Abstract
Purpose of Review
Loop diuretics are the cornerstone of the treatment of congestion in heart failure patients. The manuscript aims to summarize the most updated information regarding the use of loop diuretics in heart failure.
Recent Findings
Diuretic response can be highly variable between patients and needs to be carefully evaluated during and after the hospitalization. Diuretic resistance can lead to residual congestion which affects prognosis and can be difficult to detect. The effect of loop diuretics on long-term prognosis remains uncertain but patients with advanced heart failure typically have renal dysfunction and are more inclined to develop loop diuretic resistance, which may lead to an incomplete decongestion and thus to a worse prognosis.
Summary
Loop diuretics are the most potent diuretics available and their use is recommended in order to alleviate symptoms, improve exercise capacity, and reduce hospitalizations in patients with heart failure. Their use should be limited to the lowest dose necessary to maintain euvolemia because a low dose does not increase the risk of decompensation but reduce the risk of adverse effects and allow the up-titration of disease-modifying drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loop diuretics are the cornerstone of the treatment of congestion in heart failure (HF) patients, both in the acute and chronic settings. Achievement and preservation of an euvolemic state, by the means of an effective removal of excess extracellular fluid, is one of the mainstays of the disease management.
However, despite the undeniable benefit of loop diuretic therapy in terms of symptoms relief, they cannot be considered disease-modifying drugs, since scarce evidence is available regarding a beneficial impact on hard outcomes such as rehospitalizations for HF, mortality for cardiovascular causes, and all-cause mortality.
The Role of Loop Diuretics in Heart Failure
Congestion is defined as the retention of extracellular fluid due to a mismatch between cardiac function (high ventricular diastolic pressure), plasma volume, and venous capacitance. The gold standard to detected venous congestion is the measurement of right atria pressure (RAP) and pulmonary capillary wedge pressure (PCWP), but this methodology is not frequently adopted in a normal clinical setting.
It is important to distinguish between “clinical congestion” and “hemodynamic congestion.” Although patients present with signs and symptoms of systemic congestion such as dyspnea, rales, jugular venous reflux, and edema, this state is often preceded by hemodynamic congestion, defined as high ventricular diastolic pressures without overt clinical signs [1]. Diastolic left ventricular dysfunction has a key role in congestive heart failure and it is associated with a marked increase in all-cause mortality.
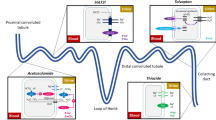
As a result, the main goal of therapy is to achieve and maintain an euvolemic state, defined as decongestion without residual volume overload. Diuretics, especially loop diuretics, are employed to attain this target, and are typically administered intravenously in the acute phase and then continued orally at the lowest dose able to maintain euvolemia [1]. Loop diuretics are the most potent diuretics available, increasing the excretion of Na+ to as much as 25% of the amount filtered; intravenous (IV) administration avoids variable bioavailability and allows for rapid onset of action, typically within 30 to 60 min. The mechanism of action for loop diuretics (e.g. furosemide, torsemide, bumetanide, and ethacrynic acid; Table 1) is by inhibiting the apical sodium/potassium/chloride transporter along the luminal membrane of the thick ascending limb of the loop of Henle. Since loop diuretic agents are organic anions that circulate in the bloodstream tightly bound to albumin, they are not filtered by the glomerular filtration barrier. To gain access to the tubular fluid and therefore to their sites of activity, they must be secreted across the proximal tubule (Fig. 1) [2].
Loop diuretic use is recommended by the latest European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure in order to alleviate symptoms, improve exercise capacity, and reduce HF hospitalizations in patients with in Heart Failure with reduced Ejection Fraction (HFrEF) (class I, level C) [4]. While loop diuretics are almost ubiquitously used in hospitalized HF, large data show that approximately 75–92% of patients with chronic stable HF also use loop diuretics chronically as home therapy [5].
Diuretic Response and Diuretic Resistance
Diuretic response can be highly variable between patients, especially in the acute phase, and needs to be carefully evaluated during and after the hospitalization. The algorithm presented in the 2019 ESC Position Paper on diuretic use in HF [1] guides the clinician towards a complete decongestion by monitoring urine output and urinary sodium concentrations and adapting the diuretic dose consequently. Actually, in clinical practice, serial changes in weight and fluid represent the usual standard approach to monitor diuretic response. Unfortunately, both are crude surrogates for the parameter of interest: sodium output. Also, critical are the practical challenges in collecting accurate cumulative fluid intake/output and weight loss during daily clinical activity [6].
Therefore, the euvolemic state needs a multiparametric assessment with clinical findings, laboratory tests, and echocardiographic parameters to be identified. During clinical follow-up, natriuretic peptides levels and bedside echocardiography should be performed to detect early signs of fluid overload, although ESC guidelines recommend echocardiography only in the presence of clinical worsening [4].
The inability to achieve decongestion despite the use of high diuretic doses is defined as diuretic resistance (DR), a condition of hampered sensitivity to diuretics resulting in ineffective diuresis and natriuresis [7]. Multiple factors can contribute to the development of DR, distal tubular sodium reabsorption being the prevalent one in most cases (due to macula densa hypertrophy as a compensatory mechanism during chronic use of loop diuretics) [8, 9]; thus, in order to overcome this phenomenon, a sequential nephron blockade strategy can be implemented, using other diuretic classes such as thiazides, thiazide-like agents (e.g., metolazone), or carbonic anhydrase inhibitors (e.g. acetazolamide, useful to reduce metabolic alkalosis induced by the other diuretics) [10, 11].
Additional factors involved in DR are renin-angiotensin-aldosterone system (RAAS) activation, sympathetic nervous system activation, and pre-existing renal impairment [1].
There is evidence that diuretic resistance, likely reflecting a more advanced disease, is associated with a worse prognosis. A study conducted by Testani et al. [11], which analyzed two distinct cohorts, observed that patients who experienced low diuretic efficiency had a substantially worse survival rate (risk for all-cause mortality in the two cohorts, respectively: HR between 1.39 and 2.86, p < 0.05). Most importantly, this finding was independent from the diuretic dose itself (whose association with all-cause mortality failed to reach statistical significance after a multivariable analysis) [11].
Residual Congestion and Recurrent Congestion
DR and improper loop diuretic treatment can lead to residual congestion, which can be difficult to detect. For this reason, patients are sometimes discharged without a complete clinical decongestion: a post hoc analysis of DOSE-AHF and CARRESS-HF trials [12] observed that 48% of patients still manifested signs and symptoms of congestion (such as orthopnea or leg edema) at discharge, the persistence of which was associated with higher mortality.
The same study concluded that, even after an effective loop diuretic therapy resulting in actual relief of congestion symptoms at discharge, 65% of patients manifested recurrent congestion after 60 days [12]. This evidence emphasizes that underlying asymptomatic hemodynamic congestion can be still present at discharge and that if not properly managed it can lead to short-term clinical re-congestion. Moreover, a higher risk of death and rehospitalizations has been observed in patients with evidence of recurrent congestion at 1 month [12]; clinical tools to avoid this occurrence are needed.
Recently a natriuretic response prediction equation (NRPE) has been developed and validated [13]. With a spot urine sample obtained 2 h after loop diuretic administration, the 6-hours cumulative total sodium output can be predicted accurately (area under the curve [AUC]> 0.9) and this tool appears to outperform traditional clinical parameters such as net fluid output and weight loss in guiding diuretic therapy (Fig. 2). Furthermore, its application has proved extremely useful in the clinical setting: total urine output, net fluid output, and weight loss clinically and statistically improved when patients diuretics dosage was titrated using the NRPE [13]
With a spot urine sample obtained 2 h after loop diuretic administration, the 6-h cumulative total sodium output and urinary output can be predicted accurately [13]
These evidences underline how loop diuretic resistance plays a major role in the efficacy of decongestive therapy, partially explaining the lack of a net proven benefit of loop diuretics on hard outcomes such as mortality and rehospitalizations for HF.
Side Effects and Drawbacks of Loop Diuretic Therapy
Worsening Renal Failure
The lack of positive effects of loop diuretics on hard endpoints can be explained by many factors, among them, one of the most debated is the loop diuretics-induced worsening of renal function (WRF, defined as an increase in serum creatinine levels and a subsequent decline in estimated Glomerular Filtration Rate, eGFR). Loop diuretics-induced WRF has been attributed to hypoperfusion of the kidney due to progressive impairment of cardiac output caused by intravascular volume depletion [14]. The development of severe WRF (defined as a rise of serum creatinine ≥0.5 mg/dl) after loop diuretic therapy is associated with an increased mortality risk, leading to a significant reduction in 18 months overall survival and hospitalization-free survival (HR 2.09 and HR 1.47, respectively) [15]. More importantly, early development of WRF in the presence of residual congestion implies a poor prognosis [16]. Wherever the WRF during loop diuretic therapy in patients with acute congestive HF is usually suggestive of a good clinical response to diuretic administration and it’s not associated with renal damage, this concept is not applicable in chronic HF.
In a study by Silva et al. [17] conducted over 6 months on outpatients with relatively stable congestive HF, the chronic use of loop diuretics was found to be associated with a 50% increase in the risk of WRF.
Similarly, Maeder et al. [15] observed a significant correlation between loop diuretic home-therapy for chronic congestive HF and the development of a severe WRF; these patients had an increased rate of all-cause mortality, cardiovascular mortality, and rehospitalization for HF. It has to be noted that the patients which experienced a more severe WRF were more likely to have more severe symptoms of congestion, higher blood urea nitrogen and NT-proBNP levels, or a baseline history of chronic kidney disease; however, even after the adjustment for these variables, a severe WRF was still associated with significantly higher risk of death (HR 2.00).
To further strengthen these evidences, it has been repeatedly observed that a discontinuation (or dose reduction) of oral loop diuretic therapy (i.e., furosemide) is accompanied by a significant increase in glomerular filtration rate, thus reflecting an improvement of renal function [18, 19]. On the other hand, intuitively, loop diuretic withdrawal leads to a significant increase in congestion markers such as natriuretic peptides (atrial natriuretic peptide, brain natriuretic peptide and NT-proBNP), therefore casting doubt on the actual net clinical benefit of this assumption [20]; however, a natriuretic peptide-guided diuretic treatment of HF has failed to show superiority in terms of effectiveness [21].
However, venous congestion seems to be the primary hemodynamic factor driving WRF rather than blood pressure, cardiac output, and wedge pressure in the setting of acute decompensated HF [22]: in the presence of elevated central venous pressure, renal function may improve with loop diuretics.
The evidence of loop diuretics nephrotoxicity is partially in contrast with a post hoc analysis from the CORONA study by Damman et al. [23], which showed that the use of loop diuretics in patients with symptomatic HFrEF was associated with an only slightly steeper decline of eGFR (0.8 ml/min/1.73m2), not enough to be considered accountable for the increased risk in mortality observed in those patients. Indeed, only in the high dose matched population did the difference in eGFR decline reach statistical significance, deviating from the natural decline of eGFR in the general population.
Electrolyte Imbalances and Metabolic Alkalosis
Another major issue concerns the electrolyte imbalances induced by the use of loop diuretic therapy, mainly hyponatremia (despite the most natriuretic diuretics are the thiazides) and hypokalemia. In particular, the hypokalemia induced by the strong kaliuretic effect of loop diuretics represents one of the main limiting factors of these drugs, requiring the association with potassium-sparing diuretics (namely mineralocorticoid receptor antagonists MRAs) or RAAS blocking agents, as well as potassium supplementation (either oral or intravenous) [1]. Mild metabolic alkalosis is a common feature of diuretic therapy, particularly at higher doses. Severe metabolic alkalosis is much less frequent and, when it occurs, it is in association with thiazide diuretic use. The generation of a metabolic alkalosis with diuretic therapy is primarily due to contraction of the extracellular fluid space caused by urinary losses of a relatively HCO3-free fluid [24].
Diuretic-induced metabolic alkalosis is best managed by administration of K+ and/or Na+ chloride, although Na+ chloride administration may be impractical in already volume-expanded patients (such as those with HF) [25]. In such cases, a carbonic anhydrase inhibitor administered IV, such as acetazolamide able to produce basic urine, may be considered to implement therapy. Metabolic alkalosis also seems to impair the natriuretic response to loop diuretics and may therefore play a role in the DR of congestive HF patients [26].
RAAS activation
By stimulating macula densa cells and juxtaglomerular cells, the diuretic and natriuretic effect of loop diuretics induces a series of hormonal compensatory mechanisms, especially involving the RAAS, whose overstimulation leads to detrimental effects such as myocardial fibrosis and ventricular remodeling, direct vascular damage, and electrolyte imbalance [27]. Moreover, this loop diuretic-mediated RAAS hyper-stimulation occurs on an HF substrate in which the reduced renal perfusion has already produced a constant hyper-production of renin.
The RAAS activation may be counterbalanced by administration of Angiotensin Converter Enzyme-inhibitors (ACE-i) or MRAs, or even by certain loop diuretics with intrinsic RAAS antagonism such as Torsemide (more on this topic later) [28].
Effect of Loop Diuretic Dose on Outcomes
Although multiple controlled trials have demonstrated the ability of diuretics to decrease physical signs of fluid retention and improve symptoms and cardiac function in patients with congestive HF [29, 30, 31], there has been scant evidence regarding their long-term effects on disease progression and prognosis.
Until now, because of ethical, logistical, and financial reasons, no large, prospective, randomized, placebo-controlled trial has been conducted to assess the effectiveness of loop diuretics in improving hard outcomes in patients with HF, especially in the acute congestive setting. As brilliantly highlighted by the well-known randomized controlled trial on the effectiveness of parachutes to prevent mortality when jumping from aircraft, it is not always necessary that the clinical choice be data-driven [32]. This is the case with the use of loop diuretics in patients with acute congestive HF: would it be ethical to conduct a randomized trial in which the control arm does not receive diuretic therapy but placebo? The effect of loop diuretics on congestive symptoms and short-term outcomes is so evident that the answer seems obvious.
Interestingly, as shown by the DOSE trial [33], among patients with acute decompensated congestive HF, no significant differences in patients’ global assessment of symptoms or in the change in renal function were identified when diuretic therapy was administered by bolus as compared with continuous infusion or at a high dose (approximately 2.5 times the outpatient dose) as compared with a low dose (equivalent to the patient’s previous oral dose). In this setting, given the steep dose-response curve of these agents, titration should be rapid, with doubling of the dose until an effective response is noted. If there is significant volume overload (>5 to 10 L) or diuretic resistance, a continuous IV infusion can be considered [34].
Opposite, with regard to chronic HF, a number of retrospective analyses reported that long-term use of diuretics was associated with increased mortality and hospitalizations [35, 36]. It should be considered, as postulated by some authors, that prescription of loop diuretics identifies patients with more advanced stages of HF and congestion, which may therefore account for their worse prognosis [37].
In a study of 813 HF patients, Dini et al. [38] reported that both the use of loop diuretics and larger doses of diuretic were associated with higher all-cause mortality rates in a propensity score-matched analysis.
Similar results come from the propensity score-matched analysis of the Digitalis Investigators Group (DIG) trial in which Ahmed et al. [39] found that loop diuretic use at baseline and throughout the study was associated with a higher risk of all-cause mortality (adjusted HR 1.32, 95% CI 1.09–1.60) compared with no-diuretic use, and the association between diuretic use and HF hospitalization was even stronger (adjusted HR 1.57, 95% CI 1.25–1.96).
Other data regarding loop diuretic dosage come from large cohort studies or registry data, where higher doses of loop diuretics were associated with worse clinical outcomes, in both acute and chronic HF, but none of these studies employed a propensity score-matched analysis allowing an accurate comparison between treatments [40, 41, 42].
On the other hand, a Cochrane review of 14 prospective trials including 221 patients showed a favorable prognostic effect of diuretic treatments [43]. The systematic review, however, comprised various types of diuretics including spironolactone. As the main action of spironolactone is blockade of the mineralocorticoid receptor, prognostic benefits may result from aldosterone antagonism rather than from intrinsic diuretic effects [44].
In one of the trials included in the above-cited Cochrane Review, Domanski et al. [36] reported data from Studies of Left Ventricular Dysfunction (SOLVD), focusing on the effect of potassium-sparing (including, but not limited to MRAs) and loop diuretics, using conventional analysis without propensity scoring match application. In their analysis, the use of loop diuretics, unlike potassium-sparing diuretic therapy, was associated with higher all-cause mortality [adjusted HR 1.28 (95% CI 1.19–1.49)].
One recent study aimed to analyze the relationships between loop diuretic dose and renal function and clinical outcomes in patients with HFrEF. As illustrated above, this is not the first study to report that the use of loop diuretic therapy in chronic HF is associated with worse outcomes, but it is the one among few that used extensive adjustment and sensitivity analysis to account for bias related to the severity of HF. In this study, loop diuretic dose at baseline was recorded in patients included in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) [23]. Changes in eGFR over time and the first occurrence of a composite outcome ( cardiovascular death or/and hospitalization for HF) were examined through a propensity score-matched analysis. Of the 5011 patients, 2550, 745, and 449 were receiving >80mg (high), 41–80mg (medium), and ≤40mg (low) of loop diuretics in furosemide-equivalent daily dosages, respectively. Compared with matched no loop-diuretic groups, eGFR declined 0.3±0.2, 0.3±0.3, and 1.2±0.5 mL/min/1.73 m2/year in the low-, medium-, and high-dose groups, respectively. Compared with matched no loop diuretic groups, hazard ratios (HR) (95% confidence intervals) for CV outcome associated with low-, medium- and high-dose groups were 1.71 (1.41–2.06), 1.99 (1.50–2.64), and 2.94 (1.95–4.41), respectively. Elevated loop diuretic dosage was particularly associated with increased risk for HF hospitalization (HR 4.80 (2.75–8.37), P < 0.001) reaching a plateau at 80 mg of furosemide: patients assuming >80mg did not experience a greater risk compared with those taking relatively lower doses of diuretics. These findings further strengthen the above-mentioned negative results and provide robust data on a large contemporary HF population. Furthermore, this study was conducted in a contemporary HF population, with high percentages of guideline-directed medical treatment use, including beta-blockers, MRAs, and ACE-inhibitors/angiotensin receptor blockers. Finally, In the CORONA trial, hospitalizations owing to HF were observed more frequently in patients treated with loop diuretics [23].
Loop Diuretics Limit Up-Titration of Disease-Modifying Drugs
Loop diuretics are often prescribed in patients with acute and chronic HF to manage symptoms and signs of congestion. As previously reviewed the use of loop diuretics can lead to a worsening of renal function and occurrence of side effects such as hypotension and orthostatic hypotension. These conditions may lead to a limited up-titration of drugs with proven disease-modifying abilities (e.g., angiotensin receptor blockers-neprylisin inhibitors ARNI, ACE-I, and MRAs) and effectiveness on long-term prognosis. Consequently, this aspect could partially explain the lack of positive effect of loop diuretics on long-term hard CV outcomes.
Maaten et al. [45] aimed to study how the use of loop diuretics might hamper the up-titration of ACE-i. Loop diuretic dose at baseline was recorded in 2338 patients with HFrEF enrolled in the BIOSTAT-CHF, an international study of HF patients eligible for up-titration of ACE-i and MRAs. The association between loop diuretic dose and up-titration of ACE-i/MRAs to target dose was adjusted for a previously published model for likelihood of up-titration and a propensity score. Higher doses of loop diuretics were associated with higher New York Heart Association (NYHA) class and higher levels of NT-proBNP, more severe signs and symptoms of congestion, more frequent MRAs use, and lower doses of ACE-i at 3 and 9 months (all P < 0.01). After propensity-score adjustment, higher doses of loop diuretics remained significantly associated with poorer up-titration of ACE-i (Beta per log for doubling of loop diuretic dose: − 1.66, P = 0.021), but not with up-titration of MRAs (P = 0.758). Higher doses of loop diuretics were independently associated with an increased risk of all-cause mortality or HF hospitalization [HR per doubling of loop diuretic dose: 1.06 (1.01–1.12), P = 0.021]. Therefore, higher doses of loop diuretics limited up-titration of ACE-i in patients with HFrEF and were associated with a higher risk of death and/or HF hospitalization, independent of their lower likelihood of up-titration and higher baseline risk.
At the same time, patients who receive optimal medical therapy are more likely to need lower loop diuretic doses. Observational studies show that patients who received maximal doses of ARNI were more likely to reduce their loop diuretic requirement [46].
Prescription of Loop Diuretics in Steady State
As highlighted by the most recent guidelines on HF treatment, the use of diuretics should be limited to the use of the lowest possible dose to maintain the state of euvolemia with the possibility of reducing diuretic dosing in the setting of increasing doses of Sodium-Glucose 2 CoTransporter inhibitors (SGLT2-i), ARNI/ACE-i/ARBs [4].
It is important to underline that HF patients do not necessarily need loop diuretics in steady state.
Sargento et al. [47] evaluated the association between daily furosemide dose prescribed during the “dry state” and long-term survival in stable, optimally medicated outpatients with HFrEF. Two hundred sixty-six consecutive outpatients with left ventricular ejection fraction <40%, clinically stable in the dry state and on optimal heart failure therapy, were followed up for 3 years. Furosemide doses were categorized as low or none (0–40 mg/day), intermediate (41–80 mg/day), and high (>80 mg). Those patients receiving the intermediate dose (hazard ratio [HR] 1/4 4.1; 95% CI: 2.57–6.64; P < .001) or high dose (HR 1/4 19.8; 95% CI: 7.9–49.6; P < .001) had a higher risk of mortality compared to those receiving a low dose. Therefore, patients receiving >40 mg/d, in a propensity score-matched cohort, had a greater risk of mortality than those receiving a low dose (HR 1/4 4.02; 95% CI: 1.8–8.8; P 1/4 .001) and those not receiving furosemide (HR 1/4 3.9; 95% CI: 0.07–14.2; P 1/4 .039). This showed that >40mg furosemide administration during the dry state in stable, optimally medicated outpatients with HFrEF is unfavorably associated with long-term survival.
Recently, Simonavičius et al. go beyond the concept of loop diuretic dose as a static parameter. TIME AHF [48•] study shows that the intensification of decongestion therapy, but not the loop diuretic dose at baseline, was related to adverse outcomes in HF. It must be noted that, in this study, patients undergoing treatment intensification, resulting in decongestion, had a better outcome than patients with persistent congestion, despite loop diuretics dose up-titration.
Moreover, a randomized study by Kapelios et al. [19] showed that lowering furosemide dose in stable chronic heart failure patients with reduced ejection fraction is not accompanied by decompensation. A major and new finding of this study was that the dose of diuretics can be safely lowered to approximately one third of the initial dose in the majority of stable, chronic HFrEF patients. Importantly, 95% of patients assigned to the dose reduction strategy remained on low doses during the entire follow-up period, without any episodes of cardiac decompensation or congestion symptoms. Moreover, NYHA functional class, body weight, and exercise capacity, expressed by peak oxygen consumption, remained unchanged compared with baseline. The most important finding, however, was a tendency towards better 1-year and 2-year prognoses associated with the decrease in the dose of diuretics in this population. Furthermore, the prolonged administration of high doses of diuretics, which, as mentioned earlier, seems to be the current prevailing clinical practice, caused a significant worsening of renal function and decrease in plasma hemoglobin, both known predictors of adverse outcomes in HF. These changes might explain how elevated doses of diuretics mediate their alleged deleterious effects in patients suffering from chronic HF. Currently, the tendency to chronically administer high doses of loop diuretics in HF seems to be attributable to the clinical misgiving that episodes of decompensation or congestion will occur. In this context, the new evidence regarding SGLT2-i (e.g., dapagliflozin, empagliflozin) which are both drugs capable of modifying the prognosis of patients with HFrEF and having a diuretic effect, brings great hope in the prospect of a safe reduction and discontinuation of loop diuretics [49]. Furthermore, the EMPEROR-reduced trial also showed that empagliflozin use was associated with improved renal function in patients with HFrEF. This finding suggests that this class of drugs may also be useful to compensate for the expected reduction in eGFR induced by loop diuretics [50].
Torsemide vs Furosemide
In current clinical practice, furosemide is by far the most commonly prescribed loop diuretic when treating congestion in patients with HF. However, evidence shows that the loop diuretic torsemide may be a valid alternative to furosemide.
A meta-analysis by Miles et al. [51] demonstrated a significantly lower risk of rehospitalizations and an improved NYHA status with torsemide as compared with furosemide. However, there was no evidence supporting the superiority of torsemide regarding all-cause mortality; instead, mortality was not affected by loop diuretic choice, allowing the authors to consider both loop diuretics interchangeably in this regard.
A larger meta-analysis by Abraham et al. [52], which included 19,280 patients across 19 studies, came to similar conclusions about the net benefit of torsemide over furosemide regarding the lower risk of hospitalization from HF and the improvement of NYHA functional status. Additionally, the analysis showed a lower cardiac mortality in patients treated with torsemide, despite not demonstrating a significant difference in all-cause mortality between the two groups: this apparent improvement in cardiac mortality is to be interpreted with caution, given the lack of evidence supporting a mortality benefit with loop diuretics.
These results are in line with the previous meta-analysis of Kido et al. [53]] as well as with the TORIC (TORasemide In Congestive heart failure) study [54], which both showed evidence of a significant reduction in NYHA class with torsemide compared with furosemide.
The ongoing ToRsemide compArisoN with furoSemide FOR Management of Heart Failure (TRANSFORM-HF) trial (ClinicalTrials.gov Identifier: NCT03296813), which is expected to enroll 6000 participants by mid-2022, will investigate the role of torsemide, compared with furosemide, in regard of the primary outcome of all-cause mortality, filling this current knowledge gap.
The reported superiority of torsemide over furosemide can have multiple explanations, taking into account both pharmacodynamics and pharmacokinetics. First, oral torsemide has a more predictable bioavailability profile (approximately 90%) as compared to oral furosemide (ranging between 10 and 100%) [1], since it is less affected by enteral absorption (which can be hampered by food or intestinal edema). Torsemide also has a longer half-life of 3 to 4 h (compared with 1 h for furosemide), ensuring more time above the threshold concentration needed to stimulate natriuresis and leading to a more constant diuresis [55]. Moreover, torsemide is the only loop diuretic with hepatic metabolism and excretion, thus not depending on renal function (likely to be reduced in a congestion setting) [55]. Lastly, torsemide is reported to have a direct vasodilatory effect, likely by increasing cAMP, cGMP, and prostacyclin levels, or by inhibiting angiotensin-II [52]; this pharmacodynamic aspect may be extremely useful in acute decompensated HF associated with elevated blood pressure values.
All these pharmacological properties may explain the observed benefit in improved diuresis and decreased fluid retention in patients treated with torsemide, as shown by the preliminary results from the TORNADO trial [56].
Additionally, torsemide has been shown to exert aldosterone receptor blocking activity, interfering with the RAAS; conversely, furosemide increases renin levels and RAAS activity with detrimental hemodynamic effects [28]. The RAAS blockade can be beneficial in many ways, notably thanks to a reduced kaliuresis (thus decreasing the risk of hypokalemia, a major limiting factor during loop diuretic treatment) and to the induction of an anti-fibrotic and anti-remodeling effect [51].
Sequential Nephron Blockade
Loop diuretics administration starts up mechanisms that lead to DR (i.e rebound sodium retention, post-diuretic effect). Furthermore, chronic use of loop diuretic cause hypertrophy and hyper-function of distal tubule cells, increasing Na+ uptake and aldosterone secretion. Sequential nephron blockade (NBD) with different diuretics acting on different segments of renal tubule should be considered to overcome the problem [10].
A randomized trial has proved a net improvement in urinary output and weight loss with the addition of thiazide diuretics in acute HF patients receiving loop diuretics who develop DR [57]. Other observational studies [58] have shown that NBD ease the switch to oral diuretic therapy. Although metolazone is the most commonly used TD in HF, there’s no evidence that one thiazide is better than another, suggesting a class effect. A prospective randomized study [59] has shown that the carbonic anhydrase inhibitor acetazolamide associated with loop diuretics improves natriuretic efficiency by 62% (defined as natriuresis per loop diuretic dose administered). Another study quantifies the net benefit of the combination therapy in approximately 100 mmol sodium excreted for every 40 mg furosemide-equivalent dose administrated [60].
On the other hand combination diuretic therapy increase the incidence of adverse effects such as WRF (without any clear adverse impact on clinical outcome in AHF), electrolyte imbalances and hypotension due to several urinary fluid loss. Disease-modifying diuretics (ACE-I; MRA, ARNI, SGLT-2) induce a sodium exertion and can allow the reduction of loop diuretic dose.
Conclusions
Loop diuretics are the mainstay of congestive heart failure therapy, but their effect on long-term prognosis remains uncertain. Several studies have shown a significant correlation between chronic loop diuretic treatment and increased rates of mortality and HF hospitalizations, most likely due to either the deterioration of the renal function or the hampered up-titration of disease-modifying drugs. Obviously, it should be taken into account that the need itself for diuretic therapy indicates a more advanced stage of the underlying disease. Whether the poorer prognosis attributed to the use of loop diuretics is mainly due to this aspect still remains a matter of debate. Surely, patients with advanced HF typically have renal dysfunction and are more inclined to develop loop diuretic resistance, which may lead to an incomplete decongestion and thus to a worse prognosis.
Loop diuretic use should be limited to the lowest dose necessary to maintain euvolemia, focusing instead on the optimization of guideline-directed medical treatment for HF: a lower loop diuretic dose in stable patients does not necessarily increase the risk of decompensation, but it can reduce the risk of adverse effects (such as decline of renal function, neurohormonal activation, or electrolyte disturbances) and allow the up-titration of disease-modifying drugs.
Finally, torsemide has been shown to have a safer profile regarding the risk of rehospitalizations; however, there is no evidence currently on a beneficial effect on all-cause mortality, which is the primary endpoint of the ongoing TRANSFORM-HF trial.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion — a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137–55.
Feig PU. Cellular mechanism of action of loop diuretics: implications for drug effectiveness and adverse effects. Am J Cardiol. 1986;57(2):14A–9A.
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure). J Am Coll Cardiol. 2005;46:e154–235.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Faggiano P, Opasich C, Tavazzi L, Achilli F, Gentile A, De Biase L, et al. Prescription patterns of diuretics in chronic heart failure: A contemporary background as a clue to their role in treatment. J Card Fail. 2003;9(3):210–8.
Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, et al. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND-HF Trial. JACC Hear Fail. 2017;5(1):1–13.
Cox ZL, Testani JM. Loop diuretic resistance complicating acute heart failure. Heart Fail Rev. 2020;25:133–45.
Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco-Bacik MA, Wilson FP, et al. Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol. 2017;28(11):3414–24.
ter Maaten JM, Rao VS, Hanberg JS, Perry Wilson F, Bellumkonda L, Assefa M, et al. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail. 2017;19(8):1014–22.
Knauf H, Mutschler E. Sequential nephron blockade breaks resistance to diuretics in edematous states. J Cardiovasc Pharmacol. 1997;29(3):367–72.
Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, et al. Loop diuretic efficiency a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7(2):261–70.
Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF). Circ Heart Fail. 2015;8(4):741–8.
Rao VS, Ivey-Miranda JB, Cox ZL, Riello R, Griffin M, Fleming J, et al. Natriuretic Equation to Predict Loop Diuretic Response in Patients With Heart Failure. J Am Coll Cardiol. 2021;77(6):695–708.
Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, et al. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation. 2018;137(19):2016–28.
Maeder MT, Rickli H, Pfisterer ME, Muzzarelli S, Ammann P, Fehr T, et al. Incidence, clinical predictors, and prognostic impact of worsening renal function in elderly patients with chronic heart failure on intensive medical therapy. Am Heart J. 2012;163(3):407–14.
Damman K, Testani JM. The kidney in heart failure: An update. Eur Heart J. 2015;36(23):1437–44.
De Silva R, Nikitin NP, Witte KKA, Rigby AS, Goode K, Bhandari S, et al. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: Contributing factors and relationship to prognosis. Eur Heart J. 2006;27(5):569–81.
McKie PM, Schirger JA, Benike SL, Harstad LK, Chen HH. The effects of dose reduction of furosemide on glomerular filtration rate in stable systolic heart failure. JACC: Heart Failure. 2014;2:675–7.
Kapelios CJ, Kaldara E, Ntalianis A, Nana E, Pantsios C, Repasos E, et al. Lowering furosemide dose in stable chronic heart failure patients with reduced ejection fraction is not accompanied by decompensation: a randomized study. Int J Cardiol. 2014;177(2):690–2.
Damman K, Ng Kam Chuen MJ, MacFadyen RJ, Lip GYH, Gaze D, Collinson PO, et al. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol. 2011;57(22):2233–41.
Mclellan J, Heneghan CJ, Perera R, Clements AM, Glasziou PP, Kearley KE, et al. B-type natriuretic peptide-guided treatment for heart failure. Cochrane Database Syst Rev. 2016;2016:CD008966.
Metra M, Felker GM, Zacà V, Bugatti S, Lombardi C, Bettari L, et al. Acute heart failure: Multiple clinical profiles and mechanisms require tailored therapy. Int J Cardiol. 2010;144(2):175–9 Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-77957850622&doi=10.1016%2Fj.ijcard.2010.04.003&partnerID=40&md5=70ec13453762b69ebb830811393f03ae.
Damman K, Kjekshus J, Wikstrand J, Cleland JGF, Komajda M, Wedel H, et al. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2016;18(3):328–36.
Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol. 2012;23(2):204–7.
Sica DA. Diuretic-related side effects: development and treatment. J Clin Hypertens (Greenwich, Conn). 2004;6:532–40.
Loon NR, Wilcox CS. Mild metabolic alkalosis impairs the natriuretic response to bumetanide in normal human subjects. Clin Sci. 1998;94(3):287–92.
Young M, Fullerton M, Dilley R, Funder J. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest. 1994;93(6):2578–83.
Yamato M, Sasaki T, Honda K, Fukuda M, Akutagawa O, Okamoto M, et al. Effects of torasemide on left ventricular function and neurohumoral factors in patients with chronic heart failure. Circ J. 2003;67(5):384–90.
Chaudhury RR, Chugh KS, Gupta GS, Sodhi P, Gupta KK. A controlled clinical trial comparing the diuretic furosemide and hydrochlorothiazide. J Assoc Physicians India. 1968;16(2):157–63.
Stewart JH, Edwards KDG. Clinical Comparison of frusemide with bendrofluazide, mersalyl, and ethacrynic acid. Br Med J. 1965;2(5473):1277–81.
Wilson JR, Reichek N, Dunkman WB, Goldberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med. 1981;70(2):234–9.
Yeh RW, Valsdottir LR, Yeh MW, Shen C, Kramer DB, Strom JB, et al. Parachute use to prevent death and major trauma when jumping from aircraft: randomized controlled trial. BMJ. 2018;363:k5094.
Engeln A. Diuretic strategies in patients with acute decompensated heart failure. J Emerg Med. 2011;40:797–805.
Chinese guidelines for the diagnosis and treatment of heart failure 2014. Chin J Cardiol 2014;42(2):98–122. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84896486149&doi=10.3760/2Fcma.j.issn.0253-3758.2014.02.004&partnerID=40&md5=79bc151854e86eacc08feb751b6cadfb
Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, Goto D, Yamada S, Yokoshiki H, et al. Loop diuretic use at discharge is associated with adverse outcomes in hospitalized patients with heart failure - a report from the Japanese cardiac registry of heart failure in cardiology (JCARE-CARD). Circ J. 2012;76(8):1920–7.
Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 2003;42(4):705–8.
Pellicori P, Cleland JGF, Zhang J, Kallvikbacka-Bennett A, Urbinati A, Shah P, et al. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther. 2016;30(6):705–8.
Dini FL, Ghio S, Klersy C, Rossi A, Simioniuc A, Scelsi L, et al. Effects on survival of loop diuretic dosing in ambulatory patients with chronic heart failure using a propensity score analysis. Int J Clin Pract. 2013;67(7):656–64.
Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2008;125(2):246–53.
Abdel-Qadir HM, Tu JV, Yun L, Austin PC, Newton GE, Lee DS. Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. Am Heart J. 2010;160(2):246–53.
Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97(12):1759–64.
Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM, et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE Registry. Cardiology. 2008;113(1):12–9.
Faris RF, Flather M, Purcell H, Poole-Wilson PA, Coats AJ. Diuretics for heart failure. Cochrane Database Syst Rev. 2016;2016:CD003838.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–17.
ter Maaten JM, Martens P, Damman K, Dickstein K, Ponikowski P, Lang CC, et al. Higher doses of loop diuretics limit uptitration of angiotensin-converting enzyme inhibitors in patients with heart failure and reduced ejection fraction. Clin Res Cardiol. 2020;109(8):1048–59.
Martens P, Verluyten L, Van de Broek H, Somers F, Dauw J, Dupont M, et al. Determinants of maximal dose titration of sacubitril/valsartan in clinical practice. Acta Cardiol. 2021;76(1):20–9.
Sargento L, Simões AV, Longo S, Lousada N, Dos Reis RP. Furosemide prescription during the dry state is a predictor of long-term survival of stable, optimally medicated patients with systolic heart failure. J Cardiovasc Pharmacol Ther. 2017;22(3):256–63.
• Simonavičius J, Maeder MT, Eurlings CGMJ, Aizpurua AB, Čelutkienė J, Barysienė J, et al. Intensification of pharmacological decongestion but not the actual daily loop diuretic dose predicts worse chronic heart failure outcome: insights from TIME-CHF. Clin Res Cardiol. 2021;110(8):1221–33. This study shows that the intensification of decongestion therapy, but not the loop diuretic dose at baseline, is related to adverse outcome in HF. It must be noted that, in this study, patients undergoing treatment intensification, resulting in decongestion, had better outcome than patients with persistent congestion, despite loop diuretics dose up-titration.
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413–24.
Miles JA, Hanumanthu BK, Patel K, Chen M, Siegel RM, Kokkinidis DG. Torsemide versus furosemide and intermediate-term outcomes in patients with heart failure: an updated meta-analysis. J Cardiovasc Med. 2019;20(6):379–88.
Abraham B, Megaly M, Sous M, Fransawyalkomos M, Saad M, Fraser R, et al. Meta-Analysis Comparing Torsemide Versus Furosemide in Patients With Heart Failure. Am J Cardiol. 2020;125(1):92–9.
Kido K, Shimizu M, Hashiguchi M. Comparing torsemide versus furosemide in patients with heart failure: A meta-analysis. J Am Pharm Assoc. 2019;59:432–8.
Cosín J, Díez J. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4(4):507–13.
Täger T, Fröhlich H, Seiz M, Katus HA, Frankenstein L. READY: relative efficacy of loop diuretics in patients with chronic systolic heart failure—a systematic review and network meta-analysis of randomised trials. Heart Fail Rev. 2019;24:461–72.
Balsam P, Ozierański K, Marchel M, Gawałko M, Niedziela Ł, Tymińska A, et al. Comparative effectiveness of torasemide versus furosemide in symptomatic therapy in heart failure patients: preliminary results from the randomized tornado trial. Cardiol J. 2019;26(6):661–8.
Channer KS, McLean KA, Lawson-Mathew P, Richardson M. Combination diuretic treatment in severe heart failure: A randomised controlled trial. Br Heart J. 1994;71(2):146–50.
Aravot DJ, Banner NR, Musumeci F, Fitzgerald M, Madden B, Khaghani A, et al. Oral metolazone plus frusemide for home therapy in patients with refractory heart failure. Lancet. 1989;333:727–8.
Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, et al. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail. 2019;21(11):1415–22.
Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70(3):265–73.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interests
All the authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Clinical Heart Failure
Rights and permissions
About this article
Cite this article
Antonietta, C.M., Calvi, E., Faggiano, A. et al. Impact of Loop Diuretic on Outcomes in Patients with Heart Failure and Reduced Ejection Fraction. Curr Heart Fail Rep 19, 15–25 (2022). https://doi.org/10.1007/s11897-021-00538-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-021-00538-7