulkarni S, Lifson JD, Carrington M, Altfeld M. Polymorphisms in interferon regulatory factor 7 reduce interferon-alpha responses of plasmacytoid dendritic cells to HIV-1. AIDS (London, England). 2011;25(5):715–7. doi:10.1097/QAD.0b013e328343c186.
Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472(7343):361–5. doi:10.1038/nature09976.
Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505(7485):691–5. doi:10.1038/nature12862. This paper demonstrates that cGAS is essential for control of both DNA and RNA viruses, suggesting its specific upregulation may be a therapeutic goal for HIV infection.
Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science (New York, NY). 2013;341(6148):903–6. doi:10.1126/science.1240933. This study is among the first to demonstrate that cGAS recognizes HIV DNA and stimulates IFNβ production, suggesting it may be key to viral control.
Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science (New York, NY). 2013;339(6121):786–91. doi:10.1126/science.1232458. In this study, cGAS was shown to respond to DNA the cytoplasm and to be necessary for IFNβ production upon stimulation by viral DNA.
Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004.
Thompson MR, Sharma S, Atianand M, Jensen SB, Carpenter S, Knipe DM, et al. Interferon gamma-inducible protein (IFI) 16 transcriptionally regulates type I interferons and other interferon-stimulated genes and controls the interferon response to both DNA and RNA viruses. J Biol Chem. 2014;289(34):23568–81. doi:10.1074/jbc.M114.554147.
Nissen SK, Hojen JF, Andersen KL, Kofod-Olsen E, Berg RK, Paludan SR, et al. Innate DNA sensing is impaired in HIV patients and IFI16 expression correlates with chronic immune activation. Clin Exp Immunol. 2014;177(1):295–309. doi:10.1111/cei.12317.
Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science (New York, NY). 2014;343(6169):428–32. In this study, the ISG IFI16, which can recognize HIV DNA, was identified as critical for bystander cell death, reflecting the connection between IFN-I signaling and CD4 T cell depletion.
Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–14. In this study, pyroptosis that occurs as a result of the recognition of HIV DNA transcripts by the ISG IFI16 was highlighted as a cause of CD4 T cell death, connecting the IFN-I signaling pathway with CD4 T cell depletion.
Cook KD, Whitmire JK. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol (Baltimore, Md : 1950). 2013;190(2):641–9.
Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, et al. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol (Baltimore, Md : 1950). 2002;169(8):4279–87.
Stackaruk ML, Lee AJ, Ashkar AA. Type I interferon regulation of natural killer cell function in primary and secondary infections. Expert Rev Vaccines. 2012;12(8):875–84.
Zanoni I, Spreafico R, Bodio C, Di Gioia M, Cigni C, Broggi A, et al. IL-15 cis presentation is required for optimal NK cell activation in lipopolysaccharide-mediated inflammatory conditions. Cell Rep. 2014;4(6):1235–49.
Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481(7381):394–8.
Waggoner SN, Daniels KA, Welsh RM. Therapeutic depletion of natural killer cells controls persistent infection. J Virol. 2014;88(4):1953–60.
Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, et al. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40(6):949–60. This study demonstrates that IFN-I induces upregulation of an NK cell inhibitory ligand on CD8 T cells, facilitating their survival NK cell activation by IFN-I.
Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, et al. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40(6):961–73. This study uses an IFNAR−/− T cells to demonstrate that IFN-I signaling through T cells modulates their susceptibility to NK cell attack by downregulating an NK cell activating ligand, suggesting that IFN-I can activate NK cells without widespread T cell death.
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7.
Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science (New York, NY). 2013;340(6129):202–7. In this study of IFN-I blockade, decreased PD-L1 and IL-10 expression, preserved splenic organization, and increased LCMV control was achieved, suggesting that immune exhaustion and lymphatic tissue destruction in chronic viral infections such as HIV could be attenuated by decreasing IFN-I signaling.
Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40(2):289–302.
Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science (New York, NY). 2013;340(6129):207–11. In this mouse model of chronic LCMV viremia and TLR activation, type I IFNs were shown to suppress antigen-specific CD4 T cell responses, and IFN-I-blockade suppressed virus replication. These results suggest that a similar approach may be beneficial in other situations of chronic viremia, such as HIV.
Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–10. doi:10.1038/nature07662.
Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PLoS ONE. 2013;8(10):e77780.
Krown SE, Real FX, Krim M, Cunningham-Rundles S, Koziner B, Myskowski PL, et al. Recombinant leukocyte A interferon in Kaposi’s sarcoma. Ann N Y Acad Sci. 1984;437:431–8.
Lane HC, Kovacs JA, Feinberg J, Herpin B, Davey V, Walker R, et al. Anti-retroviral effects of interferon-alpha in AIDS-associated Kaposi’s sarcoma. Lancet. 1988;2(8622):1218–22.
Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, Herpin B, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann Intern Med. 1990;112(11):805–11.
Rivero J, Fraga M, Cancio I, Cuervo J, Lopez-Saura P. Long-term treatment with recombinant interferon alpha-2b prolongs survival of asymptomatic HIV-infected individuals. Biotherapy. 1997;10(2):107–13.
Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, Cramer Y, et al. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon Alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J Infect Dis. 2010;201(11):1686–96. doi:10.1086/652420.
Manion M, Rodriguez B, Medvik K, Hardy G, Harding CV, Schooley RT, et al. Interferon-alpha administration enhances CD8+ T cell activation in HIV infection. PLoS ONE. 2012;7(1):e30306. doi:10.1371/journal.pone.0030306.
Obel AO, Koech DK. Outcome of intervention with or without low dose oral interferon alpha in thirty-two HIV-1 seropositive patients in a referral hospital. East Afr Med J. 1990;67(7 Suppl 2):SS71–6.
Koech DK, Obel AO. Efficacy of Kemron (low dose oral natural human interferon alpha) in the management of HIV-1 infection and acquired immune deficiency syndrome (AIDS). East Afr Med J. 1990;67(7 Suppl 2):SS64–70.
Babiuch L, Mian M, Kaminska E, Szymanska B, Georgiades JA. An interim report on the effect of natural human interferon alpha (IFN-alpha) lozenges in patients seropositive for the human immunodeficiency virus type 1 (HIV-1). Arch Immunol Ther Exp (Warsz). 1993;41(3–4):213–9.
Jordan WC. Three open-label studies of oral interferon alpha in the treatment of HIV disease. J Natl Med Assoc. 1994;86(4):257–62.
Wright SE, Hutcheson DP, Cummins JM. Low dose oral interferon alpha 2a in HIV-1 seropositive patients: a double-blind, placebo-controlled trial. Biotherapy. 1998;11(4):229–34.
Katabira ET, Sewankambo NK, Mugerwa RD, Belsey EM, Mubiru FX, Othieno C, et al. Lack of efficacy of low dose oral interferon alfa in symptomatic HIV-1 infection: a randomised, double blind, placebo controlled trial. Sex Transm Infect. 1998;74(4):265–70.
Alston B, Ellenberg JH, Standiford HC, Muth K, Martinez A, Greaves W, et al. A multicenter, randomized, controlled trial of three preparations of low-dose oral alpha-interferon in HIV-infected patients with CD4+ counts between 50 and 350 cells/mm(3). Division of AIDS Treatment Research Initiative (DATRI) 022 Study Group. J Acquir Immune Defic Syndr. 1999;22(4):348–57.
de Wit R, Danner SA, Bakker PJ, Lange JM, Eeftinck Schattenkerk JK, Veenhof CH. Combined zidovudine and interferon-alpha treatment in patients with AIDS-associated Kaposi’s sarcoma. J Intern Med. 1991;229(1):35–40.
Kovacs JA, Deyton L, Davey R, Falloon J, Zunich K, Lee D, et al. Combined zidovudine and interferon-alpha therapy in patients with Kaposi sarcoma and the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1989;111(4):280–7.
Baumann R, Tauber MG, Opravil M, Hirschel B, Kinloch S, Chave JP, et al. Combined treatment with zidovudine and lymphoblast interferon-alpha in patients with HIV-related Kaposi’s sarcoma. Klin Wochenschr. 1991;69(8):360–7.
Edlin BR, Weinstein RA, Whaling SM, Ou CY, Connolly PJ, Moore JL, et al. Zidovudine-interferon-alpha combination therapy in patients with advanced human immunodeficiency virus type 1 infection: biphasic response of p24 antigen and quantitative polymerase chain reaction. J Infect Dis. 1992;165(5):793–8.
Mildvan D, Bassiakos Y, Zucker ML, Hyslop Jr N, Krown SE, Sacks HS, et al. Synergy, activity and tolerability of zidovudine and interferon-alpha in patients with symptomatic HIV-1 infection: AIDS Clinical Trial Group 068. Antivir Ther. 1996;1(2):77–88.
Haas DW, Lavelle J, Nadler JP, Greenberg SB, Frame P, Mustafa N, et al. A randomized trial of interferon alpha therapy for HIV type 1 infection. AIDS Res Hum Retrovir. 2000;16(3):183–90. doi:10.1089/088922200309278.
Angel JB, Greaves W, Long J, Ward D, Rodriguez AE, Scevola D, et al. Virologic and immunologic activity of PegIntron in HIV disease. AIDS (London, England). 2009;23(18):2431–8.
Tavel JA, Huang CY, Shen J, Metcalf JA, Dewar R, Shah A, et al. Interferon-alpha produces significant decreases in HIV load. J Interf Cytokine Res Off J Int Soc Interf Cytokine Res. 2010;30(7):461–4.
Sun H, Buzon MJ, Shaw A, Berg RK, Yu XG, Ferrando-Martinez S, et al. Hepatitis C therapy with interferon-alpha and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis. 2014;209(9):1315–20.
Boue F, Reynes J, Rouzioux C, Emilie D, Souala F, Tubiana R, et al. Alpha interferon administration during structured interruptions of combination antiretroviral therapy in patients with chronic HIV-1 infection: INTERVAC ANRS 105 trial. AIDS (London, England). 2011;25(1):115–8.
Goujard C, Emilie D, Roussillon C, Godot V, Rouzioux C, Venet A, et al. Continuous versus intermittent treatment strategies during primary HIV-1 infection: the randomized ANRS INTERPRIM Trial. AIDS (London, England). 2012;26(15):1895–905.
Jacquelin B, Petitjean G, Kunkel D, Liovat AS, Jochems SP, Rogers KA, et al. Innate immune responses and rapid control of inflammation in African green monkeys treated or not with interferon-alpha during primary SIVagm infection. PLoS Pathog. 2014;10(7):e1004241. doi:10.1371/journal.ppat.1004241.
Vanderford TH, Slichter C, Rogers KA, Lawson BO, Obaede R, Else J, et al. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood. 2012;119(24):5750–7. doi:10.1182/blood-2012-02-411496. In an attempt to increase inflammation in natural hosts, recombinant IFNα2 was administered to sooty mangabees with chronic SIV infection. Despite decreasing viral load, immune activation and CD4 T cell counts were not affected, suggesting that increased IFN signaling may not be sufficient to drive disease progression.
Vaccari M, Fenizia C, Ma ZM, Hryniewicz A, Boasso A, Doster MN, et al. Transient increase of interferon-stimulated genes and no clinical benefit by chloroquine treatment during acute simian immunodeficiency virus infection of macaques. AIDS Res Hum Retrovir. 2014;30(4):355–62. doi:10.1089/AID.2013.0218.
Paton NI, Goodall RL, Dunn DT, Franzen S, Collaco-Moraes Y, Gazzard BG, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308(4):353–61.
Murray SM, Down CM, Boulware DR, Stauffer WM, Cavert WP, Schacker TW, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol. 2010;84(22):12082–6. doi:10.1128/jvi. 01466-10.
Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118(12):3263–72.
Routy JP, Angel J, Patel M, Kanagaratham C, Radzioch D, Kema I et al. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med. 2014.
Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511(7511):601–5. doi:10.1038/nature13554. This study demonstrates that blockade of IFN-I signaling, whether by administration of an IFN receptor antagonist or induction of an IFN-tolerant state, during acute SIV infection results in greater viral burden and accelerated disease progression, whereas ISG upregulation during challenge can prevent infection. Thus, careful consideration of the effects of vaccines and preventative approaches on IFN-I signaling and attempts to attenuate IFN-I signaling in chronic infection should be pursued cautiously.
Schellekens H, Niphuis H, Buijs L, Douw van der Krap P, Hochkeppel HK, Heeney JL. The effect of recombinant human interferon alpha B/D compared to interferon alpha 2b on SIV infection in rhesus macaques. Antivir Res. 1996;32(1):1–8.
Asmuth DM, Abel K, George MD, Dandekar S, Pollard RB, Miller CJ. Pegylated interferon-alpha 2a treatment of chronic SIV-infected macaques. J Med Primatol. 2008;37(1):26–30. doi:10.1111/j.1600-0684.2007.00221.x.
Gringeri A, Santagostino E, Mannucci PM, Siracusano L, Marinoni A, Criscuolo M, et al. Anti-alpha interferon immunization: safety and immunogenicity in asymptomatic HIV positive patients at high risk of disease progression. Cell Mol Biol (Noisy-le-grand). 1995;41(3):381–7.
Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, et al. Innate immune activation enhances hiv acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J infect Dis. 2012;206(7):993–1001.
Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–8. doi:10.1038/nature07831.
Weber R, Bonetti A, Jost J, Vogt MW, Spacey B, Siegenthaler W, et al. Low-dose zidovudine in combination with either acyclovir or lymphoblastoid interferon-alpha in asymptomatic HIV-infected patients: a pilot study. Infection. 1991;19(6):395–400.
Frissen PH, van der Ende ME, ten Napel CH, Weigel HM, Schreij GS, Kauffmann RH, et al. Zidovudine and interferon-alpha combination therapy versus zidovudine monotherapy in subjects with symptomatic human immunodeficiency virus type 1 infection. J infect Dis. 1994;169(6):1351–5.
Compliance with Ethics Guidelines
Conflict of Interest
Steven E. Bosinger and Netanya S. Utay declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection on HIV Pathogenesis and Treatment
Rights and permissions
About this article
Cite this article
Bosinger, S.E., Utay, N.S. Type I Interferon: Understanding Its Role in HIV Pathogenesis and Therapy. Curr HIV/AIDS Rep 12, 41–53 (2015). https://doi.org/10.1007/s11904-014-0244-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-014-0244-6