Abstract
Purpose of Review
Heart failure with preserved ejection fraction (HFpEF) is a leading cause of morbidity and mortality. The current mechanistic paradigm supports a comorbidity-driven systemic proinflammatory state that evokes microvascular and myocardial dysfunction. Crucially, diabetes and obesity are frequently prevalent in HFpEF patients; as such, we review the involvement of a metabolic-inflammatory circuit in disease pathogenesis.
Recent Findings
Experimental models of diastolic dysfunction and genuine models of HFpEF have facilitated discovery of underlying drivers of HFpEF, where metabolic derangement and systemic inflammation appear to be central components of disease pathophysiology. Despite a shared phenotype among these models, molecular signatures differ depending on type and combination of comorbidities present.
Summary
Inflammation, oxidative stress, hypertension, and metabolic derangements have been positioned as therapeutic targets to suppress the metabolic-inflammatory circuit in HFpEF. However, the stratification of unique patient phenogroups within the collective HFpEF subgroup argues for specific interventions for distinct phenogroups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 64.3 million people are living with heart failure (HF) globally [1], with absolute numbers poised to increase due to global population growth, ageing communities, and reductions in mortality following improvements in preventive care [2]. HF is a heterogeneous syndrome with diverse aetiology; as such, left ventricular ejection fraction (LVEF) is commonly used as a clinical marker to distinguish between various forms of HF (hereafter, referred to as subgroups) and to determine patient response to therapy. In this regard, HF patients can be categorised as those with reduced (LVEF < 40%), mid-range (LVEF 40–49%), or preserved ejection fraction (LVEF ≥ 50%) [3]. However, these criteria differ across guidelines and hamper the estimation of HF incidence, hospitalisation, and mortality rates. Interestingly, network analysis of biomarker profiles revealed distinct differences between patients with reduced (HFrEF), midrange (HFmrEF), and preserved (HFpEF) ejection fraction [4], which support differences in pathophysiology between HF subgroups, and argues for the possibility of clearly distinct syndromes.
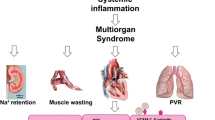
Despite these differences, there is considerable overlap between HF subgroups. Adverse remodelling and cardiac dysfunction are typical presentations in HF patients and are thought to be mediated by an inflammatory response. Indeed, an increase in circulating inflammatory markers is associated with disease severity and poorer prognosis in patient subgroups [5, 6]. However, myocardial and systemic inflammation in the setting of HFrEF is likely to be secondary in response to the primary insult of cardiomyocyte loss, while systemic inflammation in the setting of HFpEF is considered a critical mediator of coronary microvascular endothelial dysfunction, myocardial fibrosis, and increased cardiomyocyte hypertrophy and stiffness [7]. Moreover, the frequent prevalence of diabetes and obesity in HFpEF patients [8,9,10] supports the possibility of a metabolic risk–related proinflammatory state in having a defining role in the pathogenesis and progression of HFpEF, as opposed to HFrEF [4, 11] (Fig. 1).
Overview of the metabolic-inflammatory circuit in HFpEF. Metabolic comorbidities elicit a chronic proinflammatory state that evokes microvascular and myocardial dysfunction. The release of proinflammatory mediators can promote oxidative stress that subsequently impedes endothelial and cardiomyocyte function. Targeting myocardial inflammation and oxidative stress may help to restore nitric oxide bioavailability, the latter being critical for endothelial-cardiomyocyte crosstalk
In this review, we discuss recent findings that support the current HFpEF paradigm, where a metabolic comorbidity-driven proinflammatory state underlies HFpEF pathophysiology, positioning the metabolic-inflammatory circuit as an attractive therapeutic target to improve clinical outcomes of HFpEF patients.
Metabolic Comorbidities in HFpEF
HF is often accompanied by non-cardiac comorbidities which are associated with increased hospitalisation risk and worse clinical outcomes. Interestingly, evaluation of non-cardiac comorbidities revealed most comorbidities (7 out of 8) display the highest prevalence in HFpEF patients, as opposed to other HF subgroups [8]. This supports the notion that inflammation is a critical mediator of HFpEF, as comorbidities such as hypertension, diabetes, obesity, chronic obstructive pulmonary disease, and chronic kidney disease are able to elicit a chronic systemic inflammatory state. As the scope of this review is to interrogate the association between metabolic comorbidities and inflammation, preference has been given for diabetes and obesity (two comorbidities that frequently coexist). To appreciate the full extent of non-cardiac commodities and the elicited inflammatory response in HF, readers are directed to the following reviews [12•, 13].
Diabetes and obesity are frequently prominent comorbidities in HFpEF patients. Approximately 45% of HFpEF patients have diabetes [9], and several clinical studies support diabetic HFpEF patients to have greater morbidity and long-term mortality than those without diabetes [14, 15]. Although diabetes is associated with smaller LV volumes, higher mitral E/e′ ratio, poorer quality of life, and worse outcomes in both HFrEF and HFpEF patients, differences in cardiac remodelling have been observed between the two subgroups, where the former predominantly presents eccentric cardiac hypertrophy, while the latter presents concentric hypertrophy [15]. The mechanism by which diabetes promotes HF is unclear, and while vasculopathy is proposed as the main cause of morbidity and mortality in diabetic patients, cardiomyocyte dysfunction (due to mitochondrial injury, oxidative stress, impaired intracellular calcium handling, and increased inflammation and apoptosis) is also implicated in disease pathogenesis [16].
Strikingly, the prevalence of obesity is nearly twofold that of diabetes, with approximately 80% of elderly HFpEF patients being overweight or obese [10]. Obesity and related cardiometabolic traits are associated with higher risk of HFpEF than HFrEF [17]. Initially, a U-shaped relationship was observed between BMI, and both primary composite end point and all-cause mortality, where patients with the lowest and highest BMI showed increased risk of adverse outcomes [10]. However, recent findings support obesity to increase risk of all-cause mortality in HFpEF patients [18]. Interestingly, clinical evidence supports a distinct obese phenotype among HFpEF patients, where the latter (compared to non-obese controls and non-obese HFpEF patients) display increased biventricular remodelling, volume overload, RV dysfunction, pericardial restraint, and hemodynamic derangements, with worse exercise capacity and impaired pulmonary vasodilation [19]. Compared to obese non-HF and lean HFpEF patients, obese HFpEF patients display elevated levels of circulating biomarkers of volume expansion, fibrosis and systemic inflammation [20]. While these findings support an association between obesity, inflammation, and cardiac dysfunction, the mechanism by which obesity drives HFpEF pathophysiology is unclear, although expansion of epicardial adipose tissue (EAT) in obese HFpEF patients does correlate with more profound hemodynamic derangements at rest and at exercise [21]. Besides exerting mechanical stress, EAT also serves as a metabolically active depot that produces several adipokines, and which is capable of inducing a proinflammatory state associated with coronary endothelial dysfunction, cardiomyocyte stiffness, and fibrosis [22].
Causal or Association—the Role of Inflammation in HFpEF
Though several studies support an association between inflammation and HFpEF [4, 11, 23], the extent to which inflammation contributes to disease pathogenesis and progression is unclear. Indeed, the current mechanistic paradigm implicates comorbidities, or environmental and haemodynamic stressors to evoke systemic low-grade inflammation in the microvasculature which disrupts endothelium homeostasis, by initiating production of reactive oxygen species (ROS) and adhesion molecules in the latter [7]. An abundance of ROS depletes nitric oxide (NO) bioavailability, while subsequent perturbations in the NO-cGMP-PKG pathway induce cardiomyocyte hypertrophy and stiffness. Moreover, an increase in adhesion molecules facilitates macrophage infiltration which promotes collagen accumulation in the myocardium [24]. As such, the combination of increased cardiomyocytes stiffness and macrophage-mediated interstitial fibrosis are considered key inducers of diastolic dysfunction; a hallmark of HFpEF.
The possibility that inflammation does play a causal role in disease pathogenesis is supported by findings, whereby an upregulation of CCR2 (C–C Motif Chemokine Receptor 2) ligands in the settings of pressure overload and hypertension promoted myocardial infiltration of CCR2+ macrophages, the latter being associated with a proinflammatory response [24, 25]. Importantly, blockade of CCR2+ macrophage infiltration or inhibition of IL-10 (produced by these macrophages) was able to attenuate adverse remodelling and fibrosis, and improve both systolic and diastolic dysfunction [24, 25]. Conversely, the disappointing outcomes from clinical trials which evaluated anti-inflammatory therapies may suggest inflammation to play a minor role in disease pathogenesis. Given that inflammation induces cardiac fibrosis (which to a certain extent is irreversible), the introduction of anti-inflammatory therapies, especially at a late stage, may yield modest benefits; as such, the outcomes of these trials do not exclude a causal role of inflammation.
Interestingly, other studies suggest inflammation to play a partial role in disease pathogenesis, as genetic ablation of MCP-1 (monocyte chemoattractant protein 1) and CCR2, though able to prevent angiotensin II (Ang II)–induced cardiac fibrosis, was unable to alleviate adverse remodelling and diastolic dysfunction, and even worsened systolic function and LV dilation in certain settings [26, 27]. It is important to note that immune cells are responsible for evoking, as well as resolving a proinflammatory state, and this may explain why targeting these pathways can either be beneficial or detrimental. In support, canakinumab (a monoclonal antibody targeted at IL-1β) was found to reduce HF-related hospitalisation and mortality [28], while high doses of infliximab (a monoclonal antibody targeted at tumour necrosis factor (TNF-α)) worsened clinical outcomes of HF patients [29]. Collectively, these findings support a causal role of inflammation in microvascular dysfunction and myocardial fibrosis in HFpEF; however, the extent of its involvement in the development of other pathological traits needs further validation.
Evidence of a Metabolic-Inflammatory Circuit in HFpEF
Impaired diastolic function is a hallmark of HFpEF [30•]; as such, several studies have attempted to evaluate both the cardiac and vascular components of animal models of diastolic dysfunction, with preserved systolic function. A major hurdle encountered in establishing such models is the inability to preserve the HFpEF phenotype, as common HF models of pressure overload or ischemia–reperfusion injury transition rapidly to a HFrEF state. While this can be circumvented with models of hypertension or metabolic syndrome, the single endpoint of diastolic dysfunction or preserved EF may not be sufficient to categorise these models as HFpEF [31]. Other parameters such as exercise intolerance, pulmonary congestion, and concentric cardiac hypertrophy, which are frequently associated with HFpEF patients, need to be considered, and if these features are presented together with preserved EF and diastolic dysfunction, such models may be considered genuine HFpEF models [30•]. However, this is not so easy to achieve, as the complexity of the disease has made it challenging to establish models that reproduce all clinical presentations of human HFpEF.
It is important to note that HFpEF does not originate from one pathological source, but rather from an integration of several factors. In this regard, by implementing multi-hit approaches, a HFpEF phenotype that closely resembles the clinical presentation can be achieved in animals. For instance, high-fat diet (HFD) and L-NAME administration induced several systemic and cardiovascular features of HFpEF, attributed to metabolic inflammation primarily driven by iNOS (inducible nitric oxide synthase) [32•]. The inclusion of advanced ageing and female sex (both of which are prominent traits in HFpEF), coupled with HFD and Ang II administration, promoted a cardiometabolic HFpEF phenotype as evidenced by the presence of obesity, diabetes, elevated blood pressure and lung congestion, inflammation, cardiac remodelling, fibrosis, and diastolic disfunction [33]. Interestingly, a 3-hit model has also been established, where a combination of ageing, HFD, and desoxycorticosterone pivalate promoted assembly of NLRP3 (NLR family pyrin domain containing 3) inflammasome on hyperacetylated mitochondria, and this was associated with increased cardiac IL-1β and IL-18, and fibrosis [34].
Beside these rodent models, large pre-clinical models of HFpEF have also been established. The combination of hypertension, obesity, and diabetes elicited systemic inflammation and myocardial oxidative stress in female swine that were associated with myocardial stiffening and diastolic dysfunction [35]. In Ossabaw swine, western diet and aortic banding elicited a chronic inflammatory state, accompanied by diastolic dysfunction [36]. Since these multi-hit approaches employ metabolic and hypertensive stress, they are the preferred choice for modelling human HFpEF. However, single-hit models like Dahl salt–sensitive rats have also shown signs of diastolic dysfunction with elevated E/e′ ratios, and increases in left atrial (LA) diameter and cardiac hypertrophy, through activation of a cardiac NLRP3 inflammasome [37]. Aside from being used to investigate factors that either promote or prevent LV diastolic dysfunction, these experimental models can also be used to explore LA cardiomyopathy, which commonly accompanies HFpEF and is an independent predictor of mortality [38]. In support, LA cardiomyocytes from HFpEF rats displayed dysregulated TNFα/IL-10 signalling which elicited intrinsic inflammation that disrupted calcium homeostasis and promoted oxidative stress (possibly through mitochondrial fission), leading to contractile dysfunction and arrhythmogenicity [39]. Collectively, these findings support a proinflammatory state associated with HFpEF pathophysiology, and given that metabolic stress is routinely employed to evoke a HFpEF phenotype, metabolic derangements are likely to be primary drivers of the disease. The potential mechanisms by which this metabolic-inflammatory circuit mediates cardiac structural and functional alteration are discussed in the following section.
Mechanisms Underlying the Metabolic-Inflammatory Circuit
Though systemic inflammation is considered a critical mediator of HFpEF, the components and pathways involved are less recognised. While genuine HFpEF models can help to address this in the near future, other models of diastolic dysfunction have facilitated discovery of key mediators of myocardial inflammation.
Consistent with a myocardial inflammatory response, an increased infiltration of neutrophils and M1 macrophages, and proinflammatory cytokines (IL-1β, IL-6, and TNF-α) have been observed in HFpEF hearts [40]. Moreover, transcriptome analysis of the cardiac vascular fraction revealed endothelial dysfunction, systemic inflammation, and an increase in several mast cell markers [41]. The accumulation of activated mast cells in the heart was found to induce (through histamine release) cardiac microvessel disease and diastolic dysfunction, thereby positioning mast cells as a critical component in disease pathogenesis. Notably, the absence of fibrosis and cardiomyocyte abnormalities in this model reinforces the central role of inflammation and microvascular dysfunction in disease pathophysiology [41]. In other studies, proinflammatory and profibrotic macrophage polarisation was associated with diastolic dysfunction in ageing mice and was more pronounced in females than in males [42] (Fig. 1). These different myocardial immune cell populations observed between males and females [42] may explain why most elderly HFpEF patients are women [43•].
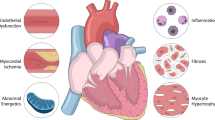
Despite being implicated in disease pathogenesis, how myocardial inflammation mediates adverse remodelling and cardiac dysfunction is less clear. An increase in myocardial stiffness and impaired cardiomyocyte relaxation are causal factors of diastolic dysfunction [44]; as such, secretion of proinflammatory and profibrotic factors by infiltrating cells may contribute to increased extracellular matrix–related myocardial stiffness that is observed in HFpEF patients (Fig. 1). In support, an increase in cardiac cardiotrophin-1 (a proinflammatory cytokine) and galectin-3 expression was associated with inflammation, fibrosis, and diastolic dysfunction, and interestingly, an elevation of both factors correlated with higher risk of cardiovascular mortality in chronic HF patients [45], but whether this may extend to HFpEF patients is unknown. Moreover, an increase in cardiac tissue factor (a transmembrane glycoprotein) elicited inflammation and cardiac hypertrophy, through ERK1/2 and STAT3 pathways [46] (Fig. 2). In other studies, inflammation evoked microvascular dysfunction through depletion of critical factors that are also required for cardiomyocyte function. For instance, stimulation of cardiac microvascular endothelial cells with TNF-α led to NO depletion [47], the latter being important for both contraction and relaxation. These findings suggest that inflammation (through microvascular dysfunction) may directly impede myofibril mechanics, and indeed, sarcomere hyperphosphorylation (primarily mediated by protein kinase C isoforms) at the Z- and M-line, and myofilament anchoring and mechano-sensing structures have been associated with diastolic dysfunction [48]. Alternatively, several studies support a casual role of metabolic derangements in diastolic calcium overload, decreased glucose transport, and elevated oxidative stress and inflammation in cardiomyocytes [49] (Fig. 1). Moreover, cardiomyocyte steatosis due to increased forkhead box O1 activity has been observed in HFpEF hearts [50•], while the uncoupling of glycolysis and glucose oxidation is considered an early event in HFpEF pathogenesis [51]. The detrimental effects exerted by the metabolic-inflammatory circuit on cardiomyocyte function may be an important mechanism in the progression of HFpEF.
Molecular mechanisms underlying the metabolic-inflammatory circuit. Systemic and local inflammation evoke an inflammation-oxidative stress feedback loop in cardiomyocytes that activates signalling networks for cardiac hypertrophy and fibrosis which induce contractile dysfunction. Anti-hypertensive agents and SGLT2 inhibitors may indirectly suppress this loop and attenuate the HFpEF phenotype
Inflammation and oxidative stress are closely related pathological processes, where one can induce the other; as such, both processes are found to simultaneously occur in various pathological settings [52]. Unsurprisingly, oxidative stress has been implicated in HFpEF pathophysiology. Chronic exposure to 3 common comorbidities elicited systemic inflammation and increased myocardial superoxide production that evoked microvascular dysfunction and reduced NO production, which led to increased fibrosis and passive cardiomyocyte stiffness, and diastolic dysfunction [35] (Fig. 1). Similarly, activation of the renin–angiotensin–aldosterone system mediated oxidative stress and inflammation with subsequent adverse remodelling and diastolic dysfunction [53]. While these findings support inflammation and oxidative stress to coexist in the setting of metabolic derangement, the latter may lie upstream as several inflammatory mediators are regulated by oxidative stress–responsive transcription factors [54]. In support, the oxidative stress–responsive cytoplasmic adapter molecule, TRAF3-interacting protein-2 (which lies upstream of NF-κB and activator protein-1), was increased in the setting of obesity, and this elicited an inflammatory response which mediated cardiac hypertrophy, fibrosis, and diastolic dysfunction [55] (Fig. 2). The possibility that oxidative stress may be an early event in HFpEF is supported by findings where NADPH oxidase-1 (NOX1)-mediated endothelial activation led to myocardial inflammation and adverse remodelling, but in the absence of diastolic dysfunction [56]. Interestingly, NOX1 was found to be highly expressed in peripheral monocytes from patients with diastolic dysfunction [56]; however, the absence of metabolic derangements in these patients suggests NOX1 elevation to be a general event and is unlikely to be specific to patients with metabolic comorbidities.
The ablation of specific genes has facilitated mechanistic understanding of HFpEF pathophysiology. Formyl peptide receptor-2 (ALXR) deficiency led to dysregulated energy metabolism and age-related obesity, with impaired myocardial strain, increased proinflammatory Ly6ChiCCR2+ macrophages in heart and spleen, and cardiorenal endothelial dysfunction [57]. Since ALXR is mainly expressed in leukocytes, these findings support a leukocyte-driven mechanism that promotes obesity, reduces life span, and induces profound interorgan non-resolving inflammation in HFpEF [58]. Moreover, augmented profibrotic TGF-β/protease-activated receptor-1 (PAR1) signalling due to PAR2 deficiency led to age-dependent diastolic dysfunction, endothelial activation, and inflammation [59•]. In other studies, reduction in osteoglycin (a small leucine-rich proteoglycan) has been observed in ageing mice which does not lead to alterations in cardiac structure or function; however, the inclusion of a hypertensive stimulus, coupled with ageing and loss of osteoglycin, exacerbated cardiac fibrosis and inflammation, which worsened diastolic dysfunction [59•]. The disruption of metabolic genes can also expedite diastolic dysfunction as cardiac-specific pyruvate dehydrogenase knockout led to reductions in myocardial glucose oxidation and increases in palmitate oxidation which impaired diastolic, but not systolic function [60]. In line with these findings, individuals that carry genetic polymorphisms that can potentially disrupt the functionality of these proteins may be at high risk of developing HFpEF.
In summary, these findings position the metabolic-inflammatory circuit to have a profound impact on cardiac function by inducing hypertrophy, fibrosis, and diastolic dysfunction (Fig. 2). Considering that most of these studies were performed in models of diastolic dysfunction, and not in genuine models of HFpEF, it is imperative that these findings are validated in the latter, as the inclusion of specific comorbidities (and sex) can promote differences in molecular signatures, despite a shared phenotype. In support, obesity was found to induce diastolic derangements in the presence of oxidative stress and hypertrophic remodelling, but in the absence of inflammation and fibrosis [61]. In the same study, hypertension minimally affected diastolic function, but had a major impact on mitochondrial function, inflammation, and fibrosis [61]. Similarly, differential gene expression from hearts of 3 models of diastolic dysfunction revealed diversity in causality and molecular processes that underlie a shared phenotype [62].
Targeting the Metabolic-Inflammatory Circuit
Given its critical role in HFpEF pathophysiology, it seems prudent to target inflammation, either by suppressing proinflammatory mediators or by augmenting anti-inflammatory pathways. However, clinical trials evaluating TNF-α inhibitors have been largely disappointing. These neutral outcomes may be due to cardiomyocytes having already entered an inflamed state, as LA cardiomyocytes from HFpEF rats show reduced TNF-α receptor expression [39]. Importantly, anti-inflammatory IL-10 treatment normalised dysfunctional calcium homeostasis in LA cardiomyocytes by attenuating oxidative stress, albeit its effect on improving global cardiac function was not evaluated [39]. Administration of a ligand to dendritic cell–specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) prevented interstitial fibrosis by inhibiting the maturation of myeloid cells, which reduced progression of diastolic dysfunction in aged females, but not in aged males [42]. This differential response to treatment may be attributed to different myocardial inflammatory components between the sexes. In other studies, the thrombin receptor antagonist, vorapaxar, reduced fibrosis and inflammation by directly inhibiting PAR1 [59•]. Interestingly, HFpEF patients who received coagulation factor X inhibitors (upstream of PAR) showed improvements in diastolic function and had reduced circulating markers of fibrosis [59•]. These findings suggest anticoagulatory agents to have pleiotropic effects extending to anti-inflammatory and anti-fibrotic properties.
Inflammation is typically accompanied by oxidative stress (and vice versa); as such, targeting oxidative stress can be another treatment strategy for HFpEF. QiShenYiQi (QSYQ) is a herbal preparation with reported benefits for several medical conditions, and may also have potential use in HFpEF, although this needs to be validated in high-quality clinical studies [63]. QSYQ attenuated myocardial microvascular inflammation and endothelial-mesenchymal transition by reactivating the NO-cGMP-PKG pathway, which prevented cardiac hypertrophy and fibrosis, and improved cardiac function [64]. Moreover, resveratrol (a potent antioxidant) protected against adverse remodelling by reducing Smad3 acetylation and transcription activity through activation of sirtuin-1 [40]. Consistent with its pleiotropic effects, resveratrol inhibited myocardial inflammation and modulated macrophage polarisation. Antioxidants can also exert direct benefits on cardiomyocytes, as the citrus fruit flavonoid, naringin, attenuated oxidative stress and inflammation [49] (Fig. 1). In other studies, chronic alpha-lipoic acid-discontinuous treatment reduced cardiometabolic derangements and attenuated diastolic dysfunction [65]. While these findings support antioxidants to attenuate the HFpEF phenotype in experimental models, it must be noted that antioxidant therapies have failed to exert protection in large-scale trials for other cardiac diseases [52, 66]. Considering their interdependence, the inability to suppress both inflammation and oxidative stress may partially explain these disappointing outcomes; as such, simultaneous use of both antioxidants and anti-inflammatory agents can be considered a potential treatment strategy.
Hypertension is a common comorbidity in HFpEF patients [30•]; as such, angiotensin receptor–neprilysin inhibition has been investigated in a large HFpEF cohort [30•]. In this study, sacubitril–valsartan or valsartan alone was unable to significantly lower risk of HF hospitalisation and death from cardiovascular causes, though some positive trends were noted in certain subgroups. Conversely, sacubitril–valsartan prevented diastolic dysfunction, and reduced fibrosis and oxidative stress in an obese model, which may suggest these agents to be more suited for obese HFpEF patients [67]. Similarly, azilsartan (an Ang II receptor antagonist) suppressed inflammation and oxidative stress, and reversed adverse remodelling and diastolic dysfunction by modulating the ACE-2/ANG 1–7/Mas R pathway [53]. Impaired conduction vasodilation is another mechanism by which microvascular dysfunction occurs, and this has been observed in HFpEF patients [68]. Interestingly, inhibition of adenosine kinase by ABT-702 enhanced conducted vasodilation which protected against development of diastolic dysfunction [68].
Attempting to normalise metabolic derangements can be a potential strategy for preventing systemic inflammation, and downstream microvascular and cardiac complications in HFpEF. In this regard, glucagon-like peptide (GLP-1) receptor agonists and sodium-glucose co-transporter-2 (SGLT2) inhibitors have been identified as favourable pharmacological agents, especially in the setting of diabetes. A meta-analysis of 4 GLP-1 receptor agonist trials revealed these classes of drugs to reduce three-point major adverse cardiovascular events, cardiovascular mortality, and all-cause mortality in diabetic patients [69]. Crucially, no study to date has evaluated GLP-1 receptor agonists in HFpEF cohorts; however, such drugs which meaningfully promote weight loss may specifically benefit obese patients. In support, liraglutide attenuated cardiometabolic dysregulation in a mouse model of HFpEF, with accompanying reductions in atrial weight and lung congestion, and improvements in cardiac structure and function [33]. Unlike GLP-1 receptor agonists, the therapeutic effects of SGLT2 inhibitors have been validated in several large trials, where these agents were found to mediate cardiovascular protection, regardless of diabetic status. SGLT2 inhibitors have been shown to improve clinical outcomes of both HFrEF and HFpEF patients. Notably, in the EMPEROR-Preserve trial, empagliflozin reduced risk of HF hospitalisation in approximately 6000 HFpEF patients, and was the first study to demonstrate significant benefits in this HF subgroup [70•]. Moreover, the protection mediated by SGLT2 inhibitors in the absence of diabetes suggests these classes of drugs are not restricted to their anti-glycaemic properties. Indeed, these agents are associated with several cardioprotective mechanisms, including reversal of endothelial activation and endothelial nitric oxide synthase deficit, and reductions in myocardial inflammation and profibrotic signalling [37, 71]; however, these are likely to be secondary to reprogramming of myocardial metabolism [72]. In support, the ketone body β-hydroxybutyrate, which is elevated post-SGLT2 inhibitor treatment [73, 74], exerted protection by attenuating NLRP3 inflammasome assembly on hyperacetylated mitochondria, which prevented proinflammatory cytokine–triggered mitochondrial dysfunction and fibrosis [34] (Fig. 2).
In summary, components of the metabolic-inflammatory circuit such as inflammation, oxidative stress, hypertension, and metabolic derangements have been positioned as potential therapeutic targets for attenuating the HFpEF phenotype, but whether these targets can be universally applied across all patients remains to be seen [22, 75, 76•]. Further work is needed to determine if targeting inflammation and oxidative stress in a broad sense, or rather specific components of these pathways [66, 77•] will be more effective as a therapeutic intervention. While SGLT2 inhibitors are gaining traction as a standardised therapeutic modality for improving outcomes of HF patients, their mode of action is unclear, and only by elucidating their cardioprotective mechanisms can healthcare professionals facilitate their rapid entry into clinical practice.
Future Directions
A proinflammatory state elicited by metabolic comorbidities is considered the primary driver of HFpEF; as such, the metabolic-inflammatory circuit has been positioned as a therapeutic target for improving clinical outcomes of HFpEF patients. Whether this approach can be universally applied across all patients is unclear, as recent findings support the existence of several phenogroups within the collective HFpEF subgroup, whose characteristics may be key determinants of therapeutic outcomes, given that some phenogroups may be responsive, while others are not. For instance, 3 distinct clinically identifiable phenogroups have been identified who display differences in circulating biomarkers, cardiac and arterial structure, and in response to therapy [75]. In other studies, unique phenotypes have been identified within the metabolic HFpEF phenogroup, where unsupervised machine learning identified 3 unique obese-inflammatory phenotypes that were associated with differences in comorbidity burden, HFpEF severity, and fibrosis [76•]. In this study, patients with a pan-inflammatory phenotype displayed the highest circulating levels of inflammatory mediators, and had more comorbidities and HF hospitalisations, while patients with a non-inflammatory phenotype had the lowest levels of inflammation and the most favourable levels of all other variables [76•]. Moreover, HFpEF patients with enlarged EAT can be classified as another unique phenogroup, which may benefit from EAT-targeted interventions [22] (Fig. 3). Importantly, though metabolic derangement is considered the primary insult, perturbations imposed by other comorbidities (and sex) and the mechanisms by which they worsen clinical outcomes need to be thoroughly investigated in models that best reproduce human HFpEF, and which are not limited to diastolic dysfunction.
Conclusions
While a metabolic-inflammatory circuit may underlie HFpEF pathophysiology, the molecular signatures in each phenogroup are likely to be different [42, 61, 62], and this may pose a major hurdle when evaluating the therapeutic benefits of pharmacological agents. As such, an argument can be made for comorbidity-specific phenotypic characterisation, as opposed to a common clustering of preserved EF, with future trials focusing on specific interventions for distinct phenogroups.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. https://doi.org/10.1016/S0140-6736(18)32279-7.
Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–41. https://doi.org/10.1056/NEJMoa1406656.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. https://doi.org/10.1002/ejhf.592.
Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, et al. Identifying Pathophysiological Mechanisms in Heart Failure With Reduced Versus Preserved Ejection Fraction. J Am Coll Cardiol. 2018;72(10):1081–90. https://doi.org/10.1016/j.jacc.2018.06.050.
Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–41. https://doi.org/10.1056/NEJM199007263230405.
Hage C, Michaelsson E, Linde C, Donal E, Daubert JC, Gan LM, et al. Inflammatory biomarkers predict heart failure severity and prognosis in patients with heart failure with preserved ejection fraction: a holistic proteomic approach. Circ Cardiovasc Genet. 2017;10(1). https://doi.org/10.1161/CIRCGENETICS.116.001633.
Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–71. https://doi.org/10.1016/j.jacc.2013.02.092.
Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, et al. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol. 2018;271:132–9. https://doi.org/10.1016/j.ijcard.2018.04.001.
Echouffo-Tcheugui JB, Xu H, DeVore AD, Schulte PJ, Butler J, Yancy CW, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure registry. Am Heart J. 2016;182:9–20. https://doi.org/10.1016/j.ahj.2016.07.025.
Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4(3):324–31. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959890.
Tromp J, Khan MA, Klip IT, Meyer S, de Boer RA, Jaarsma T, et al. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J Am Heart Assoc. 2017;6(4). https://doi.org/10.1161/JAHA.116.003989.
Murphy SP, Kakkar R, McCarthy CP, Januzzi JL, Jr. Inflammation in heart failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(11):1324–40. doi: https://doi.org/10.1016/j.jacc.2020.01.014. This review describes mechanistic links between heart failure and inflammation, and discusses clinical trials that implemented anti-inflammatory therapies.
Schiattarella GG, Sequeira V, Ameri P. Distinctive patterns of inflammation across the heart failure syndrome. Heart Fail Rev. 2021;26(6):1333–44. https://doi.org/10.1007/s10741-020-09949-5.
McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, et al. Heart failure with preserved ejection fraction and diabetes: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(5):602–11. https://doi.org/10.1016/j.jacc.2018.11.033.
Yap J, Tay WT, Teng TK, Anand I, Richards AM, Ling LH, et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. 2019;8(17): e013114. https://doi.org/10.1161/JAHA.119.013114.
Cong S, Ramachandra CJA, Mai Ja KM, Yap J, Shim W, Wei L, et al. Mechanisms underlying diabetic cardiomyopathy: from pathophysiology to novel therapeutic targets. Cond Med. 2020;3(2):82–97.
Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6(8):701–9. https://doi.org/10.1016/j.jchf.2018.05.018.
Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70(22):2739–49. https://doi.org/10.1016/j.jacc.2017.09.1111.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19. https://doi.org/10.1161/CIRCULATIONAHA.116.026807.
Kresoja KP, Rommel KP, Wachter R, Henger S, Besler C, Kloting N, et al. Proteomics to improve phenotyping in obese patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23(10):1633–44. https://doi.org/10.1002/ejhf.2291.
Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8(8):657–66. https://doi.org/10.1016/j.jchf.2020.04.016.
Elsanhoury A, Nelki V, Kelle S, Van Linthout S, Tschope C. Epicardial fat expansion in diabetic and obese patients with heart failure and preserved ejection fraction-a specific HFpEF Phenotype. Front Cardiovasc Med. 2021;8: 720690. https://doi.org/10.3389/fcvm.2021.720690.
Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13(10):1087–95. https://doi.org/10.1093/eurjhf/hfr079.
Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med. 2018;215(2):423–40. https://doi.org/10.1084/jem.20171274.
Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, et al. CCR2(+) Monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci. 2018;3(2):230–44. https://doi.org/10.1016/j.jacbts.2017.12.006.
Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49(3):499–507. https://doi.org/10.1016/j.yjmcc.2010.05.005.
Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, et al. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol. 2011;301(2):H538–47. https://doi.org/10.1152/ajpheart.01114.2010.
Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289–99. https://doi.org/10.1161/CIRCULATIONAHA.118.038010.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107(25):3133–40. https://doi.org/10.1161/01.CIR.0000077913.60364.D2.
Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297-317. https://doi.org/10.1093/eurheartj/ehz641. This study reports a new diagnostic algorithm ‘HFA-PEFF’ that can be performed in the ambulatory setting. It allows for firm diagnosis for chronic HFpEF, which often pose as a challenge.
Valero-Munoz M, Backman W, Sam F. Murine models of heart failure with preserved ejection fraction: a “Fishing Expedition.” JACC Basic Transl Sci. 2017;2(6):770–89. https://doi.org/10.1016/j.jacbts.2017.07.013.
Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568(7752):351–6. doi: https://doi.org/10.1038/s41586-019-1100-z. This study reports concomitant metabolic and hypertensive stress in mice can recapitulate numerous systemic and cardiovascular features of HFpEF in humans, with iNOS-driven dysregulation of the IRE1alpha-XBP1 pathway an important mechanism underlying cardiomyocyte dysfunction in HFpEF.
Withaar C, Meems LMG, Markousis-Mavrogenis G, Boogerd CJ, Sillje HHW, Schouten EM, et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc Res. 2021;117(9):2108–24. https://doi.org/10.1093/cvr/cvaa256.
Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, et al. Targeting mitochondria-inflammation circuit by beta-hydroxybutyrate mitigates HFpEF. Circ Res. 2021;128(2):232–45. https://doi.org/10.1161/CIRCRESAHA.120.317933.
Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN, et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res. 2018;114(7):954–64. https://doi.org/10.1093/cvr/cvy038.
Olver TD, Edwards JC, Jurrissen TJ, Veteto AB, Jones JL, Gao C, et al. Western diet-fed, aortic-banded Ossabaw swine: a preclinical model of cardio-metabolic heart failure. JACC Basic Transl Sci. 2019;4(3):404–21. https://doi.org/10.1016/j.jacbts.2019.02.004.
Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM, et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail. 2020;13(1): e006277. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006277.
Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8(2):295–303. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001667.
Bode D, Wen Y, Hegemann N, Primessnig U, Parwani A, Boldt LH, et al. Oxidative stress and inflammatory modulation of Ca(2+) handling in metabolic HFpEF-related left atrial cardiomyopathy. Antioxidants (Basel). 2020;9(9). doi: https://doi.org/10.3390/antiox9090860.
Zhang L, Chen J, Yan L, He Q, Xie H, Chen M. Resveratrol ameliorates cardiac remodeling in a murine model of heart failure with preserved ejection fraction. Front Pharmacol. 2021;12: 646240. https://doi.org/10.3389/fphar.2021.646240.
Guimbal S, Cornuault L, Rouault P, Hollier PL, Chapouly C, Bats ML, et al. Mast cells are the trigger of small vessel disease and diastolic dysfunction in diabetic obese mice. Arterioscler Thromb Vasc Biol. 2021;41(4):e193–207. https://doi.org/10.1161/ATVBAHA.121.315900.
Trial J, Diaz Lankenau R, Angelini A, Tovar Perez JE, Taffet GE, Entman ML, et al. Treatment with a DC-SIGN ligand reduces macrophage polarization and diastolic dysfunction in the aging female but not male mouse hearts. Geroscience. 2021;43(2):881–99. https://doi.org/10.1007/s11357-020-00255-4.
Tromp J, Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74(5):601–12. doi: https://doi.org/10.1016/j.jacc.2019.05.052. This study reports the associations among age, clinical characteristics and outcomes in HFpEF patients. Younger HFpEF patients tend to be obese men, died more often of cardiovascular causes, whereas elderly HFpEF patients were more likely to be women with more comorbidities, and died more often from non-cardiovascular causes.
Lin YHCS, Ong SG, Ramachandra CJA, Hausenloy DJ. New therapeutic targets to prevent diastolic dysfunction in heart failure with preserved ejection fraction. Conditioning Medicine. 2020;3(3):171–83.
Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131(14):1247–59. https://doi.org/10.1161/CIRCULATIONAHA.114.013215.
Cibi DM, Sandireddy R, Bogireddi H, Tee N, Ghani S, Singh BK, et al. Cardiac tissue factor regulates inflammation, hypertrophy, and heart failure in mouse model of type 1 diabetes. Diabetes. 2021;70(9):2131–46. https://doi.org/10.2337/db20-0719.
Juni RP, Kuster DWD, Goebel M, Helmes M, Musters RJP, van der Velden J, et al. Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. JACC Basic Transl Sci. 2019;4(5):575–91. https://doi.org/10.1016/j.jacbts.2019.04.003.
Soetkamp D, Gallet R, Parker SJ, Holewinski R, Venkatraman V, Peck K, et al. Myofilament phosphorylation in stem cell treated diastolic heart failure. Circ Res. 2021;129(12):1125–40. https://doi.org/10.1161/CIRCRESAHA.119.316311.
Uryash A, Mijares A, Flores V, Adams JA, Lopez JR. Effects of naringin on cardiomyocytes from a rodent model of type 2 diabetes. Front Pharmacol. 2021;12: 719268. https://doi.org/10.3389/fphar.2021.719268.
Schiattarella GG, Altamirano F, Kim SY, Tong D, Ferdous A, Piristine H, et al. Xbp1s-FoxO1 axis governs lipid accumulation and contractile performance in heart failure with preserved ejection fraction. Nat Commun. 2021;12(1):1684. doi: https://doi.org/10.1038/s41467-021-21931-9. This study reports the Xbp1s-FoxO1 axis as an important mechanism in the pathogenesis of cardiometabolic HFpEF, and targeting this axis can reduce myocardial lipid accumulation.
Fillmore N, Levasseur JL, Fukushima A, Wagg CS, Wang W, Dyck JRB, et al. Uncoupling of glycolysis from glucose oxidation accompanies the development of heart failure with preserved ejection fraction. Mol Med. 2018;24(1):3. https://doi.org/10.1186/s10020-018-0005-x.
Ramachandra CJA, Cong S, Chan X, Yap EP, Yu F, Hausenloy DJ. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic Biol Med. 2021;166:297–312. https://doi.org/10.1016/j.freeradbiomed.2021.02.040.
Sukumaran V, Tsuchimochi H, Tatsumi E, Shirai M, Pearson JT. Azilsartan ameliorates diabetic cardiomyopathy in young db/db mice through the modulation of ACE-2/ANG 1–7/Mas receptor cascade. Biochem Pharmacol. 2017;144:90–9. https://doi.org/10.1016/j.bcp.2017.07.022.
Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85(8):753–66. https://doi.org/10.1161/01.res.85.8.753.
Aroor AR, Habibi J, Kandikattu HK, Garro-Kacher M, Barron B, Chen D, et al. Dipeptidyl peptidase-4 (DPP-4) inhibition with linagliptin reduces western diet-induced myocardial TRAF3IP2 expression, inflammation and fibrosis in female mice. Cardiovasc Diabetol. 2017;16(1):61. https://doi.org/10.1186/s12933-017-0544-4.
Xu L, Balzarolo M, Robinson EL, Lorenz V, Verde GD, Joray L, et al. NOX1 mediates metabolic heart disease in mice and is upregulated in monocytes of humans with diastolic dysfunction. Cardiovasc Res. 2021. https://doi.org/10.1093/cvr/cvab349.
Tourki B, Kain V, Shaikh SR, Leroy X, Serhan CN, Halade GV. Deficit of resolution receptor magnifies inflammatory leukocyte directed cardiorenal and endothelial dysfunction with signs of cardiomyopathy of obesity. FASEB J. 2020;34(8):10560–73. https://doi.org/10.1096/fj.202000495RR.
Tourki B, Kain V, Pullen AB, Norris PC, Patel N, Arora P, et al. Lack of resolution sensor drives age-related cardiometabolic and cardiorenal defects and impedes inflammation-resolution in heart failure. Mol Metab. 2020;31:138–49. https://doi.org/10.1016/j.molmet.2019.10.008.
Friebel J, Weithauser A, Witkowski M, Rauch BH, Savvatis K, Dorner A, et al. Protease-activated receptor 2 deficiency mediates cardiac fibrosis and diastolic dysfunction. Eur Heart J. 2019;40(40):3318–32. doi: https://doi.org/10.1093/eurheartj/ehz117. This study reports protease-activated receptor 2 as an important regulator of profibrotic PAR1 and TGF-β signalling in the heart, and modulating this pathway may be a promising therapeutic approach to alleviate HFpEF.
Gopal K, Almutairi M, Al Batran R, Eaton F, Gandhi M, Ussher JR. Cardiac-specific deletion of pyruvate dehydrogenase impairs glucose oxidation rates and induces diastolic dysfunction. Front Cardiovasc Med. 2018;5:17. https://doi.org/10.3389/fcvm.2018.00017.
Brandt MM, Nguyen ITN, Krebber MM, van de Wouw J, Mokry M, Cramer MJ, et al. Limited synergy of obesity and hypertension, prevalent risk factors in onset and progression of heart failure with preserved ejection fraction. J Cell Mol Med. 2019;23(10):6666–78. https://doi.org/10.1111/jcmm.14542.
Altara R, Zouein FA, Brandao RD, Bajestani SN, Cataliotti A, Booz GW. In silico analysis of differential gene expression in three common rat models of diastolic dysfunction. Front Cardiovasc Med. 2018;5:11. https://doi.org/10.3389/fcvm.2018.00011.
Wang M, Shan Y, Wu C, Cao P, Sun W, Han J, et al. Efficacy and safety of Qishen Yiqi dripping pill for heart failure with preserved ejection fraction: a systematic review and meta-analysis. Front Pharmacol. 2020;11: 626375. https://doi.org/10.3389/fphar.2020.626375.
Huang Y, Zhang K, Liu M, Su J, Qin X, Wang X, et al. An herbal preparation ameliorates heart failure with preserved ejection fraction by alleviating microvascular endothelial inflammation and activating NO-cGMP-PKG pathway. Phytomedicine. 2021;91: 153633. https://doi.org/10.1016/j.phymed.2021.153633.
Pop C, Stefan MG, Muntean DM, Stoicescu L, Gal AF, Kiss B, et al. Protective effects of a discontinuous treatment with alpha-lipoic acid in obesity-related heart failure with preserved ejection fraction, in rats. Antioxidants (Basel). 2020;9(11). doi: https://doi.org/10.3390/antiox9111073.
Ramachandra CJA, Ja K, Chua J, Cong S, Shim W, Hausenloy DJ. Myeloperoxidase as a multifaceted target for cardiovascular protection. Antioxid Redox Signal. 2020;32(15):1135–49. https://doi.org/10.1089/ars.2019.7971.
Croteau D, Qin F, Chambers JM, Kallick E, Luptak I, Panagia M, et al. Differential effects of sacubitril/valsartan on diastolic function in mice with obesity-related metabolic heart disease. JACC Basic Transl Sci. 2020;5(9):916–27. https://doi.org/10.1016/j.jacbts.2020.07.006.
Davila A, Tian Y, Czikora I, Li J, Su H, Huo Y, et al. Adenosine kinase inhibition augments conducted vasodilation and prevents left ventricle diastolic dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. 2019;12(8): e005762. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005762.
Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105–13. https://doi.org/10.1016/S2213-8587(17)30412-6.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021. doi: https://doi.org/10.1056/NEJMoa2107038. This study reports empagliflozin to successfully reduce the combined risk of cardiovascular death or hospitalisation for HFpEF, regardless of patients’ diabetes status.
Cappetta D, De Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Micciche A, et al. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res. 2020;157: 104781. https://doi.org/10.1016/j.phrs.2020.104781.
Croteau D, Luptak I, Chambers JM, Hobai I, Panagia M, Pimentel DR, et al. Effects of sodium-glucose linked transporter 2 inhibition with ertugliflozin on mitochondrial function, energetics, and metabolic gene expression in the presence and absence of diabetes mellitus in mice. J Am Heart Assoc. 2021;10(13): e019995. https://doi.org/10.1161/JAHA.120.019995.
Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190–5. https://doi.org/10.2337/db15-1356.
Pietschner R, Kolwelter J, Bosch A, Striepe K, Jung S, Kannenkeril D, et al. Effect of empagliflozin on ketone bodies in patients with stable chronic heart failure. Cardiovasc Diabetol. 2021;20(1):219. https://doi.org/10.1186/s12933-021-01410-7.
Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020;8(3):172–84. https://doi.org/10.1016/j.jchf.2019.09.009.
Sabbah MS, Fayyaz AU, de Denus S, Felker GM, Borlaug BA, Dasari S, et al. Obese-inflammatory phenotypes in heart failure with preserved ejection fraction. Circ Heart Fail. 2020;13(8):e006414. doi: https://doi.org/10.1161/CIRCHEARTFAILURE.119.006414. This study reports the presence of unique obese-inflammatory phenotypes in HFpEF. Inflammation is present in some, but not all HFpEF patients, and is associated with impaired microvascular status, profibrotic state and worse function outcome.
Ramachandra CJA, Kp MMJ, Chua J, Hernandez-Resendiz S, Liehn EA, Knoll R, et al. Inhibiting cardiac myeloperoxidase alleviates the relaxation defect in hypertrophic cardiomyocytes. Cardiovasc Res. 2021. doi: https://doi.org/10.1093/cvr/cvab077. This study reports myeloperoxidase as a novel therapeutic target for alleviating diastolic dysfunction, as MPO inhibition restored MYBPC3 phosphorylation and improved relaxation in human hypertrophic cardiomyocytes.
Funding
Chrishan Ramachandra is supported by the Singapore Ministry of Health’s National Medical Research Council Open Fund-Young Individual Research Grant (NMRC/OFYIRG/0073/2018), the SingHealth Duke-NUS Academic Medicine Research Grant (AM/TP033/2020 [SRDUKAMR2033]), and the Khoo Bridge Funding Award (Duke-NUS-KBrFA/2022/0059). Myu Mai Ja Kp is supported by the National Heart Centre Singapore Centre Grant PROTECT Seed Fund (NHCS-CGSF/2022/003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yap, E.P., Kp, M.M.J. & Ramachandra, C.J. Targeting the Metabolic-Inflammatory Circuit in Heart Failure With Preserved Ejection Fraction. Curr Heart Fail Rep 19, 63–74 (2022). https://doi.org/10.1007/s11897-022-00546-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-022-00546-1