Abstract
In the USA, hepatocellular carcinoma (HCC) has the most rapidly increasing cancer incidence since 1980, has a rate of death that has increased by 43% between 2000 and 2016 and is currently the second most lethal tumor with a 5-year survival of 18%. While the expected 5-year survival after liver transplant (LT) in patients with HCC is attractive at over 70%, LT is limited by extreme shortage of organs and post-LT immunosuppression. Numerous changes to the liver allocation system for HCC in the USA have been applied since 2002. However, for the most part, USA HCC patients continue to receive similar priority for LT despite ample evidence that tumor size and number is only one of many contributors to urgency (i.e. waitlist dropout), utility (i.e. post-LT survival) and LT survival benefit. In this review, we examine where current LT criteria for HCC has resulted in overuse including 1) compensated patients with a single, small, well-treated tumor and 2) patients with HCC amenable to up-front resection. We further examine where current LT criteria for HCC has resulted in underuse including 1) patients with HCC outside of standard criteria but who have favorable markers of tumor biology based on response to local regional therapies, alpha-fetoprotein and other serum biomarker levels, 18F-FDG-PET scan results and tumor biopsy as well as 2) HCC patients with decompensated cirrhosis who have an increased risk of waitlist dropout and thus likely merit additional priority given their increased LT survival benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) incidence has been the most rapidly increasing cancer incidence in the USA since 1980 [1]. The rate of death from HCC in the USA increased by 43% (from 7.2 to 10.2 death per 100,000) between 2000 and 2016 [2]. With a 5-year survival of 18%, HCC is the second most lethal tumor in the USA after pancreatic cancer [3]. However, expected 5-year survival after liver transplantation (LT) in patients with HCC is over 70% [4], making LT an attractive treatment option. Unfortunately, LT is limited by the extreme shortage of available liver allografts and then need for lifelong immunosuppression post-LT. Precisely because of this critical shortage, organ allocation systems in the USA and elsewhere have developed prioritization criteria which attempt to balance the benefit of transplantation and the risk of post-LT HCC recurrence and subsequent reduced survival.
Numerous changes to the liver allocation system for HCC in the USA have been applied since the MELD allocation system was adopted in 2002 [5, 6]. Initially, HCC stage I (T1), defined as a single lesion between 1 and 2 cm in size, and stage II (T2), defined as either a single HCC lesion between 2 and 5 cm or 2 to 3 HCC lesions all less than or equal to 3 cm in size, was transplantable. These criteria, also known as the Milan criteria, were assigned model for end-stage liver disease (MELD) exception scores of 24 and 29 points, respectively. This was reduced to 20 and 24 respectively in 2003 in an attempt to reduce over-prioritization of individuals with HCC, and then in 2005, T1 was further reduced to no MELD exception points, and T2 were given 22 [7, 8]. Despite the United Network for Organ Sharing (UNOS) continuing to refine by standardizing the system for LT explant pathology and establishing more rigorous imaging criteria [9], individuals with HCC still appeared over-prioritized with lower waitlist dropout and a higher LT rate along with inferior long-term post-LT survival than non-HCC individuals. As a result and in an attempt to equalize access to LT between HCC and non-HCC individuals [10], a system of delaying granting of a MELD exception score of 28 for 6 months after LT listing was adopted in 2015 to allow HCC tumor biology to naturally select out those with more aggressive tumor biology and presumably higher risk of post-LT recurrence [11]. After the 6 months of waiting and granting of MELD exception score of 28, candidates that remained within T2 or less HCC were granted 10% increase in MELD exception score to a CAP of 34, called the MELD escalator.
In an effort to further improve post-LT outcomes for individuals with HCC, UNOS adopted in 2017 a policy where candidates with T2 HCC but with an alpha-fetoprotein (AFP) greater than 1000 ng/mL are not eligible for standardized MELD exception until AFP falls below 500 after local-regional therapy (LRT) [12]. This change was a result of data showing that LT candidates with HCC within T2 criteria with high AFP have a high post-LT recurrence and mortality rate [13]. It is worth noting that several other AFP cutoffs have been proposed for incorporation into transplant criteria including 100 ng/mL [14,15,16], 200 ng/mL [17], and 400 ng/mL [18,19,20] along with the currently utilized 1000 ng/mL [13, 14, 21, 22]. At the same time, UNOS adopted a policy of allowing individuals with HCC outside of T2 criteria who are successfully down-staged into T2 criteria with LRT to proceed with MELD exception points. Based off the University of California, San Francisco (UCSF) down-staging criteria [21], UNOS defined its down-staging criteria to include (1) one lesion greater than 5 cm or less than or equal to 8 cm, or (2) 2 to 3 lesions each less than 5 cm and a total diameter of all lesions less than or equal to 8 cm, or (3) 4 to 5 lesions each less than 3 cm and a total diameter of all lesions less than or equal to 8 cm [12, 23]. Additional criteria that have found to have non-significant differences compared to T2 in terms of post-LT survival include UCSF criteria (single HCC less than or equal to 6.5 cm or 2 to 3 HCCs less than or equal to 4.5 cm with total tumor diameter less than or equal to 8 cm) [24, 25], Up-to-7 criteria (HCC having the number 7 as the sum of the size in cm of the largest tumor and the number of tumors) [26], total tumor volume (TTV) criteria + AFP (TTV < 115cm3 and AFP less than 400 ng/mL) [18, 27], and the AFP-French model (points system based on tumor size, number of tumors, and AFP cutoff levels at 100 ng/mL and 1000 ng/mL) [14].
Despite the policy changes implemented by UNOS, waitlist mortality for traditional non-exception point candidates continued to exceed mortality for HCC exception point candidates [28, 29] and geographic disparities in access to liver allografts for individuals with HCC continued to widen [30]. To address this, UNOS adopted in 2019 a policy where candidates with T2 HCC (including those successfully down-staged) who had AFP within criteria and after 6 months waiting would be granted an exception score of the median MELD at transplant within 250 nautical miles of the transplant center (MMaT/250) minus 3 or MMaT-3. On the basis of simulations provided by the Scientific Registry of Transplant Recipients (SRTR) [31, 32], all patients who qualify for MELD exceptions will receive MMaT-3. The MMaT/250 is calculated by UNOS every 180 days based on the previous 365-day cohort. Expected to lead to MELD “deflation,” this UNOS policy is hoped to place LT candidates with similar medical urgency, with or without HCC, on similar footing regardless of geography and with no significant effect on posttransplant mortality.
While T2 criteria, UNOS down-staging criteria, AFP cutoffs, 6-month waiting periods, and MMaT-3 are crucial to understanding how individuals with HCC in the USA can be listed and eventually undergo LT, these specifics do not tell us who with HCC should be listed for LT. Studies have shown that additional specificities must be considered at the time of LT listing for HCC, as competitive non-transplant options exist for the large majority of patients with well-preserved liver function [33, 34]. The concepts of urgency (the risk of dying before receiving a LT), utility (maximization of post-LT outcomes), and particularly transplant benefit are crucial in deciding which individual with HCC should be listed for LT. Transplant benefit is the net benefit in survival achieved by subtracting the survival that could be achieved by non-transplant options from the absolute post-LT survival [35]. Based solely on an approach meant to maximize transplant benefit, the current US criteria for LT among individuals with HCC may be counterintuitive [36, 37].
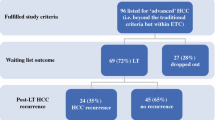
With this backdrop, this review will address situations where LT for HCC may be overused and situations where LT for HCC may be underused (see Fig. 1).
Current Transplant Criteria for HCC—Overuse
Hepatocellular carcinoma (HCC) is the only generally accepted indication for solid organ transplantation in cancer, and LT theoretically offers the best treatment by providing the most complete oncologic resection with the added benefits of replacing a diseased liver and restoring hepatic function. On the other hand, LT remains a technically demanding procedure with a well-established short-term mortality and morbidity and is fraught with persistent shortage of deceased donors with ongoing increase in demand. The number of HCC waitlist registrations in the USA had risen by nearly 2000 from 2005–2009 to 2010–2014 [30]. HCC now accounts for nearly 25% of all LTs performed in the USA, a number steadily rising from < 5% before MELD system implementation in 2002 to 10–15% in 2002–2008 [28, 38, 39]. Given the increasing demands of deceased donor LT for HCC, maximizing transplant benefit in individuals with HCC is of paramount importance [34].
Reduced Urgency: Compensated HCC Patients with Single Small Tumors
The main way LT is overused for HCC is by maximizing utility by selecting patients with very low risk of post-LT HCC recurrence and thereby maximizing post-LT survival. While this approach theoretically could improve transplant benefit, it often undercuts urgency as those patients with the lowest risk of post-LT HCC recurrence have a low risk of LT waitlist dropout. In a single center study, a subgroup of listed HCC individuals with a single 2 to 3 cm tumor, AFP < 20 ng/mL after first LRT, and a complete response to first LRT had a very low risk of waitlist dropout at < 2% at 2 years [40]. This subgroup, which accounted for nearly 20% of the HCC listed patients in the cohort, is unlikely to derive immediate benefit from LT. In another single center study of 315 HCC individuals listed for liver transplant, those with T2 disease who had complete response to LRT had similar waitlist drop rates to patients with T1 disease, and the response to LRT significantly affected both the recurrence rate of 176 listed individuals and the overall intention-to-treat survival [41]. The authors similarly concluded that the response to LRT is a potentially effective tool for prioritizing HCC patients for LT and may even allow selection of HCC individuals who would derive minimal benefit from LT. In a follow-up study looking at UNOS data in long wait time regions of 1, 5, and 9, 2052 individuals with T2 HCC listed between 2011 and 2014 were followed, and predictors of waitlist dropout were examined [42•]. Probabilities of waitlist dropout were 18.3% at 1 year and 27% at 2 years for the cohort. In multivariate analysis, factors associated with a lower risk of waitlist dropout including MELD-Na less than 15, Child-Turcotte-Pugh class A cirrhosis, single 2 to 3 cm lesion, and AFP < 20 ng/mL (all p < 0.01). The subgroup that had all four of these characteristics included 245 individuals (~ 12%), and they had a 1-year probability of dropout of 5.5% compared to 20% for all others (p < 0.01). Further, on explant, the low dropout risk group was more likely to have complete tumor necrosis (35.5% vs. 24.9%, p = 0.01) and less likely to exceed Milan criteria (9.9% vs. 17.7%, p = 0.03) [42•]. These studies primarily show that there are a group of HCC individuals who are listed for LT who either could be prioritized less than they currently are or even delisted and closely observed as performing LT on them is an overuse of the treatment option and does not maximize urgency nor transplant benefit. It has been proposed that individuals with HCC with very low risk of waitlist dropout, defined as single small HCC with resultant complete response after LRT, could be given lower priority such as MMAT – 5 after 6 months of waiting compared to other higher risk of waitlist dropout individuals [42•].
Liver Transplantation Versus Hepatic Resection
Another place where LT is overused for HCC is in patients that have HCC that would be amenable to hepatic resection. Individuals with HCC that do not have cirrhosis and have disease amenable to hepatic resection should not undergo LT, a recommendation supported by all major liver societies [43]. Individuals with cirrhosis and HCC are generally only amenable to hepatic resection if they have well-preserved liver function (including Child-Turcotte-Pugh class A cirrhosis with a total bilirubin less than or equal to 1 mg/dL and a MELD score less than 10), no evidence of prohibitive portal hypertension (including a hepatic vein wedge pressure less than 10 and/or platelets greater than 100 K/μl), single lesion HCC, or within T2 criteria and excellent performance status [6]. In a meta-analysis that compared overall intention-to-treat (ITT) survival and disease-free survival from LT and hepatic resection in individuals with T2 HCC, hepatic resection was favored with ITT 5-year overall survival (OR of 0.60, 95% CI 0.35–1.02) and ITT 5-year disease-free survival (OR of 0.18, 95% CI 0.06–0.53) [44]. This underscores that while a direct comparison of LT and hepatic resection on overall- and disease-free survival should always favor LT, an ITT comparison where those who dropped off the waitlist are factored tends to favor hepatic resection. Another study has shown that individuals with single, small (less than 3 cm), HCC who undergo hepatic resection have overall- and disease-free survival that are comparable to those who undergo LT [45]. Further, a multi-national study compared 3286 HCC individuals who received LT (n = 1218) or hepatic resection (n = 2068) and projected changes in overall survival based on varying rates of wait-list drop-out [46••]. The authors found that based on their data, for individuals with a single, less than 3 cm, HCC with preserved hepatic function (MELD less than or equal to 10) who lived in an area where wait-list drop-out was ~ 20%, overall survival between hepatic resection and LT were similar. These studies point out that LT may be overused for HCC when utility is prioritized over urgency, and thereby TB is not maximized.
In clinical practice, it may be hard to convince some individuals with HCC to undergo hepatic resection if they are also a candidate for LT. However, the strategy of salvage LT, where if LT is pursued under potentially favorable LT prioritization should HCC recur after hepatic resection, may help assuage this situation. The most recent Organ Procurement and Transplant Network policy states that patients with cirrhosis who presented with T2 resectable HCC who underwent complete resection but developed either T1 (biopsy proven) or T2 HCC within 2 years following complete resection can immediately be awarded MMAT – 3 exception points upon liver transplant listing without a 6-month delay period [47]. Multiple studies have supported this approach for T1 or T2 HCC amenable to hepatic resection [48,49,50,51,52], including a cost-effectiveness analysis [53].
Current Transplant Criteria for HCC—Underuse
The rising incidence of HCC coupled with the ongoing use of the restrictive Milan criteria for candidate selection has led to significant interest in expanding acceptable transplant criteria to offer LT to a wider population of HCC patients. However, in areas with organ shortages including the USA, it is generally accepted that post-LT outcomes for HCC patients should be similar to non-HCC patients [54] with the concern that expanding criteria to allow for greater tumor burden could compromise post-LT outcomes [55••]. In order to identify HCC patients beyond Milan criteria for which LT is currently being under-utilized, an accurate determination of tumor biology should be obtained to properly determine recipient benefit.
Expanding Transplant Criteria Using Serum Biomarkers, Tumor Biopsy, or PET Scan
In terms of biomarkers, AFP is the most extensively studied with post-LT survival declining at an AFP of ~ 20 ng/ml [13, 22, 56] with worse survival as AFP increases. A combination of AFP and tumor burden parameters, such as with the Metroticket 2.0 [55••] and the French AFP model [14], discriminates post-LT prognosis significantly better than using tumor burden alone. For example, using the Metroticket calculator, a patient with a single 7-cm tumor but AFP of 5 mg/mL would have an estimated 5-year post-LT HCC-specific survival > 85%, which exceeds proposed utility thresholds for post-LT survival and HCC recurrence. In addition to AFP, there are several markers of tumor biology in HCC patients beyond Milan criteria that can help predict post-LT survival and thus can be used to expand access to LT. Multiple centers have shown acceptable post-LT survival in HCC patients beyond Milan criteria by excluding those with poorly differentiated tumor via needle biopsy [57,58,59] though agreement of biopsy with explant pathology in terms of tumor differentiation grade is not ideal [60, 61]. Additional approaches largely from Eastern centers performing live donor LT (LDLT) for HCC beyond Milan criteria include 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) scan and measuring des-gamma-carboxy prothrombin (DCP) and lectin-reactive alpha-fetoprotein (AFP-L3) [62, 63] with cutoffs of 7.5 ng/mL and 35% respectively, associated with worse post-LT survival. 18F-FDG-PET tumor positivity, especially if the tumor to non-tumor ratio is > 2, has been associated with worse recurrence-free survival after LDLT [64]. The National Cancer Center Korea criteria includes total tumor diameter < 10 cm and negative 18F-FDG-PET scan with 5-year post-LDLT survival of 84% in recipients meeting these criteria compared to only 60% in those exceeding these criteria [65]. Similarly, DCP has been incorporated into the Japanese extended criteria [66] where patients beyond Milan criteria but with < 10 tumors, largest tumor size < 5 cm, and DCP < 7.5 ng/mL have 5-year post-LDLT survival > 80% compared to 42% in those beyond these criteria.
Maximizing Transplant Survival Benefit by Accounting for Liver Function
Currently in the USA, all HCC patients within Milan criteria are assigned the same allocation priority (i.e., MMAT-3) regardless of tumor characteristics or liver function. However, when approaching waitlist survival, both tumor and liver-related factors should be considered. For example, a decompensated HCC patient with MELD score in the 20s has a much higher risk of waitlist dropout compared to a well-compensated HCC patient who has less urgency for LT and thus reduced transplant survival benefit. Specifically, using the European HCC and LT (EurHeCaLT) project, Lai et al [67•] studied over 2100 HCC patients and found that MELD score < 13 decreased the survival benefit of LT. Additionally, Berry and Ioannou [34] found that HCC patients derive a significantly lower 5-year survival benefit from LT than non-HCC patients.
Several proposed models have suggested ways to increase priority for listed HCC patients based on waitlist dropout risk factors including MELD, AFP, and tumor size and number [68,69,70,71]. However, a major concern in adopting a proposal that gives additional priority to HCC patients with increased tumor burden and AFP is selecting aggressive tumors for LT with higher rate of post-LT HCC recurrence. Additionally, nearly all listed HCC patients receive LRT while awaiting LT [72], with increasing tumor burden in this setting associated with inferior post-LT outcome [73,74,75,76]. While this issue remains unresolved, one potential solution to avoid “underuse” and improve LT survival benefit would be to account for MELD-Na score above a certain threshold in decompensated HCC patients with increased urgency without giving additional priority for elevated AFP or increased tumor burden despite LRT.
Are There Upper Limits to Attempted Tumor Down-Staging?
The rationale of tumor down-staging, or a reduction in tumor burden using LRT to meet acceptable LT criteria (e.g., Milan), is to select candidates with favorable tumor biology based on objective response to LRT. In HCC patients meeting pre-specified upper limits of tumor burden who are successfully down-staged, several studies have found similar post-LT survival compared to those always within Milan criteria [19, 21, 77]. Accordingly, in order to standardize criteria for down-staging, in 2017 UNOS/OPTN adopted the UCSF/Region 5 down-staging protocol with patients successfully down-staged to within Milan criteria eligible for automatic priority listing for LT [78]. These initial down-staging selection criteria include single lesion <8 cm, or 2–3 lesions < 5 cm with total tumor diameter < 8 cm, or 4–5 nodules all < 3 cm with total tumor diameter < 8 cm.
Not surprisingly, liberalizing tumor size and number cutoffs beyond these down-staging inclusion criteria leads to a lower rate of successful down-staging, higher rates of waitlist dropout, and, of significant concern, worse post-LT survival [77, 79,80,81]. In the UNOS database, 3-year post-LT survival was 71% in the “all-comers” group with initial tumor burden exceeding UNOS down-staging criteria compared to 83% among patients always within Milan criteria [77]. Given these concerns, “all-comers” who are successfully down-staged to Milan criteria are evaluated by the National Liver Review Board on a case-by-case basis rather than receiving automatic MELD exception.
In order to successfully extend initial tumor size and number criteria for attempted down-staging, it is likely that more stringent AFP cutoffs are needed. In patients initially beyond Milan criteria requiring down-staging, AFP at LT > 100 ng/mL predicts higher risks of HCC recurrence and death, with a 3-year post-LT survival of 60% versus 81% for those with an AFP < 20 ng/mL [77]. Additionally, in “all-comers” with an AFP at LT > 20 ng/mL who are successfully down-staged on pre-LT imaging, 3-year post-LT survival was only 50% [77]. Lai et al. [82] recently assessed upper limits of tumor burden for attempted down-staging in combination with pre-treatment AFP and identified the following criteria leading to successful LT- AFP ≤ 20 ng/mL combined with up-to-twelve (i.e., # of tumors plus largest tumor in cm up to 12), AFP 21–200 with up-to-ten, and AFP 201–500 with up-to-seven. Besides combining pre-treatment tumor size and number with AFP cutoffs, additional considerations to successfully expand LT to a subset of “all-comers” include mandating a longer period of stability before LT to select less aggressive tumors and using stringent exclusion criteria for LT such as the development of new lesions during the period of observation [80].
Conclusions
Several recent UNOS/OPTN HCC policy changes standardizing transplant wait times, excluding LT candidates with AFP > 1000 ng/mL until reduction to < 500 with LRT, and granting automatic exception for patients meeting down-staging criteria have been important steps forward in the selection of HCC candidates for LT. However, for the most part, HCC patients meeting Milan criteria in the USA continue to receive similar priority for LT despite ample evidence that tumor size and number is only one of many contributors to urgency (i.e., waitlist dropout) and utility (i.e., post-LT survival) to determine LT survival benefit. Compensated patients with a single, small well-treated tumor, comprising ~ 20% of listed HCC patients, have extremely low risk of waitlist dropout, and thus LT is likely being overused for them. Similarly, up-front resection should be considered rather than LT when feasible, especially for patients with a single tumor up to ~ 3 cm. In the case of post-resection recurrence, salvage LT is likely an option with such patients able to bypass the mandatory 6-month wait before MELD exception. On the other end of the spectrum, expanding selection criteria to increase access to LT for those with tumor burden beyond Milan criteria can be accomplished by incorporating markers of tumor biology, including response to LRT (i.e., down-staging), AFP and other serum biomarkers, 18F-FDG-PET scan, and tumor biopsy. Finally, decompensated HCC patients with elevated MELD-Na score have increased risk of waitlist dropout and thus likely merit additional priority given their increased LT survival benefit.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- AASLD:
-
American Association for the Study of Liver Diseases
- HCC:
-
Hepatocellular carcinoma
- ITT:
-
Intention-to-treat
- LT:
-
Liver transplantation
- LRT:
-
Local-regional therapy
- MMaT-3:
-
Median MELD at transplant minus 3
- MMaT/250:
-
Median MELD at transplant within 250 nautical miles
- MELD:
-
Model for end-stage liver disease
- SRTR:
-
Scientific Registry of Transplant Recipients
- TTV:
-
Total tumor volume
- UNOS:
-
United Network for Organ Sharing
- US:
-
United States
- UCSF):
-
University of California, San Francisco
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Howlader N, Noone AM, Krapcho M, et al. (2019) SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site
Centers for Disease Control and Prevention, National Center for Health Statistics. Trends in liver cancer mortality among adults aged 25 and over in the United States, 2000–2016. July 2018 (https://www.cdcgov/nchs/products/databriefs/db314.htm).
Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring survival. J Natl Cancer Inst 2017;109.
Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9.
Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70.
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50.
Freeman RB Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4(Suppl 9):114–31.
Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127:S261–7.
Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–82.
Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61:1643–50.
Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10:1643–8.
Organ Procurement and Transplantation Network. Changes to HCC criteria for auto approval. Washington, DC, USA: United States Department of Health and Human Services; 2017; Available at: https://optn.transplant.hrsa.gov/media/1922/liver_hcc_criteria_for_auto_approval_20160815.pdf. [Accessed 20 October 2017].
Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945–51.
Duvoux C, Roudot-Thoraval F, Decaens T, et al. (2012) Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 143:986–94 e3; quiz e14–5
Levi DM, Tzakis AG, Martin P, et al. Liver transplantation for hepatocellular carcinoma in the model for end-stage liver disease era. J Am Coll Surg. 2010;210:727–34 35-6.
Grat M, Krasnodebski M, Patkowski W, et al. Relevance of pre-transplant alpha-fetoprotein dynamics in liver transplantation for hepatocellular cancer. Ann Transplant. 2016;21:115–24.
Hong G, Suh KS, Suh SW, et al. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol. 2016;64:852–9.
Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation. Hepatology. 2015;62:158–65.
Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–57.
Lai Q, Avolio AW, Manzia TM, et al. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transpl. 2012;26:E125–31.
Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968–77.
Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634–45.
Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80.
Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–403.
Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–96.
Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43.
Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–8.
Massie AB, Caffo B, Gentry SE, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11:2362–71.
Northup PG, Intagliata NM, Shah NL, Pelletier SJ, Berg CL, Argo CK. Excess mortality on the liver transplant waiting list: unintended policy consequences and model for end-stage liver disease (MELD) inflation. Hepatology. 2015;61:285–91.
Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Increasing liver transplantation wait-list dropout for hepatocellular carcinoma with widening geographical disparities: implications for organ allocation. Liver Transpl. 2018;24:1346–56.
SRTR Analysis Report, ‘Data Request from the OPTN Liver and Intestinal Organ Transplantation committee.’ Presented December 14, 2016.
Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 annual data report: liver. Am J Transplant. 2019;19(Suppl 2):184–283.
Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–55.
Berry K, Ioannou GN (2015) Comparison of liver transplant-related survival benefit in patients with versus without hepatocellular carcinoma in the United States. Gastroenterology 149:669–80; quiz e15–6.
Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–81.
Vitale A, Morales RR, Zanus G, et al. Barcelona clinic liver cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654–62.
Cillo U, Vitale A, Polacco M, Fasolo E. Liver transplantation for hepatocellular carcinoma through the lens of transplant benefit. Hepatology. 2017;65:1741–8.
Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology. 2014;60:1957–62.
Organ Procurement and Transplantation Network. National data: liver transplants 2017. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed July 1, 2018.
Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl. 2013;19:1343–53.
Cucchetti A, Cescon M, Bigonzi E, et al. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl. 2011;17:1344–54.
• Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Predictors of low risk for dropout from the liver transplant waiting list for hepatocellular carcinoma in long wait time regions: Implications for organ allocation. Am J Transplant. 2019;19:2210–8 Using UNOS data, study identified that individuals with hepatocellular carcinoma listed for liver transplant with native MELD-Na less than 15, Child-Turcotte-Pugh class A cirrhosis, single 2 to 3cm lesion and AFP less than 20ng/mL had lower risk of wait-list dropout and therefore may be a group with minimal transplant benefit.
Manzini G, Henne-Bruns D, Porzsolt F, Kremer M. Is there a standard for surgical therapy of hepatocellular carcinoma in healthy and cirrhotic liver? A comparison of eight guidelines BMJ Open Gastroenterol. 2017;4:e000129.
Menahem B, Lubrano J, Duvoux C, et al. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: an attempt to perform an ideal meta-analysis. Liver Transpl. 2017;23:836–44.
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–82.
•• Pinna AD, Yang T, Mazzaferro V, et al. Liver Transplantation and Hepatic Resection can Achieve Cure for Hepatocellular Carcinoma. Ann Surg. 2018;268:868–75 Multi-national study comparing liver transplant and hepatic resction for HCC that also projected changes in overall survival based on varying rates of wait-list drop-out. Found that individuals with a single, less than 3cm, HCC with perserved hepatic function who lived in an area where wait-list drop-out was ~20%, overall survival between hepatic resection and liver transplant were similar.
Organ Procurement and Transplantation Network. Guidance to liver transplant programs and the national liver review board for: adult meld exceptions for hepatocellular carcinoma (HCC). https://optn.transplant.hrsa.gov/media/2846/liver_guidance_hcc_201706.pdf . Accessed August 15, 2020.
de Haas RJ, Lim C, Bhangui P, et al. Curative salvage liver transplantation in patients with cirrhosis and hepatocellular carcinoma: an intention-to-treat analysis. Hepatology. 2018;67:204–15.
Bhangui P, Allard MA, Vibert E, et al. Salvage versus primary liver transplantation for early hepatocellular carcinoma: do both strategies yield similar outcomes? Ann Surg. 2016;264:155–63.
Yadav DK, Chen W, Bai X, et al. Salvage liver transplant versus primary liver transplant for patients with hepatocellular carcinoma. Ann Transplant. 2018;23:524–45.
Lee SY, Konstantinidis IT, Eaton AA, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB (Oxford). 2014;16:943–53.
Zheng J, Kuk D, Gonen M, et al. Actual 10-year survivors after resection of hepatocellular carcinoma. Ann Surg Oncol. 2017;24:1358–66.
Lim KC, Wang VW, Siddiqui FJ, et al. Cost-effectiveness analysis of liver resection versus transplantation for early hepatocellular carcinoma within the Milan criteria. Hepatology. 2015;61:227–37.
Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–22.
•• Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128–39 Data driven model called Metroticket 2.0 that predicts competing risks of death after liver transplantation for hepatocellular carcinoma based on AFP and tumor burden parameters.
Mehta N, Heimbach J, Harnois DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3:493–500.
Cillo U, Vitale A, Grigoletto F, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant. 2007;7:972–81.
Sapisochin G, Goldaracena N, Laurence JM, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: a prospective validation study. Hepatology. 2016;64:2077–88.
Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–32.
Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–92.
Court CM, Harlander-Locke MP, Markovic D, et al. Determination of hepatocellular carcinoma grade by needle biopsy is unreliable for liver transplant candidate selection. Liver Transpl. 2017;23:1123–32.
Lee JH, Cho Y, Kim HY, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg. 2016;263:842–50.
Chaiteerakij R, Zhang X, Addissie BD, et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2015;21:599–606.
Hsu CC, Chen CL, Wang CC, et al. Combination of FDG-PET and UCSF criteria for predicting HCC recurrence after living donor liver transplantation. Transplantation. 2016;100:1925–32.
Lee SD, Lee B, Kim SH, et al. Proposal of new expanded selection criteria using total tumor size and (18)F-fluorodeoxyglucose - positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: the National Cancer Center Korea criteria. World J Transplant. 2016;6:411–22.
Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053–60.
• Lai Q, Vitale A, Iesari S, et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology. 2017;66:1910–9 European HCC and LT (EurHeCaLT) project showing that in a cohort of over 2,100 individuals with HCC, native MELD score less than or equal to 13 significantly decreased the survival benefit of liver transplantation.
Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416–21.
Toso C, Dupuis-Lozeron E, Majno P, et al. A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list. Hepatology. 2012;56:149–56.
Alver SK, Lorenz DJ, Marvin MR, Brock GN. Projected outcomes of 6-month delay in exception points versus an equivalent model for end-stage liver disease score for hepatocellular carcinoma liver transplant candidates. Liver Transpl. 2016;22:1343–55.
Sasaki K, Firl DJ, Hashimoto K, et al. Development and validation of the HALT-HCC score to predict mortality in liver transplant recipients with hepatocellular carcinoma: a retrospective cohort analysis. Lancet Gastroenterol Hepatol. 2017;2:595–603.
Brondfield MN, Dodge JL, Hirose R, Heimbach J, Yao FY, Mehta N. Unfair advantages for hepatocellular carcinoma patients listed for liver transplant in short-wait regions following 2015 hepatocellular carcinoma policy change. Liver Transpl. 2020;26:662–72.
Kim DJ, Clark PJ, Heimbach J, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant. 2014;14:1383–90.
Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108–18.
Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–7.
Millonig G, Graziadei IW, Freund MC, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–9.
Mehta N, Dodge JL, Grab JD, Yao FY. National experience on down-staging of hepatocellular carcinoma before liver transplant: influence of tumor burden, alpha-fetoprotein, and wait time. Hepatology. 2020;71:943–54.
OPTN. Organ Procurement and Transplantation Network (OPTN) Policies. https://optn.transplant.hrsa.gov/media/1922/liver_hcc_criteria_for_auto_approval_20160815.pdf . Accessed July 22, 2020.
Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transpl. 2015;21:1142–52.
Sinha J, Mehta N, Dodge JL, Poltavskiy E, Roberts J, Yao F. Are there upper limits in tumor burden for down-staging of hepatocellular carcinoma to liver transplant? Analysis of the all-comers protocol. Hepatology. 2019;70:1185–96.
Murali AR, Romero-Marrero C, Miller C, et al. Predictors of successful downstaging of hepatocellular carcinoma outside Milan criteria. Transplantation. 2016;100:2391–7.
Lai Q, Vitale A, Halazun K, et al. Identification of an upper limit of tumor burden for downstaging in candidates with hepatocellular cancer waiting for liver transplantation: a west-east collaborative effort. Cancers (Basel). 2020;12.
Acknowledgments
The authors would like to acknowledge Francis Yao, MD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Neil Mehta has served on advisory boards for FujiFilm Wako and received institutional research grant support from FujiFilm WAKO, Glycotest, and Target Pharmasolutions.
Varun Saxena declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hepatic Cancer
Rights and permissions
About this article
Cite this article
Saxena, V., Mehta, N. Current Transplant Criteria for Hepatocellular Carcinoma—Overuse or Underuse. Curr Hepatology Rep 19, 470–477 (2020). https://doi.org/10.1007/s11901-020-00555-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-020-00555-5