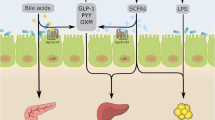
Abstract
Purpose of Review
Obesity and diabetes are worldwide epidemics. There is also a growing body of evidence relating the gut microbiome composition to insulin resistance. The purpose of this review is to delineate the studies linking gut microbiota to obesity, metabolic syndrome, and diabetes.
Recent findings
Animal studies as well as proof of concept studies using fecal transplantation demonstrate the pivotal role of the gut microbiota in regulating insulin resistance states and inflammation.
Summary
While we still need to standardize methodologies to study the microbiome, there is an abundance of evidence pointing to the link between gut microbiome, inflammation, and insulin resistance, and future studies should be aimed at identifying unifying mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and type 2 diabetes (T2DM) have become worldwide epidemics. In 2015, 30.3 million, representing 9.4% of Americans, had diabetes [1, 2]. Every year, 1.5 million Americans are diagnosed with diabetes. As per the latest statistics, diabetes remains the seventh leading cause of death in the USA. Obesity is intertwined with the increasing incidence of metabolic syndrome and T2DM. T2DM is associated with increased incidence of micro- and macrovascular complications and thus places a huge burden on the health care system as a whole. According to the American Diabetes Association, the total cost of diagnosed diabetes in the USA in 2017 is $327 billion.
Several studies have shown that the common underpinning of obesity, metabolic syndrome, and T2DM is dysglycemia, insulin resistance, and inflammation. In recent studies, much attention has also been focused on the role of the gut microbiota in obesity, metabolic syndrome, and T2DM, and this hypothesis forms the basis of this review [3,4,5].
The gut microbiota refers collectively to the microbial composition in the gut and contains several diverse sets of microorganisms such as bacteria, viruses, Archaea, fungi as well as phages [6,7,8,9]. While it was thought that microbiota are 10-fold more abundant in the human body, recent data point out that there is at least equal abundance of microbiota as the total number of somatic and germ cells in a human [10]. Among all of the different bacterial species, the five most abundant phyla include Firmicutes, Bacteriodetes, Verrucomicrobia, Actinobacteria, and Proteobacteria [8]. Based on the pH gradient, different microbial communities inhabit different parts of the GI tract; the proximal part has an abundance of Firmicutes (Lactobacilli) as well as Proteobacteria, while the distal part is concentrated with anaerobes such as Bacteriodetes, Verrucomicrobia, and Akkermansia [11,12,13,14].
Gut Microbiome, Obesity, and Insulin Resistance
In the last decade, several experimental studies especially in obese mouse models have demonstrated the role of the gut microbiome in obesity [6, 7, 15••, 16]. One of the first studies conducted in animal models with controlled diet, environment, and genotype showed that in C57BL/6 mice, which are genetically protected from developing obesity even after consuming a high-fat/high-carbohydrate diet, colonizing them with microbiota from obese mice resulted in a profound increase (60%) in body fat, and subsequently these mice became insulin resistant despite reduced food intake [3,4,5]. In addition, when compared to lean mice, 16S rRNA profiling demonstrated that ob/ob mice had only half the abundance of Bacteroidetes and a proportional increase in Firmicutes [3,4,5]. The hypothesis was that microbiota from obese mice were more efficient at extracting energy from diet than lean counterparts. Using shotgun sequencing, the authors showed that the genome of ob/ob mice predominantly had environmental gene tags encoding glycoside hydrolase (which degrades dietary polysaccharides and starch), as well as ATP-binding cassette transporters. Furthermore, the end products, acetate and butyrate, were significantly enriched in ob/ob mice compared to lean mice, demonstrating that the gut microbiome of these mice had an increased potential to harvest energy.
Proof of concept came from studies of fecal transplantation into germ-free mice. Ridaura et al. [17••] showed that fecal microbiota transplantation from female adult twin pairs that were discordant for obesity into germ-free mice that were fed low-fat mouse feed resulted in the set of mice that received a transplant from the obese donor having increased total body mass and fat mass, in addition to metabolic phenotypes that were associated with obesity, while the other set that received the lean twin’s microbiome prevented the development of increased body mass and obesity-associated metabolic phenotypes. They then fed them a saturated fat diet that also included increased consumption of fruits and vegetables. Significant differences in body composition were documented between ob/ob and ln/ln mice consuming this diet. Thus, while the donor phenotype can influence the microbiome of a recipient, they demonstrated that diet continues to regulate whether these mice developed a lean or obese phenotype.
In the above study, the authors also measured increased levels of butyrate and propionate in mice that were colonized with lean human gut microbiota when compared to the gut microbiota from the obese [17••]. Additionally, they were able to demonstrate that when they performed fecal microbiota transplantation from lean human donors to obese human recipients with metabolic syndrome, this resulted in significant improvement in insulin sensitivity [18]. Furthermore, in a double-blind, randomized trial of controlled intervention, they showed that treatment with probiotics such as Lactobacillus gasseri resulted in significant reduction in body weight in both overweight and obese subjects [19]. These studies point to the role of the gut microbiome in regulating body weight.
Gut Microbiome, Insulin Resistance, and Type 2 Diabetes
With regard to T2DM, one of the first studies came from the group of Larsen et al. [20] who studied 18 lean and 18 overweight males with T2DM. While the bacterial abundance was similar in both groups, the abundance of Firmicutes bacteria was significantly increased in controls compared to participants with T2DM. There was a corresponding upregulation in Bacteriodetes, but this was not statistically significant. Also, the authors did not report on the confounding effect of antidiabetic treatment on gut microbiome composition in this study.
Qin et al. [21] performed an association case-control study of metagenomes in a population of subjects with T2DM in China. Among the intestinal bacteria, compared to the controls, subjects with T2DM showed a decrease in Clostridum species, Fecalibacteria and Roseburia, all butyrate-producing bacteria of the Firmicutes phylum. There was a concomitant increase in Bacteriodetes species and Escherichia coli. Pathway analysis showed that in T2DM, there is increased membrane sugar as well as branched-chain amino acid transport and sulfate reduction. These results appear to suggest that butyrate-producing pathogens afford protection against T2DM [22, 23].
Similar to the study by Larsen et al. in males, Karlsson et al. [24] studied the fecal microbiome of 145 older women, of whom 53 had T2DM, 49 had impaired glucose tolerance, and 43 were normal. They reported that in women with T2DM, there was enrichment of four Lactobacillus species and decreases in the abundance of five Clostridium species. Furthermore, the abundance in Lactobacillus species correlated positively with glycated hemoglobin and glucose levels while Clostridium species correlated negatively. They also showed that Roseburia and Fecalibacterium prausnitzii, which are known butyrate producers, were associated with T2DM.
Factors that Contribute to Altered Microbial Composition in Obesity and Diabetes
Insulin resistance states such as metabolic syndrome and T2DM are associated with low-grade subclinical inflammation [25, 26]. While alterations in gut microbiome in the metabolic syndrome are reported, the relationship between inflammation, gut microbiome, and metabolic derangements is not well studied. Mice that were fed a diet rich in fiber have been shown to have increased levels of short-chain fatty acids and developed less allergic lung inflammation than mice fed a low fiber diet. Also, treatment with one of the short-chain fatty acids, propionate, resulted in an abundance of macrophage and dendritic cells.
Inflammation plays a key role in metabolic disease involving insulin resistance such as obesity, metabolic syndrome and T2DM [27,28,29]. In one of the pioneering studies, Cani et al. [30••, 31, 32] demonstrated a link between the gut microbiome and the pro-inflammatory state of the metabolic syndrome. In mice fed high fat diet, there was increased endotoxinemia, and this was associated with decreased abundance of gram-negative Bacteriodes and gram-positive Clostridia and bifidobacteria. In support of these preclinical findings, the DESIR study examined the development and pattern of metabolic syndrome and associated complications and also reported dysbiosis of the gut microbiome, evidenced by decreased bacterial DNA content and increased abundance of Proteobacteria in those patients that progressed to have cardiovascular events [33].
Two studies in humans provided further proof of concept. In both of these studies [34, 35], the authors assessed the fecal gut microbiome of 12 obese participants that enrolled in a weight loss program for 1 year, and followed a low-calorie diet that was either fat restricted or carbohydrate restricted. They reported increased abundance of Firmicutes and decreased Bacteriodetes in obese compared to lean individuals whose microbiome signature showed remarkable stability over the year. Both diets caused weight loss and this correlated to decreased content of Firmicutes as well as to increased amounts of Bacteroidetes (3–15%). Thus, all of the studies point to the gut microbiome as a contributing factor to obesity. Kalliomäki et al., in a prospective study of children that were followed from birth up to the age of 7 years [36], collected fecal specimens at 6 and 12 months of age. They showed increased Bifidobacterium taxa and decreased Staphylococcus aureus in normal weight compared to overweight or obese children.
The administration of antibiotics has an opposite effect on gut microbiome [37]. Toll-like receptors (TLRs) are a family of key pattern recognition receptors that aid cells in recognizing ligands such as endotoxin and mediating inflammation and immunity. We and others have shown increased expression and activity of TLRs that are present on cell surfaces in patients with obesity, diabetes, and metabolic syndrome [38,39,40]. Recently, studies have focused on the role of the gut microbiome in regulating TLR-mediated insulin resistance. TLR5-deficient mice are hyperphagic and develop obesity, insulin resistance, and features of the metabolic syndrome, a process that is associated with dysregulation of interleukin-1β signaling [41]. When gut microbiota of these mice are transplanted into wild-type TLR5 mice, the recipient mice also influence the gut microbiome in a way that predisposes to the metabolic syndrome. Similarly, TLR2-deficient mice are reported to have increased Firmicutes and decreased Actinobacteriai, and they subsequently develop insulin resistance, obesity, and metabolic syndrome. Treatment with antibiotics decreased the abundance of Firmicutes and eventually improved insulin action and sensitivity. Furthermore, since Bifidobacterium can result in increased gut permeability, it is possible that a dysregulated microbiome could lead to a leaky gut, thereby yielding increased metabolic endotoxemia and increased TLR activity, begetting more inflammation. We have previously shown that both TLR2 and TLR levels and activity are increased in monocytes of patients with metabolic syndrome and diabetes. Furthermore, when either TLR2 or TLR is deficient, there is decreased preponderance of diabetic complications such as diabetic nephropathy [42,43,44,45,46,47,48]. Thus, the gut microbiome may also contribute to insulin resistance and associated diabetic vasculopathies, and this area will be a key area of future investigation.
Inflammasomes also regulate inflammation by sensing endogenous or exogenous damage-associated molecular patterns referred to as DAMPs [49, 50]. These proteins exist as multiprotein complexes and convert pro-inflammatory cytokines such as interleukin (IL)-1β and IL-18 to their active forms in response to “alarm” signals. In NLRP6 deficiency [49, 51], there are decreased IL-18 levels and altered fecal microbiota, characterized by increased abundance of Bacteroidetes (Prevotellaceae). We have recently shown that NLRP3 inflammasome activation in the diabetic milieu increases monocyte activation and alters gut microbiota resulting in gut dysbiosis, all of which are eliminated via knockout of the inflammasome pathway [51].
Anti-Diabetic Therapy and Gut Microbiome
There are several therapies that are used to treat insulin resistance and diabetes, one of the most popular of these and used as first-line therapy is metformin. Some of the beneficial effects of metformin could be attributed to alteration in gut microbiota [52, 53, 54•, 55]. Metformin therapy, in addition to improving the glycemic profile of mice fed a high-fat diet, also increases abundance of Akkermansia, a mucin-degrading bacterium, when compared to controls fed a high-fat diet without metformin. Human studies from Danish, Swedish, and Chinese participants with T2DM on metformin therapy have corroborated these findings [52, 53, 54•, 55]. Multivariate analysis has shown that there are significant differences in gut composition between metformin-untreated participants with T2DM vs controls and significant increases in Escherichia species and decrease in Intestinibacter after metformin therapy.
Morbid obesity can be improved by gastric bypass or bariatric surgery. Diet-induced obese (DIO) C57BL/6 J mice that were fed a high-fat diet underwent Roux-en-Y gastric bypass (RYGB) surgery, sham surgery, or sham surgery along with caloric restriction [56,57,58]. RYGB altered gut microbial composition as early as 1 week post-surgery and stabilized after 5 weeks. Mainly, RYGB produced enrichment of Bacteroidetes, Verrucomicrobia, and Proteobacteria. In a set of provocative experiments, when the authors inoculated lean, germ-free mice from RYGB donors, there was a significant reduction in body weight, improved insulin sensitivity, and decreased triglycerides [56,57,58]. Whether other widely used antidiabetic drugs such as glucagon-like peptide 1 (GLP-1) receptor agonists and GLP-1 degradation inhibitors [59,60,61,62] act via altering microbiota awaits results of large trials.
Conclusions
The last few decades have shed light on the role of the gut microbiome in linking inflammation and insulin resistance. We are just at the tip of the iceberg of understanding host-microbiome interactions and specific mechanisms of modulation. Methodologies to identify gut microbial composition and function need to be standardized to allow the performance of meta-analyses, and facilitate the understanding of the role of mechanistic pathways involving short-chain fatty acids, propionate, butyrate, bile acids, lipopolysaccharide, TLRs, and NLRP inflammasomes in the pathogenesis of complications of obesity, metabolic syndrome, and diabetes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel. Executive summary: guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association task force on practice guidelines. Based on a systematic review from the obesity expert panel, 2013. Obesity (Silver Spring). 2013;2014(Suppl 2):S5–39.
Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;2:93–6.
Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–23.
Bäckhed F, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20.
Dethlefsen L, Eckburg PB, Bik EM, Relman DA. Assembly of the human intestinal microbiota. Trends Ecol Evol. 2006;21(9):517–23.
Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev. 2000;6:776–88.
Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A. 1998;95:6578–83.
Rajilic-Stojanovic M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 2014;38(5):996–1047.
Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–73.
Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS One. 2001;8(10):e74957.
Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32.
Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol. 2005;11(8):1131–40.
Scheithauer TP, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5(9):759–70.
•• Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31 This was one of the pioneering studies to demonstrate that the gut microbiota from obese can harvest increased energy from diet and thus contribute to the pathophysiology of obesity.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4.
•• Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214 Studies in this report emphasize the strong microbiota-by-diet interactions and illustrate how a poor diet (high saturated fat and low in fruits and vegetables) can select against human gut bacterial taxa associated with lean body mass.
Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6.
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64(6):636–43.
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667.
Hur KY, Lee M-S. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198–203.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103.
Zand H, Morshedzadeh N, Naghashian F. Signaling pathways linking inflammation to insulin resistance. Diabetes Metab Syndr. 2017;Suppl 1:S307–9.
Verma S, Hussain ME. Obesity and diabetes: an update. Diabetes Metab Syndr. 11(1):73–79.
Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13(3):435–4.
Pirola L, Ferraz JC. Role of pro- and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J Biol Chem. 2017;8(2):120–8.
Keane KN, Calton EK, Carlessi R, Hart PH, Newsholme P. The bioenergetics of inflammation: insights into obesity and type 2 diabetes. Eur J Clin Nutr. 2017;71(7):904–12.
•• Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72 One of the first studies that demonstrates that increased endotoxin results in increased inflammation, weight gain and diabetes.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81.
Pedersen C, Gallagher E, Horton F, Ellis RJ, Ijaz UZ, Wu H, et al. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br J Nutr. 2016;116:1869–77.
Amar J, Lange C, Payros G, Garret C, Chabo C, Lantieri O, et al. Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: the DESIR study. PLoS One. 2013;8:e54461.
Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2009;26:5–11.
Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity. 2001;20:738–47.
Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8.
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2010;108(Suppl 1):4554–61.
Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99(1):39–48.
Gupta S, Maratha A, Siednienko J, Natarajan A, Gajanayake T, Hoashi S, et al. Analysis of inflammatory cytokine and TLR expression levels in type 2 diabetes with complications. Sci Rep. 2017;7(1):7633.
Rempel JD, Packiasamy J, Dean HJ, McGavock J, Janke A, Collister M, et al. Preliminary analysis of immune activation in early onset type 2 diabetes. Int J Circumpolar Health. 2013;5:72.
Carvalho BM, Guadagnini D, Tsukumo DM, Schenka AA, Latuf-Filho P, Vassallo J, et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55:2823–34.
Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, Ropelle ER, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212.
Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–8.
Jialal I, Huet BA, Kaur H, Chien A, Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care. 2012;35:900–4.
Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–7.
Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol. 2011;31:1796–804.
Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35:14S20S.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
Zupancic ML, Cantarel BL, Liu Z, Drabek EF, Ryan KA, Cirimotich S, et al. Analysis of the gut microbiota in the old order Amish and its relation to the metabolic syndrome. PLoS One. 2012;7:e43052.
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85.
Pahwa R, Balderas M, Jialal I, Chen X, Luna RA, Devaraj S. Gut microbiome and inflammation: a study of diabetic Inflammasome-knockout mice. J Diabetes Res. 2017;2017:6519785.
Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–35.
De la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40:54–62.
• Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–6 Provides evidence of microbial mediation of the therapeutic effects of metformin through short-chain fatty acid production. Overall, the study emphasizes the need to disentangle gut microbiota signatures of T2DM from those that receive metformin and other antidiabetic medication.
Devaraj S, Venkatachalam A, Chen X. Metformin and the gut microbiome in diabetes. Clin Chem. 2016;62(12):1554–5.
Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, Verger EO, Hedjazi L, Bouillot JL, Chevallier JM, Pons N, Le Chatelier E, Levenez F, Ehrlich, SD, Dore J, Zucker JD, Clément K. major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2018.
Duboc H, Nguyen CC, Cavin JB, Ribeiro-Parenti L, Jarry AC, Rainteau D, et al. Roux-en-Y gastric-bypass and sleeve, gastrectomy induces specific shifts of the gut microbiota without altering the metabolism of bile acids in the intestinal lumen. Int J Obes (Lond). 2018. https://doi.org/10.1038/s41366-018-0015-3.
Ejtahed HS, Angoorani P, Hasani-Ranjbar S, Siadat SD, Ghasemi N, Larijani B, et al. Adaptation of human gut microbiota to bariatric surgeries in morbidly obese patients: a systematic review. Microb Pathog. 2018;116:13–21.
Zhao L, Chen Y, Xia F, Abudukerimu B, Zhang W, Guo Y, et al. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front Endocrinol (Lausanne). 2018;9:233.
Claus SP. Will gut microbiota help design the next generation of GLP-1-based therapies for type 2 diabetes? Cell Metab. 2017;26(1):6–7.
Zietek T, Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Front Immunol. 2016;7:154.
Greenhill C. Gut microbiota: Firmicutes and Bacteroidetes involved in insulin resistance by mediating levels of glucagon-like peptide 1. Nat Rev Endocrinol. 2015;11(5):254.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Xinpu Chen and Sridevi Devaraj declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Therapies and New Technologies in the Treatment of Diabetes
Rights and permissions
About this article
Cite this article
Chen, X., Devaraj, S. Gut Microbiome in Obesity, Metabolic Syndrome, and Diabetes. Curr Diab Rep 18, 129 (2018). https://doi.org/10.1007/s11892-018-1104-3
Published:
DOI: https://doi.org/10.1007/s11892-018-1104-3