Abstract
Purpose of Review
This review provides a literature update and practical outline for the management of diabetes and stress hyperglycemia for adult surgical patients in the pre- and intraoperative settings.
Recent Findings
Hyperglycemia in surgical patients has been associated with increased risk of complication in both diabetic and non-diabetic patients in the perioperative setting. While current recommended perioperative blood glucose target is < 180 mg/dL (10 mmol/L), optimal outcomes may require different treatment targets for diabetic versus non-diabetic patients. Hemoglobin A1C level is associated with elevated risk of hyperglycemia and adverse outcomes, but there is insufficient evidence to recommend routine preoperative testing or optimal values in elective surgical patients. Day of surgery blood glucose testing and treatment are recommended in the perioperative period, and anesthetic management includes appropriate patient selection for use of subcutaneous insulin, intravenous insulin infusions, and insulin pumps. Additionally, administration of both intravenous and perineural dexamethasone is associated with increased blood glucose levels and clinicians should consider the risk benefit ratio in surgical patients. For enhanced recovery after surgery protocols, further evidence is needed to support routine use of carbohydrate loading in diabetic patients.
Summary
Optimal perioperative care includes screening at-risk patients, use of preoperative oral hypoglycemics and home insulin, anesthetic type and medication selection, blood glucose testing, and treatment for hyperglycemia in the operating room. Partnerships with surgery and endocrinology teams aid optimal postoperative management and discharge planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hospital hyperglycemia (blood glucose ≥ 140 mg/dL, 7.8 mmol/L) is commonly encountered in the perioperative environment, estimated to occur in 20–40% of non-cardiac, 35% of vascular, and 80% of cardiac surgery patients [1,2,3]. The stress of surgery has been demonstrated to increase blood glucose levels in both diabetic and non-diabetic patients and is associated with a multitude of adverse clinical outcomes, including higher risk of wound infection, delayed wound healing, pneumonia, sepsis, cardiovascular complication, and acute kidney injury [1, 4•, 5]. While evidence suggests that patients with controlled blood glucose levels demonstrate improved postoperative outcomes versus those who remain hyperglycemic [6••, 7], the optimal glucose management strategy is still subject to debate. This review provides a practical outline for perioperative management of diabetes and stress hyperglycemia in adult surgical patients.
Perioperative Hyperglycemia, Pathophysiology
The stress of an invasive procedure or surgery alters the normally well-regulated balance between hepatic glucose production and glucose utilization in the body. Counterregulatory hormones produced by the body’s stress response (epinephrine and cortisol) increase hepatic glucose production and promote gluconeogenesis [8], while also enhancing lipolysis and free fatty acid (FFA) formation [9]. FFAs and inflammatory molecules, including tumor necrosis factor-α (TNF-α), interfere with peripheral glucose uptake and create a state of relative insulin resistance [10, 11]. Although the pathophysiology is not entirely understood, elevated blood glucose levels have been demonstrated to impair neutrophil function, increase reactive oxygen species, induce mitochondrial injury, and produce endothelial dysfunction.
Stress Hyperglycemia
Stress hyperglycemia is defined as a transient hyperglycemic state which occurs in a patient without a history of glucose intolerance [9]. As the acute surgical stress or illness subsides, hyperglycemia resolves, and patients return to a euglycemic state. Following 170 surgical patients considered to be at risk for hyperglycemia (age ≥ 45 years and/or BMI ≥ 25 kg/m2), we observed that 6.4%, with normal preoperative A1C, experience at least one BG ≥ 180 mg/dL (10 mmol/L) during hospitalization (Table 1). The severity of hyperglycemia is aggravated by the invasiveness of the surgical procedure, use of inotropic medications, anatomic location of the surgery, and the type of anesthesia. Cardiac surgical procedures with the use of cardiopulmonary bypass (CPB) circuit, thoracic and abdominal surgery, epinephrine infusions, and general anesthesia appear to be more frequently associated with elevated cortisol and catecholamine states and resultingly, hyperglycemia.
Non-diabetic patients suffering hyperglycemia in the perioperative period are at higher risk of developing a complication than those with diagnosed diabetes [2, 13]. However, the initial data examining non-diabetic patients compared to diabetic patients notably lacks differentiation of undiagnosed disease at the time of surgery. Longitudinal studies indicate up to one third of patients who experience hyperglycemia in the hospital have impaired glucose tolerance on further testing [14], suggesting the presence of undiagnosed pre-diabetes or diabetes. Additionally, patients without a diagnosis of diabetes are less likely to be treated with insulin when hyperglycemic [4•].
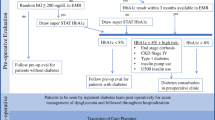
Over a 24-month screening period, 4204 preoperative surgical patients presented to our institution with risk factors for DM2 (age ≥ 45, BMI ≥ 25 kg/m2) but without a history of disease. A1C testing revealed that 27.2% of screened patients had pre-diabetes and 3.9% undiagnosed diabetes. Those with elevated A1C were more likely to suffer hyperglycemia during their perioperative course (Table 1) and less likely to be treated with insulin for BG > 180 mg/dL (10 mmol/L). Measurement of A1C presents the opportunity to diagnose and treat surgical patients during their operative course and also ensure continued care on hospital discharge.
A1C Testing
The majority of patients with diabetes have DM2, affecting 12.2% of the adult population in the USA [15]. Approximately 25% of those afflicted by the disease (7.2 million individuals) are unaware that they carry a diagnosis, and an additional 84 million Americans have pre-diabetes [15].
A1C levels are used in the outpatient setting to measure a patient’s 3-month average blood glucose level. The American Diabetes Association (ADA) 2019 Standards of Medical Care recommend checking A1C twice per year in well-controlled diabetic patients and every 3 months in poorly controlled diabetic patients. An A1C should be performed in the hospital setting in all patients with diagnosed DM and those with blood glucose > 140 mg/dL (7.8 mmol/L) if not done 3 months prior to admission [16••]. The American Diabetes Association defines pre-diabetes as an A1C 5.7–6.4% and the presence of diabetes as A1C > 6.5% [17••]. It is important to recognize that this is a disease which belongs on a continuous spectrum and often is insidious and asymptomatic at the time of diagnosis. Conditions that interfere with accurate A1C testing are listed in Table 2 and should be considered before making a formal diagnosis of diabetes.
Current recommendations target A1C levels < 7% for young, non-pregnant patients, and < 8% for older patients with multiple comorbidities. These values, however, are defined for long-term disease management to reduce the risk of heart disease, peripheral vascular disease, and stroke. Perioperative targets are less clear. Increasing A1C is associated with adverse outcomes, including increased risk of wound infections [18], longer length of stay (LOS), and intensive care unit (ICU) admission [19••]. A recent retrospective study at Duke University examined the relationship between preoperative A1C, perioperative blood glucose level, and mortality in 13,000 surgical cases. Preoperative A1C correlated with perioperative blood glucose levels in both cardiac and non-cardiac surgery. Perioperative mortality demonstrated a linear relationship in non-cardiac surgery, increasing from 1 to 1.6% when blood glucose increased from 100 (5.5 mmol/L) to 200 mg/dL (11.1 mmol/L). In cardiac surgery, the relationship was U-shaped: mortality was 4.5% at 100 mg/dL (5.5 mmol/L), 1.5% at 140 mg/dL (7.8 mmol/L), and 6.9% at 200 mg/dL (11.1 mmol/L). However, after controlling for blood glucose (as well as body mass index, age, and sex), elevated preoperative A1C was not associated with mortality in cardiac or non-cardiac surgery [6••]. Perioperative blood glucose control may limit the risk of perioperative complication in patients with higher A1C [7]. Thus, preoperative A1C remains unknown and there is insufficient information to suggest case cancelation for a particular A1C.
Testing does provide several pieces of useful information in the perioperative setting and may offer benefit to patients:
-
1.
Diagnosed DM without testing in the prior 3 months
-
2.
At risk for pre-diabetes or diabetes (age ≥ 45 years, BMI ≥ 25 with one additional risk factor) [17••]
-
3.
Day of surgery fasting BG ≥ 126 mg/dL
-
4.
Hospital hyperglycemia, BG ≥ 140 mg/dL
Results will identify those patients with previously undiagnosed disease, indicate baseline disease control in those with known diabetes, and predict the risk of developing hospital hyperglycemia. It does not, however, provide information on blood glucose variability or propensity for hypoglycemia.
Preoperative Hyperglycemia
Patients presenting for elective surgery with severe hyperglycemia (BG ≥ 300 mg/dL, 16.6 mmol/L) should be evaluated prior to proceeding to the operating room. Type of diabetes, degree of hyperglycemia, and A1C results should be reviewed in the context of the type of surgery, patient’s baseline health (frailty, immunosuppression, malnutrition, comorbidities), and compliance with medical therapy. Currently, no guidelines exist for the postponement of elective surgery due to hyperglycemia except for in the setting of severe dehydration, diabetic ketoacidosis (DKA), or hyperosmolar nonketotic states [20]. Serum bicarbonate and electrolytes may provide valuable diagnostic information identifying these states. In the absence of these findings, an insulin infusion or subcutaneous insulin should be initiated to achieve blood glucose levels < 300 mg/dL (16.6 mmol/L). If blood glucose levels drop as expected, insulin treatment should be continued, and surgery can proceed. If elevated blood glucose levels persist, it is reasonable to postpone surgery for further treatment.
Type of Anesthesia
There is evidence that anesthetic technique has an effect on blood glucose levels in the perioperative period. Neuraxial techniques such as epidural and spinal anesthesia blunt the sympathetic response to surgical stimuli. Both diabetic and nondiabetic patients undergoing hip arthroplasty who received spinal anesthesia were found to have lower perioperative blood glucose levels compared to those who had general anesthesia [21]. A meta-analysis of 11 randomized controlled trials (RCT) found that patients who had combined general-epidural anesthesia had lower perioperative blood glucose levels than patients who had general anesthesia alone (difference of 22.7 mg/dL, 1.26 mmol/L) [22].
The impact of varied types of general anesthetics has also been studied to determine the impact on BG levels. A RCT of 60 nondiabetic patients undergoing inguinal hernia repair found lower cortisol and blood glucose levels in patients who received total intravenous anesthesia with propofol compared to those who received a volatile anesthetic [23]. This effect was seen both intraoperatively and 24 h into the postoperative period. Animal modeling has demonstrated BG levels increase with sevoflurane anesthesia, but are stable under propofol anesthesia and similar to animals receiving surgery without anesthesia [24]. These results are likely attributable to volatile anesthetics’ (sevo- and isoflurane) impairment on glucose tolerance in non-diabetic patients [25].
When deciding on an anesthetic technique, a number of factors must be considered including the type of surgery, surgical length and complexity, patient comorbidities, patient preference, and anesthetic cost.
Preoperative Glycemic Management
Oral Hypoglycemics
Preoperative management of oral diabetic medications is based upon the type and duration of surgery and length of pre- and postoperative fasting. There is a paucity of data examining oral hypoglycemic agents in patients on the day of surgery. A small study in ambulatory surgery patients demonstrated that those who continue oral hypoglycemic medication on the day of surgery had lower perioperative BG levels with fewer episodes of hyperglycemia than those who discontinue oral agents on the day of surgery [26]. There was no difference in the incidence of hypoglycemia between groups. The study did not, however, look at patient outcomes and was insufficiently powered to distinguish between the 2 study medications (metformin and sulfonylureas). Oral agent used in the preoperative period is summarized in Table 3.
Metformin
Metformin is the preferred initial treatment agent for type 2 diabetes [28] and is the most extensively used oral hypoglycemic agent in the USA. The Society for Ambulatory Anesthesia (SAMBA) recommends patients continue metformin until the day of surgery but hold their dose on the day of surgery [20]. This is consistent with 2019 ADA Standards of Medical Care [16••]. However, the Joint British Diabetes Societies Guidelines recommend continuing metformin if the patient will only miss a single meal [29]. For those undergoing same-day procedures with immediate return to meals, metformin may offer benefit in maintaining glycemic control.
Metformin-associated lactic acidosis (MALA) has been the perceived risk using this agent in surgical patients. A Cochrane meta-analysis of 347 trials examining predominantly outpatients using metformin found no evidence of increased risk of lactic acidosis [30]. A retrospective study of 1284 diabetic patients who continued metformin prior to cardiac surgery also demonstrated no increased risk of lactic acidosis nor adverse events including in-hospital mortality, cardiac, renal, or neurologic morbidities in cardiac surgery patients [31]. However, caution should be exercised in those with renal dysfunction (GFR ≤ 30 mL/min) and/or undergoing procedures with the administration of intravenous (IV) contrast because these factors are known to increase the risk of MALA in the perioperative period.
Secretagogues
Secretagogues, including sulfonylureas, should be held the day of surgery to decrease the risk of hypoglycemia [20, 32].
Sodium Glucose Co-transporter 2 Inhibitors
Sodium glucose co-transporter 2 (SGLT-2) inhibitors are now recommended as the second-line diabetes agents in diabetic patients with atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease [28]. Providers can expect to see these used in increasing frequency in the perioperative period. Euglycemic diabetic ketoacidosis (eDKA) has been reported in patients taking SGLT-2 inhibitors, most frequently in the setting of fasting states and/or stressful events such as acute illness or surgery [33,34,35]. As a result, recent recommendations by the American College of Endocrinology (ACE) and American Association of Clinical Endocrinologists (AACE) advise holding SGLT-2 inhibitors 24 h prior to surgery [36].
The incidence of DKA in patients using SGLT-2 inhibitors is estimated to be 0.02–0.03% in patients with DM2, and 0.07% when including DM1 and DM2 [33]. Several studies report blood glucose levels on the diagnosis being within normal range making the diagnosis potentially less recognizable [34, 35]. However, in a review of 17,596 patients reporting 12 cases of DKA, 10 patients had a blood glucose level reported at presentation that was > 300 mg/dL (16.7 mmol/L) [33]. Half of the cases of DKA reported in this study were found in patients with latent autoimmune diabetes of adulthood (LADA), and the study authors postulate that patients with low β-cell reserve to be unable to produce sufficient insulin to suppress ketogenesis [33]. While more research is needed to understand the underlying mechanisms of SGLT-2-associated DKA, caution may be warranted in those undergoing pancreatic surgery or with underlying pancreatic disease as these patients may be at higher risk of developing DKA when taking SGLT-2 inhibitors [35, 37].
Fasting also places patients at risk because there is a metabolic shift from carbohydrate to fat oxidation that predisposes patients to an increased risk of DKA. Post-surgical symptoms commonly overlap manifestations of DKA including nausea, vomiting, fatigue, and abdominal pain. Additionally, the lack of severe hyperglycemia may not provide a diagnostic clue. Vigilance must be exercised when caring for surgical patients taking SGLT-2 inhibitors, and work-up initiated to evaluate persistent symptoms, signs of dehydration, and/or metabolic acidosis (low serum bicarbonate, low pH on blood gas, and/or anion gap). Positive urine or serum ketones further support the diagnosis.
Incretins
Medications that modify the incretin hormone, including DPP-4 inhibitors and GLP-1 agonists, are a class of agents that, in addition to lowering blood glucose levels, have been shown to have a number of beneficial cardiovascular effects. There is evidence that DPP-4 inhibitors improve heart function in patients with heart disease, reduce blood pressure, and reduce postprandial lipid levels [38,39,40]. RCTs have demonstrated that both DPP-4 inhibitors and GLP-1 agonists to be safe for the management of hospitalized medicine and surgery patients [41, 42].
A large trial (n = 277) examined the safety and efficacy of sitagliptin in medical and surgical patients with a wide range of presenting A1C. Patients were randomized to once-daily basal-bolus insulin plus sitagliptin versus basal-bolus insulin alone. There was no difference in mean daily BG levels or incidence in hypoglycemia between groups; however, the sitagliptin arm required a lower total daily dose of insulin as well as decreased number of correctional injections [43]. An open-label multicenter RCT examining linagliptin, specifically in surgical patients with admission BG 140–400 mg/dL (7.8–22.2 mmol/L), resulted in fewer hypoglycemic events as compared to basal-bolus therapy [44]. Continuing DPP-4 medications in diabetic surgical patients on the day of surgery is not more likely to cause hypoglycemia than insulin alone, and may decrease the number of injections needed to keep blood glucose levels within a target range. These agents can be safely taken on the day of surgery and continued throughout the surgical period.
GLP-1 agonists have additionally been studied in the hospital setting, including patients undergoing coronary artery bypass grafting (CABG). Mean blood glucose levels between those randomized to exenatide plus insulin infusion, versus insulin infusion only, do not demonstrate superiority in achieving target blood glucose levels. Subjects in the exenatide arm did receive less insulin, and time to insulin commencement was longer [45]. Liraglutide initiation the day prior to non-cardiac surgery (as compared to an insulin infusion or 50% of home-dose basal insulin) demonstrated lower blood glucose levels on the first postoperative day, no difference in hypoglycemia, and no association with postoperative complications [46]. However, in this study, as well as a RCT examining surgical patients administered twice daily exenatide, GLP-1 agonist use was associated with increased nausea [42]. This side effect has been reported in the outpatient setting, but appears to be transient and can be prevented with gradual up-titration of the medication. Nausea may be less pronounced in individuals on long-standing therapy versus day of surgery initiation. Caution should however be exercised in patients at high risk for impaired bowel motility following surgery, and if perceived risk is high, holding GLP-1 agonists may be prudent.
Insulin
DM1 and DM2 patients should continue their insulin therapy in the preoperative period to minimize hyperglycemia on the day of surgery. An observational study examining fasting patients on the morning of surgery revealed that patients that hold evening basal insulin arrive with significantly higher blood glucose levels than those taking 50–75% of their basal insulin. Those taking no basal insulin presented for surgery with an average blood glucose level of 274 mg/dL (15.2 mmol/L) [47].
A 25% reduction in long-acting insulin dose the evening before surgery improves glycemic control without an increase in hypoglycemia on the day of surgery [47, 48] and is recommended by the Society for Ambulatory Anesthesia (SAMBA) [20]. NPH and mixed insulin should also be dosed at 80% of normal dose the evening prior to surgery. Prandial insulin should be held when fasting begins.
The morning of surgery, patients on twice-daily dosing of basal insulin should take 80% of their morning dose. If morning blood glucose is > 120 mg/dL (6.7 mmol/L), NPH and mixed insulin should be reduced to 50% of usual dose [49]. To minimize the risk of hypoglycemia, NPH and mixed insulin are held if BG < 120 mg/dL (6.7 mmol/L) [27].
Ultra-long-acting insulins, including insulin glargine U300 (Toujeo) and insulin degludec (Tresiba), are a new class of basal insulin gaining popularity for treatment of diabetes. Glargine U300 is three times as potent as glargine U100 (300 units/mL compared to 100 units/mL), allowing for smaller injection volumes. Glargine U300 has a slower onset of action (6 h) compared to glargine U100 (2–4 h) and longer duration of action (30 h) with a decreased peak effect [50].
Insulin degludec, which comes in both 100 units/mL and 200 units/mL formulation, has a 2-h onset, 25-h half-life, and 42 h duration of action at steady state, which is typically reached in 2–3 days. Analyzed in the outpatient setting over the course of 1 year, insulin degludec was associated with fewer episodes of hypoglycemia (BG < 56 mg/dL, 3.1 mmol/L) compared to insulin glargine U100 [51]. Rates of severe hypoglycemia appeared similar between groups, but there were too few incidents to be comparatively assessed.
Data regarding ultra-long-acting insulin use in the inpatient or surgical setting is lacking, and there are limited dosing recommendations in the perioperative period. Given the available data and known pharmaco-dynamics and kinetics of the drugs, ultra-long-acting insulin can be managed similarly to other basal insulins. A 20% reduction in usual dose is recommended prior to surgery. Further studies are underway to evaluate dosing regimens for ultra-long-acting insulin in hospitalized medical and surgical patients.
Intraoperative Glycemic Management
Management of intraoperative blood glucose will vary based on a patient’s diabetes status, home management, type and duration of surgery, anesthetic technique, and anticipated postoperative fasting duration. Although there is no consensus on the ideal blood glucose level, a number of societies recommend maintaining blood glucose levels < 180 mg/dL (10 mmol/L), including SAMBA [20], the AACE [52], the Society for Thoracic Surgery [53], and the Joint British Diabetes Society [29]. The 2019 ADA Guidelines recommend treatment initiation at 180 mg/dL (10 mmol/L) with an acceptable perioperative BG range 80–180 mg/dL (4.4–10.0 mmol/L) [16••]. The Society of Critical Care Medicine recommends maintaining blood glucose < 150 mg/dL (8.3 mmol/L) in critically ill patients [54]. For patients without a diagnosis of diabetes, data in the cardiac surgery population showed improved outcomes in non-diabetic patients whose blood glucose was controlled between 110 and 140 mg/dL (6.1–7.8 mmol/L) [55]. There is a paucity of data examining outcomes in general surgery patients when blood glucose targets are maintained in the 110–140 mg/dL vs. 140–180 mg/dL range.
Hyperglycemia (blood glucose > 180 mg/dL, 10 mmol/L) should be treated with subcutaneous (SC) rapid-acting insulin or continuous infusion of regular insulin. Insulin infusions have been demonstrated to be the best method for achieving glycemic targets in critically ill patients [52] and are thus recommended in patients presenting to the operating room undergoing procedures with anticipated hemodynamic changes, fluid shifts, significant blood loss, need for inotropes or vasopressors, and lengthy operative times [27]. These factors impact the absorption and distribution of SC insulin and result in unpredictable pharmacodynamics. The short half-life of regular IV insulin allows for rapid titration of the medication to adjust for changing conditions in the operating room.
In non-critically ill patients undergoing surgeries without anticipated hemodynamic instability, large fluid shifts, or prolonged postoperative fasting times, or in short ambulatory procedures (less than 4 h), SC insulin is recommended over IV insulin infusion for treatment of hyperglycemia [20, 52]. Advantages of SC rapid-acting insulin include ease of administration, low rate of hypoglycemia, and efficacy in correcting hyperglycemia [56].
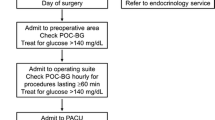
SC insulin dosing should be based on a patient’s anticipated insulin sensitivity. The following formula can be used to calculate the correctional dose of SC insulin: measured glucose − 100/insulin sensitivity factor. Insulin sensitivity factor is 1800 divided by patient’s total daily dose (TDD) of insulin, which includes basal, prandial, and correctional insulin. If TDD is not available or the patient is on oral medication, an insulin sensitivity factor can be estimated based on the patient’s medical history. Table 4 provides an example of our institutional correctional insulin scale. This can be used on the day of surgery to calculate subcutaneous insulin for those who meet the criteria. When using rapid-acting insulin, blood glucose should be checked at least every 2 h. Dosing of rapid-acting insulin should not occur more frequently than every 2 h to decrease the risk of hypoglycemia from insulin stacking, and no more than two doses should be provided to an anesthetized patient to limit the risk of hypoglycemia [27]. See Fig. 1 for the intraoperative hyperglycemia treatment algorithm used at our institution.
Special consideration should be given to type I DM patients presenting for surgery. While often diagnosed in childhood, DM1 can develop at any age and is the result of an autoimmune process that destroys the beta cells of the pancreas. Insulin is required to treat DM1 and prevent ketoacidosis in the perioperative period. Dosing should be matched to the patient’s sensitivity scale because these patients have higher rates of both hyper- and hypoglycemia as compared to DM2 [57].
Intraoperative Blood Glucose Testing
Hypoglycemia
Level 1 hypoglycemia is defined as BG ≤ 70 mg/dL (3.0 mmol/L) [16••] and can be difficult to recognize in the anesthetized patient. Symptoms such as tremor, anxiety, palpitations, or sweating are not present in those under general anesthesia. Care must be taken to appropriately monitor patients with diabetes and those administered insulin in the perioperative period. Factors that have been demonstrated to contribute to intraoperative hypoglycemia include aggressive target blood glucose levels, poor team communication, administration of prandial insulin in fasting patients, failure to monitor patients appropriately, drug error, and interruption of enteral or parental nutrition [58, 59]. Strategies to reduce the risk of hypoglycemia include close BG monitoring, moderate (versus intensive) BG targets, and clear communication between healthcare providers [58, 59]. Institutions should standardize algorithms for the treatment of hypoglycemia and define immediate correctional medications/fluids, frequency of repeat BG testing, and discontinuation of insulin therapy. An example of an operating room algorithm for the management of intraoperative hypoglycemia is outlined in Fig. 1.
Intravenous Dexamethasone
Dexamethasone is administered to prevent postoperative nausea and vomiting (PONV), to augment analgesics in reducing postoperative pain, and to reduce post-extubation stridor and airway edema. Given its propensity to exacerbate hyperglycemia, clinicians should evaluate its risk versus benefit ratio in the perioperative period.
The literature evaluating steroid use in anesthetized patients commonly delineates doses into intravenous dexamethasone equivalents: low dose < 8 mg, intermediate dose 8–16 mg, and high dose > 16 mg. The varied dosing ranges have been examined, and while data is mixed, increasing dexamethasone dose appears to increase the risk of developing hyperglycemia in both diabetic and non-diabetic patients [60]. At the three varied dosing ranges, there is a significant rise in blood glucose levels as compared to patients not administered dexamethasone, with the weighted mean difference being approximately 14 mg/dL (0.8 mmol/L) in non-diabetic and 30 mg/dL (1.7 mmol/L) in diabetic patients.
DM2 patients randomized to dexamethasone 8 mg IV as prophylactic therapy for PONV demonstrated a mean BG of 30 mg/dL (1.7 mmol/L) higher than those randomized to 4 mg IV ondansetron. The non-diabetic patients also demonstrated an increase in blood glucose (23.5 mg/dL, 1.3 mmol/L) [61]. However, while doses upwards of 8 mg IV of dexamethasone are used for prophylactic therapy, the current recommended dose of dexamethasone by SAMBA is 4–5 mg [20]. Compared to 4–5 mg, 8–10 mg IV dexamethasone is associated with a 25-mg/dL (1.4 mmol/L) greater increase in blood glucose [62].
Given the elevated blood glucose levels seen in patients administered IV dexamethasone in conjunction with the drug’s immunologic side effects [63], concerns have been raised regarding increased risk of surgical site infection (SSI). A 2017 meta-analysis of RCTs examining various doses of dexamethasone in the perioperative period found no difference in the incidence of infection [60]. The ENIGMA-II trial, an international RCT examining postoperative cardiac events in patients undergoing nitrous anesthetic, performed a post hoc sub-analysis of 2178 (of 5499) patients receiving dexamethasone (En-DEX) and found no increase in infection in those given dexamethasone [64]. It is notable, however, that the patients were not randomized to dexamethasone in this study and anesthesiologists were less likely to choose this agent as PONV prophylaxis in diabetic patients or those with concurrent infection. Subgroup analysis by diabetic status indicated no difference in wound infection.
The Perioperative Administration of Dexamethasone and Infection (PADDI) Trial is a large RCT currently underway to specifically examine the impact of dexamethasone on SSI (Clinical Trials.gov).
Perineural Dexamethasone
Perineural dexamethasone is a common adjuvant to local anesthetics for peripheral nerve blockade in upper extremity surgery because it prolongs the duration of sensory block and may be effective in reducing postoperative pain and opioid consumption [65]. Compared to intravenous dexamethasone administered to patients undergoing extremity block, perineural dexamethasone displays equivalent block duration and reduction of pain intensity.
There are limited studies evaluating the impact of perineural dexamethasone and blood glucose levels. Duration of analgesia is the most common primary study outcome with limited studies examining intravenous versus perineural dexamethasone on hyperglycemia as a secondary outcome. A comparison of 30 mL 0.5% ropivacaine for interscalene block to 0.5% ropivacaine with concomitant administration of 10 mg perineural dexamethasone resulted in an increase of mean postoperative blood glucose of 3.8 mg/dL (0.2 mmol/L) [66]. Of note, diagnosed diabetes was an exclusion criterion for the study. At smaller doses, 5 mg dexamethasone given IV or perineurally with interscalene block also caused a statistical increase in blood glucose values from baseline (9.6 mg/dL, 0.5 mmol/L, in the IV group, and 7.4 mg/dL, 0.4 mmol/L, in the perineural group) [67]. Patients with “uncontrolled diabetes mellitus” were excluded from the study, but this was not defined by the authors and blood glucose levels were not analyzed by diabetes status.
The minimal data available examining the impact of perineural dexamethasone on blood glucose limits recommendations for its use in diabetic patients; however, trials are underway examining its impact on blood glucose levels during the surgical period which will better determine its safety in patients at risk for hyperglycemia.
Insulin Pumps and Continuous Glucose Monitors
Insulin pumps (also called continuous subcutaneous insulin infusion therapy, CSII) are being used increasingly for patients with both DM1 and DM2 to deliver continuous basal rapid-acting insulin. Up to 400,000 DM1 patients in the USA use insulin pumps [68]. Traditional insulin pumps have a reservoir attached to the body with tubing and can be programmed to deliver bolus doses at mealtimes. Insulin patch pumps also have a reservoir, but are wirelessly controlled by a separate device, including continuous blood glucose monitors (CGM). Often referred to as sensor-augmented pumps, CGM and CSII can be integrated to suspend insulin delivery if glucose levels reach a preset low threshold level.
While strategies to maintain CSII in the inpatient setting have been published [69], there is limited data on the use of CSII on the day of surgery. Validated approaches have not been published extensively, and there is wide variation in practice [70]. Therapeutic options for patients on insulin pumps include continuing the pump throughout the perioperative period, discontinuing the pump, and using an intravenous insulin infusion or using injectable subcutaneous insulin in lieu of or in addition to the insulin pump. The course of action depends upon the type of surgery, the ability of the provider to safely operate the pump, the expected fasting period, and the timing required until the patient can again safely control the pump following surgery.
There are a number of limitations for CSII use in the perioperative environment similar to the constraints listed for intraoperative injectable SC rapid-acting insulin. CSII should be converted to an intravenous insulin infusion in patients with critical illness, hemodynamic instability, large fluid shifts, and operative time > 3 h (Fig. 1). Given the unpredictable complexity of the case and the severity of patient illness, emergency surgery patients with an insulin pump should be converted to intravenous insulin infusion. Access to the pump should be considered by the surgical team; if the pump or insertion site is in the surgical field, it will need to be removed. As per the device manufacturer’s recommendations, the device can be inserted in an alternative location (arm or leg) for abdominal surgeries [71]. When monopolar electrocautery is to be used, the electrical arc (monopolar electrocautery tip to grounding pad) should not encompass the pump. It may be best to remove the pump to prevent damage to the device if the pump cannot be moved to avoid the electric arc. Pump manufacturers recommend the device be removed to avoid exposure to radiation; patients undergoing procedures involving computed tomography, magnetic resonance imaging, or fluoroscopy will need alternative insulin therapy. Additionally, plans need to be made to transition pump care back to the patient when recovered from anesthesia.
The SAMBA consensus statement advocates the use of CSII continuation [20]. If the pump is not in the surgical field, and the patient and surgery meet the criteria for SC insulin use, a patient’s home pump can be continued in the operating room. It is paramount in this case that the anesthesiologist managing the device is familiar and comfortable with the pump. A retrospective study of 92 surgical cases found intraoperative mean BG levels between CSII and insulin infusion to be the same [72]. Sobel et al. demonstrated in 43 surgical cases treated with CSII a first mean postoperative BG of 173 mg/dL (9.6 mmol/L) in DM1 and 182 mg/dL (10.1 mmol/L) in DM2 (target study BG ≤ 200 mg/dL, 11.1 mmol/L). No patient suffered from an episode of intraoperative hypoglycemia [73]. Additional subcutaneous insulin was provided by the anesthesiologist for BG > 200 mg/dL (11.1 mmol/L), or an insulin infusion was initiated if the procedure lasted > 2 h and patient had one BG measurement > 250 mg/dL (13.9 mmol/L).
Blood glucose should be tested hourly to ensure euglycemia. Hyperglycemia can be treated with a bolus delivered directly from the pump given no more frequently than every 2 h. Correctional boluses are limited to a maximum number of two doses to limit insulin stacking. If hyperglycemia persists despite bolus dosing and basal infusion, the pump should be suspended and an IV insulin infusion started. Providers should also be aware that DM1 patients often aim for tight control (80–110 mg/dL, 4.4–6.1 mmol/L) which is too low for a hospital setting. The basal rate may need to be adjusted to 80% of its baseline in surgical patients if the pump is continued [16••]. The pump should be stopped in the operating room for BG < 100 mg/dL (5.5 mmol/L).
CGM devices are typically minimally invasive and measure glucose in the interstitial fluid, most commonly every 5 min [68, 74]. There is limited data exploring the accuracy of sensors during hypotension, hypothermia, and hypoxia, events that may be encountered during a surgical course. There are also a number of other medications used frequently in the perioperative period (mannitol, heparin, acetaminophen, and dopamine) which may interfere with CGM results. The Consensus Statement on inpatient use of CGM does not specifically address patients in the operating room; however, the panel cautions the use of these devices in critically ill patients [74]. At this time, arterial blood gas samples, venous phlebotomy, and point-of-care testing remain the standard testing modality in operating rooms in patients with CGM devices. Continued investigation is underway examining the accuracy and safety of CGM in the operating room.
Enhanced Recovery After Surgery Carbohydrate Loading
Enhanced recovery after surgery (ERAS) programs are multimodal perioperative care pathways designed to achieve early recovery following a surgical procedure. These often include preoperative carbohydrate (CHO) loading, frequently maltodextran solutions, given the evening before surgery (800 mL with 100 grams (g) CHO) and again in the morning 2–3 h prior to surgery (400 mL with 50 g CHO). CHO-rich fluids are advised in ERAS pathways to prevent dehydration, improve patients’ sense of well-being [75], and keep patients in a “fed” versus “starved” state. Widespread adoption of carbohydrate drinks prior to surgery has also been demonstrated to decrease insulin resistance, maintain glycogen reserve, and decrease protein breakdown following surgery [76]. Additionally, clear liquids administered up to 2 h prior to surgery are accepted safe care and supported by practice guidelines from the American Society of Anesthesiologists (ASA) and European Society of Anesthesiology [77, 78].
The ERAS society guidelines support carbohydrate loading up until 2–4 h prior to surgery in multiple procedure types including gynecologic, colorectal, and thoracic [79,80,81]. The recommendations are less clear for patients with known diabetes, and at this time, CHO loading has only a class IIb recommendation in cardiac surgery patients [82]. A 2014 Cochrane analysis of 1976 patients in 27 trials using complex carbohydrates concluded that compared to placebo or fasting, there was a small reduction in length of stay (LOS). However, it did not decrease perioperative complications, impact patient reports of well-being, or decrease the incidence of PONV [83]. Of note, 26 of the included studies did not represent patients with impaired glucose tolerance: 1 excluded DM1, 20 excluded all types of diabetes, and 4 excluded patients with abnormal fasting glucose. A network meta-analysis of 43 trials additionally assessed varied doses of carbohydrate load to the fasting state and found a moderate reduction in LOS [84]. However, compared to water, CHO loading demonstrated no reduction in LOS and failed to influence the rate of complications. This questions if allowing water (safe in diabetic patients) may provide the same benefit as preoperative carbohydrate drinks.
A recent placebo-controlled RCT did demonstrate that oral CHO load was effective to avoid BG > 180 mg/dL in the perioperative period, but did not affect the risk of postoperative infection. Diabetic patients were excluded from the study [85]. Current ADA guidelines recommend against carbohydrate drinks (including sports drinks) in diabetic patients to limit the development of hyperglycemia. Currently, there is a paucity of evidence examining preoperative carbohydrates in diabetic patients to determine if the same hyperglycemic effect occurs on the day of surgery. More data is needed to determine if routine use of preoperative CHO loading is safe in patients with diabetes.
Conclusion
Hyperglycemia is a common occurrence in the perioperative period and is associated with increased risk of complications. Correcting hyperglycemia (BG > 180 mg/dL, 10 mmol/L) with insulin administration has been shown to improve outcomes. While current targets range from 110 to 180 mg/dL (6.1–10 mmol/L), this may change in varied populations as further data emerges. Managing perioperative hyperglycemia is a multi-disciplinary effort, and consequently, it is important for hospitals and medical providers to create appropriate institutional protocols for hyperglycemia screening, monitoring, and treatment to improve patient care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. 2010;33(8):1783–8.
Long CA, Fang ZB, Hu FY, Arya S, Brewster LP, Duggan E, et al. Poor glycemic control is a strong predictor of postoperative morbidity and mortality in patients undergoing vascular surgery. J Vasc Surg. 2019;69(4):1219–26.
Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–21.
• Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261(1):97–103 Surgical outcome database an evaluation of surgical demonstrating that hyperglycemia in non-diabetic patients is significantly associated with poor outcomes.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82.
•• van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782–8 Retrospective analysis of > 13,000 cardiac and non-cardiac surgical patients demonstrating that when blood glucose is controlled for, A1C may not be associated linearly with 30-day mortality.
Goodenough CJ, Liang MK, Nguyen MT, Nguyen DH, Holihan JL, Alawadi ZM, et al. Preoperative glycosylated hemoglobin and postoperative glucose together predict major complications after abdominal surgery. J Am Coll Surg. 2015;221(4):854–61 e1.
Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, et al. Glucose metabolism and catecholamines. Crit Care Med. 2007;35(9 Suppl):S508–18.
Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–807.
Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253–9.
Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91(11):4854–8.
Duggan EW, O'Reilly-Shah VN, Tsegka KG, Galindo RJ, Umpierrez GE. HbA1c screening characterizes undiagnosed dysglycemia in surgical patients. Diabetes. 2018;67(Supplement 1). https://doi.org/10.2337/db18-1305-P.
Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8–14.
Greci LS, Kailasam M, Malkani S, Katz DL, Hulinsky I, Ahmadi R, et al. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care. 2003;26(4):1064–8.
Diabetes 2017 Report Card: US Dept of Health and Human Services; 2018 [Available from: https://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf.
•• American Diabetes A. 15. Diabetes care in the hospital: standards of medical care in diabetes-2019. Diabetes care. 2019;42(Suppl 1):S173-S81. Current recommendations from the ADA regarding testing and treatment of hospitalized diabetic patients.
•• American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes care. 2019;42(Suppl 1):S13-S28. Updated standards for screening/testing for abnormal glucose tolerance and diabetes.
Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006;141(4):375–80 discussion 80.
•• Yong PH, Weinberg L, Torkamani N, Churilov L, Robbins RJ, Ma R, et al. The presence of diabetes and higher HbA1c are independently associated with adverse outcomes after surgery. Diabetes care. 2018;41(6):1172–9 Prospective, observation study which screened 7,655 inpatients with A1C, demonstrating diabetes was associated with 6-month mortality and increaseing A1C associated with major complications, ICU admission and hospital LOS.
Joshi GP, Chung F, Vann MA, Ahmad S, Gan TJ, Goulson DT, et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378–87.
Gottschalk A, Rink B, Smektala R, Piontek A, Ellger B, Gottschalk A. Spinal anesthesia protects against perioperative hyperglycemia in patients undergoing hip arthroplasty. J Clin Anesth. 2014;26(6):455–60.
Li X, Wang J, Chen K, Li Y, Wang H, Mu Y, et al. Effect of different types of anesthesia on intraoperative blood glucose of diabetic patients: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2017;96(13):e6451.
Acar D, Erkilic EK, Gumus T, Sahin D, Dincel AS, Kanbak O. The effects of different anaesthetic techniques on surgical stress response during inguinal hernia operations. Turk J Anaesthesiol Reanim. 2015;43(2):91–9.
Kitamura T, Ogawa M, Kawamura G, Sato K, Yamada Y. The effects of sevoflurane and propofol on glucose metabolism under aerobic conditions in fed rats. Anesth Analg. 2009;109(5):1479–85.
Tanaka T, Nabatame H, Tanifuji Y. Insulin secretion and glucose utilization are impaired under general anesthesia with sevoflurane as well as isoflurane in a concentration-independent manner. J Anesth. 2005;19(4):277–81.
Gasanova I, Meng J, Minhajuddin A, Melikman E, Alexander JC, Joshi GP. Preoperative continuation versus interruption of oral hypoglycemics in type 2 diabetic patients undergoing ambulatory surgery: a randomized controlled trial. Anesth Analg. 2018;127(4):e54–e6.
Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology. 2017;126(3):547–60.
American Diabetes A. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care 2019;42(Suppl 1):S90-S102.
Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, et al. NHS diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med. 2012;29(4):420–33.
Salpeter SR, Greyber E, Pasternak GA, Salpeter Posthumous EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;1:CD002967.
Duncan AI, Koch CG, Xu M, Manlapaz M, Batdorf B, Pitas G, et al. Recent metformin ingestion does not increase in-hospital morbidity or mortality after cardiac surgery. Anesth Analg. 2007;104(1):42–50.
Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38(9):1680–6.
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–93.
Hine J, Paterson H, Abrol E, Russell-Jones D, Herring R. SGLT inhibition and euglycaemic diabetic ketoacidosis. Lancet Diabetes Endocrinol. 2015;3(7):503–4.
Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American Association of Clinical Endocrinologists and American College of endocrinology position statement on the association of Sglt-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22(6):753–62.
Pace DJ, Dukleska K, Phillips S, Gleason V, Yeo CJ. Euglycemic diabetic ketoacidosis due to sodium-glucose cotransporter 2 inhibitor use in two patients undergoing pancreatectomy. J Pancreat Cancer. 2018;4(1):95–9.
Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3(2):195–201.
Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48(5):592–8.
Matikainen N, Manttari S, Schweizer A, Ulvestad A, Mills D, Dunning BE, et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49(9):2049–57.
Umpierrez GE, Gianchandani R, Smiley D, Jacobs S, Wesorick DH, Newton C, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care. 2013;36(11):3430–5.
Fayfman M, Galindo RJ, Rubin DJ, Mize DL, Anzola I, Urrutia MA, et al. A randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. Diabetes Care. 2019;42(3):450–6.
Pasquel FJ, Gianchandani R, Rubin DJ, Dungan KM, Anzola I, Gomez PC, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5(2):125–33.
Perez-Belmonte LM, Gomez-Doblas JJ, Millan-Gomez M, Lopez-Carmona MD, Guijarro-Merino R, Carrasco-Chinchilla F, et al. Use of linagliptin for the management of medicine department inpatients with type 2 diabetes in real-world clinical practice (Lina-Real-World Study). J Clin Med. 2018;7(9).
Besch G, Perrotti A, Mauny F, Puyraveau M, Baltres M, Flicoteaux G, et al. Clinical effectiveness of intravenous exenatide infusion in perioperative glycemic control after coronary artery bypass graft surgery: a phase II/III randomized trial. Anesthesiology. 2017;127(5):775–87.
Polderman JAW, van Steen SCJ, Thiel B, Godfried MB, Houweling PL, Hollmann MW, et al. Peri-operative management of patients with type-2 diabetes mellitus undergoing non-cardiac surgery using liraglutide, glucose-insulin-potassium infusion or intravenous insulin bolus regimens: a randomised controlled trial. Anaesthesia. 2018;73(3):332–9.
Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184–8.
Rosenblatt SI, Dukatz T, Jahn R, Ramsdell C, Sakharova A, Henry M, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610–7.
Likavec A MV, Greenberg J, Drum M, Sweitzer B. Comparison of preoperative blood glucose levels in patients receiving different insulin regimens. Anesthesiology. 2006;105(567).
Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 units · mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units · mL-1. Diabetes Care. 2015;38(4):637–43.
Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Munoz-Torres M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498–507.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–69.
Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, et al. The Society of Thoracic Surgeons practice guideline series: blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87(2):663–9.
Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251–76.
Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care. 2015;38(9):1665–72.
Cook CB, Boyle ME, Cisar NS, Miller-Cage V, Bourgeois P, Roust LR, et al. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital setting: proposed guidelines and outcome measures. Diabetes Educ. 2005;31(6):849–57.
Mendez CE, Umpierrez GE. Management of type 1 diabetes in the hospital setting. Curr Diab Rep. 2017;17(10):98.
Schwenk ES, Mraovic B, Maxwell RP, Kim GS, Ehrenfeld JM, Epstein RH. Root causes of intraoperative hypoglycemia: a case series. J Clin Anesth. 2012;24(8):625–30.
Finfer S, Heritier S, Committee NSM, Committee SSE. The NICE-SUGAR (normoglycaemia in intensive care evaluation and survival using glucose algorithm regulation) study: statistical analysis plan. Crit Care Resusc. 2009;11(1):46–57.
Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2017;126(2):234–48.
Tien M, Gan TJ, Dhakal I, White WD, Olufolabi AJ, Fink R, et al. The effect of anti-emetic doses of dexamethasone on postoperative blood glucose levels in non-diabetic and diabetic patients: a prospective randomised controlled study. Anaesthesia. 2016;71(9):1037–43.
Low Y, White WD, Habib AS. Postoperative hyperglycemia after 4- vs 8-10-mg dexamethasone for postoperative nausea and vomiting prophylaxis in patients with type II diabetes mellitus: a retrospective database analysis. J Clin Anesth. 2015;27(7):589–94.
Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–65.
Corcoran T, Kasza J, Short TG, O'Loughlin E, Chan MT, Leslie K, et al. Intraoperative dexamethasone does not increase the risk of postoperative wound infection: a propensity score-matched post hoc analysis of the ENIGMA-II trial (EnDEX). Br J Anaesth. 2017;118(2):190–9.
Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11:CD011770.
Desmet M, Braems H, Reynvoet M, Plasschaert S, Van Cauwelaert J, Pottel H, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111(3):445–52.
Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore). 2016;95(23):e3828.
Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care. 2018;41(8):1579–89.
Bailon RM, Partlow BJ, Miller-Cage V, Boyle ME, Castro JC, Bourgeois PB, et al. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract. 2009;15(1):24–9.
Nassar AA, Boyle ME, Seifert KM, Beer KA, Apsey HA, Schlinkert RT, et al. Insulin pump therapy in patients with diabetes undergoing surgery. Endocr Pract. 2012;18(1):49–55.
Boyle ME, Seifert KM, Beer KA, Apsey HA, Nassar AA, Littman SD, et al. Guidelines for application of continuous subcutaneous insulin infusion (insulin pump) therapy in the perioperative period. J Diabetes Sci Technol. 2012;6(1):184–90.
Corney SM, Dukatz T, Rosenblatt S, Harrison B, Murray R, Sakharova A, et al. Comparison of insulin pump therapy (continuous subcutaneous insulin infusion) to alternative methods for perioperative glycemic management in patients with planned postoperative admissions. J Diabetes Sci Technol. 2012;6(5):1003–15.
Sobel SI, Augustine M, Donihi AC, Reider J, Forte P, Korytkowski M. Safety and efficacy of a peri-operative protocol for patients with diabetes treated with continuous subcutaneous insulin infusion who are admitted for same-day surgery. Endocr Pract. 2015;21(11):1269–76.
Wallia A, Umpierrez GE, Rushakoff RJ, Klonoff DC, Rubin DJ, Hill Golden S, et al. Consensus statement on inpatient use of continuous glucose monitoring. J Diabetes Sci Technol. 2017;11(5):1036–44.
Hausel J, Nygren J, Lagerkranser M, Hellstrom PM, Hammarqvist F, Almstrom C, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344–50.
Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–8.
Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. 2017;126(3):376–93.
Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Soreide E, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28(8):556–69.
Nelson G, Bakkum-Gamez J, Kalogera E, Glaser G, Altman A, Meyer LA, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations-2019 update. Int J Gynecol Cancer. 2019.
Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations: 2018. World J Surg. 2019;43(3):659–95.
Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115.
Engelman DT, Ben Ali W, Williams JB, Perrault LP, Reddy VS, Arora RC, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019.
Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev. 2014;8:CD009161.
Amer MA, Smith MD, Herbison GP, Plank LD, McCall JL. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg. 2017;104(3):187–97.
Gianotti L, Biffi R, Sandini M, Marrelli D, Vignali A, Caccialanza R, et al. Preoperative oral carbohydrate load versus placebo in major elective abdominal surgery (PROCY): a randomized, placebo-controlled, multicenter. Phase III Trial Ann Surg. 2018;267(4):623–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elizabeth Duggan and York Chen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hospital Management of Diabetes
Rights and permissions
About this article
Cite this article
Duggan, E., Chen, Y. Glycemic Management in the Operating Room: Screening, Monitoring, Oral Hypoglycemics, and Insulin Therapy. Curr Diab Rep 19, 134 (2019). https://doi.org/10.1007/s11892-019-1277-4
Published:
DOI: https://doi.org/10.1007/s11892-019-1277-4