Abstract
The outcome of umbilical cord blood transplantation for adult patients with hematologic malignancies now rivals that of matched unrelated donor transplantation. However, delayed hematopoietic and immunologic recovery remains a source of significant morbidity and mortality. Multiple strategies are now being studied to overcome these limitations. One strategy involves ex vivo expansion of the umbilical cord blood unit prior to transplantation. A second strategy involves exposure of the umbilical cord blood graft to compounds aimed at improving homing and engraftment following transplantation. Such a strategy may also address the problem of slow hematopoietic recovery as well as the increased risk of graft failure. Many of these strategies are now being tested in late phase multi-center clinical trials. If proven cost-effective and efficacious, they may alter the landscape of donor options for allogeneic stem cell transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation using umbilical cord blood (UCB) is a highly effective alternative to stem cells collected from healthy adult donors.
There are significant qualitative and quantitative differences in the umbilical cord blood graft compared to the adult donor graft. Most importantly, the CD34+ hematopoietic stem and progenitor cell doses are significantly lower. The lower cell dose has the most significant impact on adult recipients, resulting in an increased incidence of graft failure and delayed hematopoietic recovery. While there remains room for improvement, the advent of dual umbilical cord blood transplantation has, in large part, addressed the problem of excessive graft failure. Co-infusion of T cell depleted related haplo-identical donor stem cells along with the UCB graft is another technique being studied as a way to shorten the neutropenic period [1, 2]. Early engraftment of the haplo-identical donor graft is later immunologically rejected by engraftment of the UCB graft, which is then expected to provide long-term hematopoiesis.
Despite the remaining limitations, the outcome of UCB transplantation rivals that of mobilized peripheral blood or bone marrow transplantation from unrelated adult donors [3, 4]. Resolution of the remaining limitations could increase the desirability of the UCB transplantation for adult recipients, perhaps even escalating it to a preferred graft source in patients without a fully matched family member.
Addressing the problem of suboptimal stem cell dose within the UCB graft through ex vivo expansion has met with both practical and technical challenges. Before the advent of dual UCB, cells for ex vivo culture initiation were derived from a small aliquot of the transplanted single UCB graft. With the cell dose already marginal for most adult recipients, this strategy was only appropriate for the limited number of adult recipients with an available UCB unit that exceeded the minimum cell dose requirement. Furthermore, there was no definitive method for assessing the impact of the manipulated cell fraction, particularly the ex vivo expanded hematopoietic stem cell. These hurdles compromised early attempts at ex vivo expansion [5].
New techniques to exploit the proliferative and homing capacity of hematopoietic stem cells in an ex vivo culture system have emerged in recent years. Many of these technologies are now being tested in early and late phase clinical trials. If proven safe, effective, and practical, these strategies may indeed change the landscape of allogeneic stem cell transplantation.
Hematopoietic Stem/Progenitor Cell Manipulation
Until the advent of dual umbilical cord blood transplantation, the biggest impediment to development of novel hematopoietic stem cell expansion technologies was the ability to safely test and assess the approach in the clinical setting. The emergence of dual UCB transplantation has changed this, paving the way for innovative clinical trials of novel methods of cord blood graft engineering (Table 1) [6, 7]. As long as the patient receives a single UCB unit of adequate size, a second experimentally manipulated graft can be ethically co-infused and assessed for efficacy. Multiple strategies of ex vivo UCB manipulation have emerged. If lineage committed hematopoietic progenitor cells are targeted for expansion, the period of bone marrow aplasia that complicates intensive chemotherapy can be reduced or eliminated. However a second, unmanipulated UCB graft containing long-term repopulating hematopoietic stem cells will always need to be co-infused to provide durable hematopoiesis. If long-term repopulating cells are targeted for expansion, the manipulated graft can be expected to provide both short-term and long-term hematopoiesis, thereby eliminating the need for transplantation of a second unmanipulated unit. Another strategy aims not to expand hematopoietic stem/progenitor cells but to provide them with a homing and engraftment advantage following transplantation. This advantage could both improve kinetics of engraftment as well as reduce the relatively high risk of graft failure that continues to plague the field of UCB transplantation.
Notch-Mediated Expansion
Since the early 1990s, the Notch gene family has been implicated in modulation of early hematopoietic stem cell fate [8]. In vitro studies suggested that activation of Notch signaling promotes retention of the most primitive hematopoietic stem cell phenotype during culture. This led investigators at the Fred Hutchinson Cancer Research Center in Seattle Washington to develop a serum-free ex vivo hematopoietic stem cell (HSC) culture system consisting of immobilized Delta1 Notch ligand along with early acting HSC cytokines (stem cell factor [SCF], thrombopoietin [TPO], Flt3 ligand, IL-3, and IL-6) [9]. Of note, cultures were initiated with CD34+ cells isolated from the UCB unit. The final expanded product contained no T cells.
In the context of a phase I trial using a myeloablative dual umbilical cord blood transplant configuration, patients received one unmanipulated UCB unit and a second unit that was expanded for approximately 16 days in the presence of Notch ligand and cytokines. CD34+ hematopoietic stem–progenitor cells expanded a mean 164-fold during the culture period. In a report describing the outcome of the first ten patients treated on the trial, patients received an expanded cord blood graft containing an average of 6 × 106 CD34+ cells/kg [10]. This compared to 0.24 × 106 from the unmanipulated cord blood graft. The large number of hematopoietic progenitor cells contained within the expanded cord blood graft resulted in a median time to neutrophil engraftment (absolute neutrophil count (ANC) >500) of 16 days (range 7–34 days). This compared to 26 days for an historical control population that received standard myeloablative dual cord blood transplantation. Peripheral blood chimerism analysis confirmed that the expanded cord blood unit was responsible for early neutrophil engraftment. However, by 3 months following transplantation, hematopoiesis was derived by the unmanipulated cord blood unit in all but one of the evaluable subjects. While it is possible that transient hematopoiesis from the expanded UCB graft is a consequence of loss of long-term repopulated cells during ex vivo culture, it is more likely that the T cell replete unmanipulated unit mounted a cellular immune response leading to rejection of the expanded graft [11, 12].
Because of the logistical complexity associated with production of a patient-specific expanded cord blood graft, Delaney and colleagues are currently assessing the safety and efficacy of an “off-the-shelf” graft. With this strategy, cord blood transplant recipients receive an unmanipulated single or double UCB transplantation as per standard of care. In addition, the patient receives a fully HLA mismatched third UCB graft that was previously expanded and cryopreserved [13]. The intent is for this mismatched third graft to provide early neutrophil recovery after which it is rejected by the incoming unmanipulated, T cell replete UCB graft. This approach is currently being studied in the context of a multi-center phase II trial (Clinicaltrials.gov NCT01175785).
StemEx® Copper Chelator Expansion
Based on pre-clinical evidence that low concentrations of copper prevents stem cell differentiation in an in vitro culture system, Gamida Cell developed StemEx® which combines the copper chelator tetraethylenepentamine (TEPA) with early and late acting hematopoietic cytokines [14, 15]. The StemEx® UCB graft is derived from a single UCB unit. A fraction of the unit (contained in a separate segment of the cryobag) is thawed and expanded for 21 days in the presence of TEPA. On the day of transplantation, the recipient received the expanded fraction along with the unmanipulated fraction. StemEx® was first studied and found to be safe and feasible in a phase I/II trial performed at the MD Anderson Cancer Center [16]. Based on this early experience, Gamida Cell launched a multinational phase II/III registration trial designed to test the hypothesis that more rapid neutrophil engraftment facilitated by StemEx® would positively impact survival at 100 days following transplantation.
The study accrued 101 subjects with high-risk leukemia and lymphoma from 25 stem cell transplant centers in the USA, Europe, and Israel. Study patients were compared to a contemporaneous control group from the Center for International Blood and Marrow Transplant Research. The control population received myeloablative unmanipulated dual UCB transplantation between 2006 and 2010. The cumulative incidence of engraftment failure was 8.1 % for the StemEx® group and 14.5 % for the controls (p = 0.086). The median time to neutrophil engraftment (ANC >500) was 21 days in the StemEx® group and 28 days in the control group (p < 0.0001). The survival at 100 days for StemEx® recipients was 84.2 % compared to 74.6 % for conventional dual UCB transplant recipients (p = 0.035) [17]. The study suggests that when compared to a registry-based retrospective patient cohort, transplantation of the StemEx® single UCB graft results in improvement in a number of important clinical endpoints. Furthermore, Gamida Cell confirmed feasibility of conducting and completing a large, multinational cell therapy trial.
NiCord® Expansion
NiCord® is an ex vivo expanded cell product from an entire unit of umbilical cord blood that utilizes a small molecule, nicotinamide, as an epigenetic approach to inhibit differentiation and to increase the functionality of hematopoietic stem and progenitor cells expanded in ex vivo cultures. When nicotinamide is added to stimulatory hematopoietic cytokines, umbilical cord blood–derived hematopoietic progenitor cell cultures demonstrate an increased frequency of phenotypically primitive CD34+CD38− cells. The cells also demonstrate increased migration toward stromal cell–derived factor 1. In a pre-clinical murine transplant model, they home to and engraft in the bone marrow with higher efficacy [18].
The unique feature of the NiCord® UCB graft is that it consists of a hematopoietic stem–progenitor cell fraction that has been expanded for 21 days along with a non-cultured T cell containing fraction that is collected and re-frozen following thaw. Thus, the NiCord® graft retains immunologic potency to augment both engraftment and immunologic recovery.
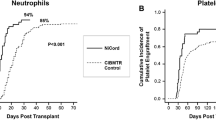
The phase I study of NiCord® commenced in 2010 at Duke University in Durham, North Carolina, and Loyola University in Chicago, Illinois. Eligible patients had high-risk hematologic malignancies and were candidates for dual umbilical cord blood transplantation. One unit was selected for NiCord® expansion and the other to be infused without manipulation. Recipients were conditioned with total body irradiation (1350 cGy) and fludarabine with or without cyclophosphamide. Key endpoint measures were compared to an historical control cohort of patients who received myeloablative conditioning followed by an unmanipulated dual UCB transplantation. Eleven patients received the NiCord® graft on this study. Full or partial neutrophil and T cell engraftment derived from NiCord® was observed in eight patients. With a median follow-up of 24 months, NiCord® engraftment has remained stable in all patients. Two additional patients engrafted with the unmanipulated unit. Patients engrafting with NiCord® achieved significantly earlier neutrophil recovery (median of 11 vs 25 days, p = 0.001; Fig. 1), and platelet recovery (30 vs 41 days, p = 0.012), compared to controls [19].
This study confirms the presence of both long-term and short-term repopulating cells within the NiCord® expanded cord blood graft. With this knowledge, Gamida Cell has launched a follow-up trial whereby recipients receive myeloablative conditioning followed by a single NiCord® expanded graft (Clinicaltrials.gov NCT01816230). To address practical and logistical concerns surrounding the transport of a fresh cell therapy product, the graft will be cryopreserved following expansion and before delivery to the transplant center.
Mesenchymal Cell Co-culture (Mesoblast)
Ex vivo expansion of unfractionated, previously cryopreserved umbilical cord blood is extremely challenging. Initiation of the culture with a purified population of CD34+ cells significantly enhances the expansion efficiency but also adds to the complexity of the assay [20]. In order to avoid CD34+ cell selection, McNiece and colleagues developed a culture system utilizing mesenchymal stromal cells (MSCs) [21]. It is hypothesized that both contact-dependent and soluble factors provided by the MSCs more closely resemble the bone marrow micro-environment. Refinement of this technique ultimately led to development of a GMP-grade mesenchymal precursor product that was brought to the clinic by the group at MD Anderson Cancer Center. The 14-day UCB expansion process consists of co-culture with the adherent MSCs for 7 days in the presence of hematopoietic cytokines and an additional 7 days in the presence of cytokines only.
Thirty-one adults with hematologic malignancies were treated with a dual UCB transplantation. The conditioning regimen was myeloablative, consisting of melphalan, thiotepa, fludarabine, and rabbit anti-thymocyte globulin. One of the units was infused without manipulation and the other following expansion as described above. The outcome of these patients was compared to that of 80 historical controls who received a standard myeloablative dual UCB transplantation and whose outcome was reported to the Center for International Blood and Marrow Transplant Research data registry [22]. The cultured unit expanded by a median factor of 12.2 and CD34+ cells were expanded by a factor of 30.1. The expanded unit provided a median CD34+ cell dose of 0.95 × 106/kg recipient body weight. In comparison, the unmanipulated unit provided a median CD34+ cell dose of 0.38 × 106/kg. The median time to neutrophil recovery was 15 days (range, 9 to 42) and the median time to platelet recovery was 42 days (range, 15 to 62). This compared favorably to the CIBMTR registry cohort who engrafted neutrophils on median day 24 (range, 12–52) and platelets on median day 49 (range, 18–264). Peripheral blood chimerism analysis performed in the peri-engraftment period demonstrated presence of the expanded unit in 46 % of the subjects. By 6 months, the expanded unit was detectable in 13 % of the subjects. By 1 year following transplantation, hematopoiesis was provided by the unmanipulated unit in all the subjects.
HSC835 Aryl Hydrocarbon
Boitano and colleagues screened a library of 100,000 compounds resulting in identification of a purine derivative [StemRegenin 1 (SR1)] with marked ability to expand human CD34+ hematopoietic stem and progenitor cells ex vivo [23]. For this technology, a purified population of CD34+ cells is used to initiate the cell culture. During a 3-week culture in serum-free media containing SR1 supplemented with thrombopoietin, stem cell factor, flt3 ligand, and interleukin-6, the CD34+ cells expand 1118-fold relative to input cells. Removal of SR1 from the culture system led to rapid differentiation, highlighting the importance of SR1 in the culture system. Cultured cells also produced high-level engraftment in immunodeficient mice. A 3-week expansion of 300 UCB CD34+ cells provided a comparable level of human engraftment in NSG mice as 10,000 uncultured UCB CD34+ cells. These results demonstrate that SR1 promotes expansion of UCB CD34+ cells that contribute to both early and sustained engraftment.
The first clinical results were presented at the 2014 annual meeting of the American Society of Hematology [24]. The phase I/II study enrolled recipients of myeloablative (TBI/fludarabine/cyclophosphamide) UCB transplantation. The first 15 patients received both an SR1 expanded and an unmanipulated UCB graft. An additional two patients received a single SR1 expanded graft. Recipients also received the CD34-negative, T cell-containing fraction of the expanded UCB unit, similar to what was done in the NiCord® trial. The SR1 expanded graft contained a median 12.3 × 106 CD34+ cells/kg (range 2.3–48.5 × 106/kg). This compares to recipients of standard double UCB graft without manipulation (n = 111) who received a median of 0.5 × 106 CD34+ cells/kg (0.1–2.1). Eleven of the 17 patients demonstrated a predominance of the SR1 expanded unit. The remainder showed predominance of the unmanipulated unit. Neutrophils recovered at a median of 11 days (6–23) for those engrafting with the SR1 expanded unit as compared to 23 days (14–30) for those in whom the unmanipulated unit predominated. Engraftment of the SR1 expanded unit has been stable, with the longest follow-up being 2 years. The SR1 expanded graft, now called HSC835 (Novartis Pharmaceuticals), continues to be studied in the context of single myeloablative UCB transplantation at the University of Minnesota (Clinicaltrials.gov NCT01930162)
Prostaglandin e2 Co-culture
The previously discussed technologies are aimed at addressing the low stem cell content of the UCB by ex vivo expansion of both short-term and long-term hematopoietic progenitor cells. In contrast, the prostaglandin e2 co-culture methodology was conceived as an inexpensive, safe, and practical method for augmenting the homing and engraftment potential of UCB stem cells without expansion. The method utilizes 16,16-dimethyl prostaglandin E2 (dmPGE2) previously identified in a zebra fish stem cell model to be a critical regulator of hematopoietic stem cell homeostasis [25]. From this pre-clinical work, it was hypothesized that brief ex vivo exposure of UCB to dmPGE2 could improve patient outcomes by increasing the “effective dose” of hematopoietic stem cells without significant toxicity. This hypothesis was tested in a phase I study conducted at the Dana Farber Cancer Institute in Boston. The investigators enrolled recipients of non-myeloablative dual UCB transplantation. One unit was infused following a brief exposure to dmPGE2 and the other was infused without manipulation. Two cohorts of patients were enrolled. In cohort 1, the investigational unit was incubated with 10 mM of dmPGE2 for 60 min at 4 °C (nine patients). Cohort 2 consisted of 12 patients in whom the larger of the two UCB units was incubated with the same concentration dmPGE2, but for 120 min instead of 60 min and at 37 °C instead of 4 °C.
While patients in cohort 1 experienced no safety concerns, there was no demonstrable benefit of the dmPGE2-treated unit. However, the optimized co-culture conditions utilized for patients in cohort 2 resulted in more rapid median time to neutrophil engraftment compared to historical controls (17.5 vs. 21 days, p = 0.45). Furthermore, 10 of 12 patients showed complete and sustained engraftment of the dmPGE2-treated units. While it is possible that the engraftment advantage afforded to the manipulated unit is a consequence of it being the larger of the two infused grafts, it is also possible that co-culture with PGE2 did indeed improve homing of the resident stem cells. No safety concerns emerged from this small phase I trial [26]. Based on promising results emerging from this trial, the technology is now being studied in a randomized phase II trial supported by Fate Therapeutics (Clinicaltrials.gov NCT01627314).
C3a Complement Co-culture
Complement fragment 3a (C3a) is a product of proteolytic cleavage of complement protein C3. Along with a myriad of immunoregulatory properties, C3a also sensitizes human hematopoietic stem and progenitor cells to homing properties of stromal derived factor 1 (SDF-1) through binding of the C3a receptor present on the cell surface. Pre-clinical murine transplant models demonstrated that incubation of hematopoietic stem cells with C3a prior to transplantation into lethally irradiated hosts accelerates engraftment kinetics [27, 28].
Based on these promising pre-clinical data, a phase I/II study was performed at the University of Minnesota to assess safety and efficacy of C3a in promoting engraftment of UCB stem cells [29]. Adults receiving non-myeloablative dual umbilical cord blood transplantation were eligible for the trial. One of the two UCB grafts was incubated at room temperature with GMP-grade C3a prior to transplantation. Twenty-nine patients were treated on the study. Mild infusion-related side effects were observed following infusion of the C3a primed units. Because the patients were conditioned with a non-myeloablative regimen and therefore experienced early autologous reconstitution, the impact of C3a on engraftment kinetics could not be assessed. Of the 27 patients who achieved neutrophil engraftment, 9 had hematopoiesis derived from the C3a primed unit and 18 from the unmanipulated unit. Thus, C3a treatment did not appear to provide an engraftment advantage.
Fucosylation
Like dmPGE2 and C3a co-culture, ex vivo fucosylation is a technique under development that is designed to augment homing of umbilical cord blood stem cells to the bone marrow stroma. The rationale for this strategy hinges on the fact that UCB stem cells do not home as avidly to the bone marrow as those from adult bone marrow or mobilized peripheral blood. This defect in bone marrow homing may, in part, be due to lack of binding to the critical adhesion molecules P- and E-selectin which are expressed on bone marrow endothelial cells [30]. Fucosylation of selectin ligands expressed on UCB stem cells increases affinity to P- and E-selectin. Thus, in pre-clinical models of UCB transplantation, engraftment of UCB stem cells into immunodeficient mice can be improved with pre-transplant ex vivo fucosylation [31, 32].
Phase I testing of this technique was recently reported by the group from MD Anderson [33]. Twenty-two patients with high-risk hematologic malignancies received myeloablative bone marrow conditioning followed by transplantation with two UCB units. One unit was infused without manipulation while the other was incubated for 30 min in the presence of guanosine diphosphate fucose along with the enzyme fucosyltransferase-VI, a technique shown to improve cell surface fucosylation of hematopoietic stem cells. Engraftment kinetics were compared to a historical cohort transplanted in a similar fashion with two unmanipulated UCB units. A statistically significant reduction in time to neutrophil and platelet engraftment was observed in recipients of a fucosylated graft (17 vs 26 days, p = 0.0003, and 35 vs 45 days, p = 0.0065, respectively). Interestingly, faster blood count recovery was not dependent on engraftment or dominance of the treated unit, which occurred in only 9 of the 22 subjects. This suggests that both units following infusion share beneficial effects of fucosylation.
Conclusion
In the early 2000s, the advantages of UCB transplantation were only being appreciated in the pediatric population where outcomes were comparable to that of adult unrelated donor transplantation. Since then, a better understanding of the minimum safe transplantable cell dose, improved quality and characterization of the UCB inventory, and the advent of dual UCB transplantation has broadened the applicability to the adult population. However, limitations remain and chief among them is the kinetics of hematopoietic recovery following UCB transplantation. Ex vivo manipulation of the UCB graft prior to transplantation holds great promise in addressing these limitations. Clinical data is already available from multiple techniques demonstrating more rapid neutrophil and platelet recovery. Short-term safety has also been demonstrated. Confirmation of long-term safety and reduction in the graft failure rate will require longer follow-up and larger studies. With the development of improved techniques for the transplantation of haplo-identical related donor grafts, the major challenge going forward will be demonstration of cost-effectiveness and practicality of ex vivo UCB stem cell manipulation.
References
Sebrango A et al. Haematopoietic transplants combining a single unrelated cord blood unit and mobilized haematopoietic stem cells from an adult HLA-mismatched third party donor. Comparable results to transplants from HLA-identical related donors in adults with acute leukaemia and myelodysplastic syndromes. Best Pract Res Clin Haematol. 2010;23(2):259–74.
Liu H et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438–45.
Peffault de Latour R et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19(9):1355–60.
Smith AR et al. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009;15(9):1086–93.
Jaroscak J et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell system. Blood. 2003;101(12):5061–7.
Scaradavou A et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121(5):752–8.
Barker JN et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–7.
Milner LA et al. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood. 1994;83(8):2057–62.
Delaney C et al. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106(8):2693–9.
Delaney C et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–6.
Gutman JA et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115(4):757–65.
Moretta A et al. In vitro evaluation of graft-versus-graft alloreactivity as a tool to identify the predominant cord blood unit before double cord blood transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18(7):1108–18.
Delaney C. Infusion of Non-HLA matched, off-the-shelf ex vivo expanded cord blood progenitor cells in patients undergoing myeloablative cord blood transplantation is safe and decreases the time to neutrophil recovery. Biol Blood Marrow Transplant. 2012;18(2):S203.
Peled T et al. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increases their engraftment potential in NOD/SCID mice. Exp Hematol. 2004;32(6):547–55.
Peled T et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6(4):344–55.
de Lima M et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008.
Stiff PM et al. StemEx® (copper chelation based) ex vivo expanded Umbilical Cord Blood Stem Cell Transplantation (UCBT) accelerates engraftment and improves 100 day survival in myeloablated patients compared to a registry cohort undergoing double unit UCBT: results of a multicenter study of 101 patients with hematologic malignancies. Blood. 2013;122(295):3060.
Peled T et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40(4):342–55.e1.
Horwitz ME et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124(7):3121–8.
Briddell RA et al. Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood. 1993;82(6):1720–3.
McNiece I et al. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6(4):311–7.
de Lima M et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–15.
Boitano AE et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–8.
Wagner JE et al. StemRegenin-1 (SR1) Expansion Culture Abrogates the Engraftment Barrier Associated with Umbilical Cord Bood Transplantation. Abstract to be presented at the 56th American Society of Hematology Meeting and Exposition. 2014.
North TE et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–11.
Cutler C et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122(17):3074–81.
Ratajczak J et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103(6):2071–8.
Reca R et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101(10):3784–93.
Brunstein CG et al. Complement fragment 3a priming of umbilical cord blood progenitors: safety profile. Biol Blood Marrow Transplant. 2013;19(10):1474–9.
Hidalgo A et al. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110(4):559–69.
Robinson SN et al. Fucosylation with fucosyltransferase VI or fucosyltransferase VII improves cord blood engraftment. Cytotherapy. 2014;16(1):84–9.
Xia L et al. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104(10):3091–6.
Popat U et al. Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood. 2015;125(19):2885–92.
Robinson SN et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rgamma(null) mice. Exp Hematol. 2012;40(6):445–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mitchell Horwitz has received research support from and has served as a consultant for Gamida Cell Ltd.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Stem Cell Transplantation
Rights and permissions
About this article
Cite this article
Horwitz, M.E. Ex Vivo Expansion or Manipulation of Stem Cells to Improve Outcome of Umbilical Cord Blood Transplantation. Curr Hematol Malig Rep 11, 12–18 (2016). https://doi.org/10.1007/s11899-015-0297-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-015-0297-7