Abstract
Purpose of review
Insulin resistance (IR) is recognized to play an important role in the pathogenesis of dyslipidemia. This review summarizes the complex interplay between IR and dyslipidemia in people with and without diabetes.
Recent findings
IR impacts the metabolism of triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and very low-density lipoprotein cholesterol (VLDL-C) by several mechanisms. Trials with insulin sensitizing therapies, including biguanides and thiazolidinediones, have provided inconsistent results on lipid lowering in people with and without diabetes. In this review, we focus on the pathophysiological interplay between IR and dyslipidemia and recapitulate lipid and lipoprotein data from insulin-sensitizing trials.
Summary
Further research elucidating the reciprocal relationship between IR and dyslipidemia is needed to better target these important risk factors for cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance (IR) is an important metabolic component of obesity, metabolic syndrome, type 2 diabetes (T2DM), and even type 1 (T1DM) and is associated with elevated risk for micro- and macrovascular complications. Whereas hyperglycemia, hypertension, kidney disease, and dyslipidemia are considered the traditional risk factors of cardiovascular disease (CVD) in diabetes, there is an increasingly recognized relationship between IR and CVD even in the absence of diabetes [1,2,3].
Despite the substantial link between IR and CVD, the mechanism underlying this relationship remains insufficiently understood. IR is associated with changes in lipid and lipoprotein metabolism which result in atherogenic dyslipidemia and has been proposed to contribute to an increased risk of CVD [4]. Beyond changes in lipid and lipoprotein metabolism, IR is also associated with changes in mean particle size for lipoproteins. For example, nuclear magnetic resonance analysis has demonstrated larger mean particle size for VLDL and smaller size for LDL and HDL in IR individuals as compared to their insulin sensitive counterparts [5]. Despite abundant data supporting strong relationships between IR and dyslipidemia, it remains unclear whether IR leads to dyslipidemia or vice versa. The sequence of this relationship is further complicated by the notion that clinical and metabolic phenotypes of IR may differ by diabetes status. For example, the clinical and metabolic features of IR in people with T1DM are quite different from those characteristics in obese people with T2DM and/or metabolic syndrome.
To better understand the relationship between IR and dyslipidemia, we must define the effects of IR on lipids, lipoproteins, and related enzymes, and vice versa, i.e., whether dyslipidemia impacts insulin sensitivity or vice versa.
Pathogenesis of IR and Lipid Metabolism
VLDL and TG Metabolism
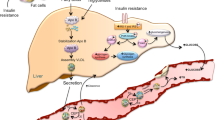
IR plays an important role in VLDL metabolism, including effects to increase hepatic VLDL triglyceride (TG) synthesis [6, 7] (Table 1). The increased VLDL TG synthesis is then variably linked to increased hepatic apo B-100 production [6, 7, 26]. Collectively, this results in hypertriglyceridemia, variable increases in particle number reflected by VLDL apo B-100, and lower HDL-C concentrations [26]. IR is also associated with increases in hepatic triglyceride lipase (HTGL) which may result in accelerated clearance of HDL and reductions in HDL-C [27]. Furthermore, HTGL activity has recently been proposed to be an important regulator of insulin clearance [8]. A major factor in the mechanism of both IR and increased VLDL-TG production is an accelerated rate of lipolysis of stored TG-derived free fatty acids (FFA) from adipose tissue with resultant increases in FFA flux to the liver [9]. Moreover, although insulin is an important stimulator of adipose tissue lipoprotein lipase (ATLPL) [10, 11••], a pathway that reflects the provision of TG-rich lipoprotein (VLDL, chylomicron)-derived FFA for adipose tissue uptake and storage, there is a shift to the right in the insulin (ATLPL) dose response curves in IR states [28]. Accordingly, IR may reduce VLDL breakdown and consequently increase hypertriglyceridemia. Moreover, decreases in skeletal muscle LPL in T2DM may also contribute to reductions in TG-rich lipoprotein TG clearance [29]. In fact, the LPL gene has been proposed to be a candidate gene for IR [30], and overexpression of LPL has been shown to increase whole-body insulin sensitivity in animal models [31]. Low circulating adiponectin concentrations, which may also contribute to IR, are also associated with increased VLDL production and HDL catabolism [32, 33]; however, these effects may occur independent of IR [32,33,34].
The data reviewed in the preceding paragraph suggest that IR impacts TG metabolism in a major way, but there are also studies supporting the reciprocal, i.e., that lipid accumulation results in IR. For example, there is evidence linking hepatic TG accumulation with hepatic IR [35]. Moreover, increased plasma FFA are associated with IR [36, 37] through intramyocellular and intrahepatic accumulation of TG and other metabolites [38•]. TG is not considered a signaling lipid, and thus, it is thought to be more likely that diacylglycerol, the synthetic precursor of TG, ceramide, and other lipids are implicated in the pathogenesis of hepatic IR through several mechanisms including reduced insulin receptor tyrosine kinase activity, insulin receptor destabilization, and reduced insulin-stimulated glycogen synthase activity [39,40,41,42]. FFA may also mediate IR through pro-inflammatory effects [12, 43].
Chylomicron Metabolism
Chylomicrons synthesized and released by the intestine allow transport of diet-derived TG to other tissues in the postprandial state. Whereas VLDL contains apo B-100, apo B-48, a truncated form of the holoprotein, is the exclusive apo B in chylomicrons [13, 14]. In the vasculature, chylomicrons are hydrolyzed by LPL releasing their fatty acids to peripheral cells. IR-related reduction in LPL activity also influences hydrolysis of chylomicron TGs [15]. This is particularly evident if excessive hepatic VLDL saturates all available LPL binding sites in the endothelium [16, 17]. Adults with T2DM who demonstrate a reduced level of ATLPL activity have an exaggerated postprandial chylomicron response [18]. An additional consideration is the physiological role of glucagon-like peptides in chylomicron processing and postprandial chylomicron excursion [19].
HDL Metabolism
Insulin has important effects on HDL metabolism, and low concentrations of HDL-C are commonly observed in IR states [17, 20]. IR is thought to contribute to low HDL-C concentrations by several mechanisms. First, IR is associated with increased exchange of TG from chylomicrons and VLDL for cholesterol esters from HDL particles, thus reducing HDL-C, a process regulated by cholesteryl ester transfer protein (CETP) [21]. Second, decreased LPL activity results in reduced hydrolysis of TG from chylomicrons and VLDL which may further limit the contribution of TG-rich lipoprotein-derived HDL particles [17, 20]. Third, increased HTGL activity in IR states is associated with enhanced HDL clearance and therefore lower concentrations of HDL-C [17, 20]. Fourth, low concentrations of HDL-C may also be due to reduced synthesis and secretion of apo A-I from liver and intestine [22].
LDL Metabolism
Compared to VLDL metabolism, IR appears to have a more modest effect on LDL metabolism. Insulin is known to upregulate LDL receptor activity [23], and administration of insulin may increase the catabolism of LDL-C with a small reduction in LDL-C, as observed in people with T1DM [24]. IR may also play an important role in the metabolism of the atherogenic small dense LDL particles [25], considered but unproven to be independent biomarkers for atherosclerosis [25, 44].
IR and Dyslipidemia in Obesity, T2DM, and Metabolic Syndrome
Dyslipidemia in obese individuals with T2DM and/or metabolic syndrome is characterized by elevation of TG, reduction in HDL-C, increases in apo B-100, non-HDL-C, and small dense LDL and HDL. In the European Group for the Study of Insulin Resistance (EGIR), insulin sensitivity quantified by hyperinsulinemic-euglycemic clamp technique strongly correlated with TG concentrations [45]. In particular, hypertriglyceridemia and low HDL-C are characteristic of metabolic syndrome, and in fact, the ratio of TG/HDL-C has been used as a surrogate of IR [46,47,48]. Low HDL-C and hypertriglyceridemia are thought to occur in up to at least 1/3 of people with metabolic syndrome [49]. It is also important to appreciate that early dyslipidemia may not be evident in the fasting state. For example, people with T2DM who have a normal fasting TG and optimal glycemic control experience a greater postprandial rise in VLDL, apo B-48, apo B-100, cholesterol, and TG concentrations compared with their non-diabetic peers [50].
Youth-onset T2DM is increasing in prevalence and incidence worldwide and becoming a major public health burden [51•]. Further, youth-onset T2DM is considered a more aggressive disease than adult-onset T2DM with more rapid deterioration in β cell function and a greater lifetime risk for comorbidities and complications independent of diabetes duration [52, 53]. Furthermore, the recently completed Restoring Insulin Secretion (RISE) Study demonstrated that youth with impaired glucose tolerance or recently diagnosed T2DM have lower insulin sensitivity and reduced insulin clearance compared with adults [54]. Consistent with worse IR, dyslipidemia is also more prevalent in youth-onset T2DM. In fact, the prevalence of dyslipidemia was reported to be 82% in 1340 people with youth-onset T2DM, which included 41% with hypercholesterolemia, 53% hypertriglyceridemia, 59% low HDL-C, and 65% high LDL-C [55].
Combined Hyperlipidemia
Combined hyperlipidemia is a disorder related to increases in total cholesterol and TG, and generally associated with IR. In the MESA study, the adjusted odds of combined hyperlipidemia was greater than 2-fold higher in participants with overweight and obesity compared with normal weight individuals and greater than 4-fold higher in quartiles 2 through 4 of IR compared to quartile 1 [56]. Moreover, in 26 Japanese patients with apo E2/E2 and familial dysbetalipoproteinemia, mean total cholesterol was 256 mg/dl, TG 374 mg/dl, and remnant cholesterol 49 mg/dL, respectively. Because patients with apo E2/E2 who manifest the familial dysbetalipoproteinemia lipid phenotype also have other etiologies of overproduction of VLDL and TG, it is not surprising that 54% of this cohort had T2DM, 66% metabolic syndrome, and 42% coronary heart disease [57].
IR and Dyslipidemia in T1DM
Using gold standard techniques, we and others have clearly demonstrated that IR is a prominent feature of T1DM in adolescents [58••, 59] and adults [60••] with T1DM. This IR in T1DM occurs irrespective of obesity and metabolic syndrome features [58••, 59,60,61,62,63]. Moreover, IR confers higher risk for a more atherogenic lipoprotein profile [64, 65] and micro- and macrovascular complications in T1DM youth [66] and adults [67, 68].
The classic diabetic dyslipidemia characterized by elevated TG, small dense LDL, and low HDL-C [69] is seldom observed in modern cohorts of adults with T1DM. In fact, adults with T1DM have lipid values typically similar to or better than their non-diabetic peers, with lower total cholesterol, LDL-C, TG, and even higher levels of HDL-C [70]. However, data in youth with T1DM demonstrate higher prevalence of dyslipidemia [71]. For example, the Diabetes-Patienten-Verlaufsdokumentation (DPV) registry reported hypercholesterolemia in 29% of youth with T1DM [72, 73], and T1DM Exchange data demonstrated elevated LDL-C in 28% of youth with T1DM with suboptimal glycemic control [74]. The higher prevalence of dyslipidemia in youth compared to adults with T1DM is likely attributable to worse glycemic control, higher rates of obesity [75], and lower insulin sensitivity in adolescents [76, 77].
Despite having lipid concentrations comparable to adults without diabetes, people with T1DM are afflicted by increased risk of atherosclerotic CVD (ASCVD) [78,79,80,81,82,83], which is at least partially attributed to an increased atherogenic lipid profile, independent of LDL-C concentration [81, 84, 85]. Possible mechanisms for the increased atherogenic lipid profile of T1DM include differences in lipoprotein particle size, lipoprotein subfraction cholesterol distribution, LDL-C oxidation, COX2 expression, inflammatory response to lipids, and increased transvascular and macrophage lipid transport, in addition to variably greater concentrations of lipoprotein(a) (Lp(a)), apo B-100, and non-HDL-C in patients with T1DM [65, 86,87,88,89].
While people with T1DM and T2DM are at greater risk of ASCVD events and death from ASCVD compared to the general population, it is important to acknowledge that the pathophysiology underlying ASCVD may differ in T1DM vs T2DM. In fact, the atherosclerotic plaques in T1DM are thought to have different features than those found in T2DM, including softer, less lipid-laden, and more concentric plaques associated with greater calcification and inflammation [90,91,92,93,94,95].
Insulin Sensitizers and Dyslipidemia
Insulin sensitizers are proposed to improve lipid and lipoprotein metabolism by several potential mechanisms including inhibition of both intestinal and hepatic sterol regulatory element-binding protein-1c (SREBP-1c), thereby decreasing the synthesis of TG-rich lipoproteins [96,97,98,99,100]. While effects of metformin on lipid metabolism are generally modest, multiple mechanisms may contribute; these include metformin-mediated changes in the microbiome associated with reduced lipid absorption [101, 102], inhibition of bile acid absorption with resultant increases in LDL receptor-mediated LDL clearance [103, 104], and reduced phosphorylation of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR) via AMPK activation [105, 106].
Insulin Sensitizers and Dyslipidemia in T1DM
There have been a few trials of insulin sensitizers in youth and adults with T1DM with lipid lowering as secondary outcomes. The REducing with MetfOrmin Vascular Adverse Lesions in T1DM (REMOVAL) study of adults with T1DM did not show reduction in LDL-C following 3 years of metformin therapy [107]. A randomized controlled trial in youth with T1DM (8–18 years) by Anderson et al. found no significant effect of 12 months of metformin therapy on LDL-C, HDL-C, total cholesterol, TG, or adiponectin [108]. Another randomized control trial in overweight/obese youth with T1DM demonstrated no change in LDL-C, HDL-C VLDL-C, TG, and total cholesterol with 26 weeks of metformin therapy in youth with T1DM [109•, 110]. Consistently with these data, we recently demonstrated in the Effects of MEtformin on CardiovasculaR Function in AdoLescents with Type 1 Diabetes (EMERALD) Study that 3 months of metformin therapy did not change LDL-C, HDL-C, TG, and total cholesterol in youth with T1DM [111].
Insulin Sensitizers and Dyslipidemia in T2DM
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study, a multicenter randomized controlled trial in youth-onset T2DM, did not report significant changes in total cholesterol, LDL-C, HDL-C, or TG across three treatment arms: metformin, metformin + rosiglitazone or metformin, and lifestyle [112••]. In contrast, in a more recent clinical trial in newly diagnosed adults with T2DM, participants were classified into two groups following 3 months of metformin therapy: responders (HbA1c reduction ≥ 1% from baseline) and non-responders. All participants received atorvastatin, gemfibrozil or atorvastatin, and gemfibrozil daily. Responders experienced a greater decrease in LDL-C to HDL-C ratio and total cholesterol to HDL-C ratio compared to non-responders, which may suggest that response to metformin therapy may influence therapeutic outcomes of atorvastatin on atherogenic lipid markers [113]. Finally, a metabolic analysis in the population-based KORA cohort demonstrated that metformin therapy was associated with lower concentrations of three acyl-alkyl PCs and LDL-C, likely due to AMPK activation [114].
Conclusion
IR adversely affects lipid and lipoprotein metabolism and is strongly associated with dyslipidemia. The mechanisms by which IR influences lipid metabolism are complex and may depend on the disease state associated with the IR, i.e., obesity, metabolic syndrome, T2DM, and T1DM. The relationship between IR and dyslipidemia is likely reciprocal and the direction of the causality remains incompletely defined. Data suggest that there are different phenotypes of IR in T1DM vs. T2DM, and an understanding of how these metabolic phenotypes influence lipid metabolism is needed to better target diabetic dyslipidemia and prevent ASCVD. Accordingly, carefully designed mechanistic human studies are needed to advance our understanding of how different phenotypes of IR impact dyslipidemia and ASCVD risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
West KM, Ahuja MM, Bennett PH, Czyzyk A, De Acosta OM, Fuller JH, et al. The role of circulating glucose and triglyceride concentrations and their interactions with other "risk factors" as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care. 1983;6(4):361–9.
Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the strong heart study. Arterioscler Thromb Vasc Biol. 2000;20(3):830–5.
Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–61.
Grundy SM. Small LDL, atherogenic dyslipidemia, and the metabolic syndrome. Circulation. 1997;95(1):1–4.
Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the insulin resistance atherosclerosis study. Circulation. 2005;111(25):3465–72.
Grundy SM, Mok HY, Zech L, Steinberg D, Berman M. Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J Clin Invest. 1979;63(6):1274–83.
Kissebah AH, Alfarsi S, Adams PW. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in man: normolipemic subjects, familial hypertriglyceridemia and familial combined hyperlipidemia. Metabolism. 1981;30(9):856–68.
Labadzhyan A, Cui J, Peterfy M, Guo X, Chen YI, Hsueh WA, et al. Insulin clearance is associated with hepatic lipase activity and lipid and adiposity traits in Mexican Americans. PLoS One. 2016;11(11):e0166263.
Boden G. Fatty acids and insulin resistance. Diabetes Care. 1996;19(4):394–5.
Eckel RH, Prasad JE, Kern PA, Marshall S. Insulin regulation of lipoprotein lipase in cultured isolated rat adipocytes. Endocrinology. 1984;114(5):1665–71.
•• Sadur CN, Eckel RH. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J Clin Invest. 1982;69(5):1119–25 One of the first studies using a hyperinsulinemic-euglycemic clamp to study metabolism of lipids and lipoproteins.
Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–11.
Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab. 2013;24(8):391–7.
Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50(Suppl):S86–90.
Brown CM, Layman DK. Lipoprotein lipase activity and chylomicron clearance in rats fed a high fat diet. J Nutr. 1988;118(11):1294–8.
Medh JD, Fry GL, Bowen SL, Ruben S, Wong H, Chappell DA. Lipoprotein lipase- and hepatic triglyceride lipase- promoted very low density lipoprotein degradation proceeds via an apolipoprotein E-dependent mechanism. J Lipid Res. 2000;41(11):1858–71.
Garg A. Insulin resistance in the pathogenesis of dyslipidemia. Diabetes Care. 1996;19(4):387–9.
Annuzzi G, Giacco R, Patti L, Di Marino L, De Natale C, Costabile G, et al. Postprandial chylomicrons and adipose tissue lipoprotein lipase are altered in type 2 diabetes independently of obesity and whole-body insulin resistance. Nutr Metab Cardiovasc Dis. 2008;18(8):531–8.
Xiao C, Dash S, Morgantini C, Adeli K, Lewis GF. Gut peptides are novel regulators of intestinal lipoprotein secretion: experimental and pharmacological manipulation of lipoprotein metabolism. Diabetes. 2015;64(7):2310–8.
Garg A, Haffner SM. Insulin resistance and atherosclerosis. Diabetes Care. 1996;19(3):274.
de Vries R, Borggreve SE, Dullaart RP. Role of lipases, lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in abnormal high density lipoprotein metabolism in insulin resistance and type 2 diabetes mellitus. Clin Lab. 2003;49(11–12):601–13.
Brinton EA, Eisenberg S, Breslow JL, Human HDL. Cholesterol levels are determined by apoA-I fractional catabolic rate, which correlates inversely with estimates of HDL particle size. Effects of gender, hepatic and lipoprotein lipases, triglyceride and insulin levels, and body fat distribution. Arterioscler Thromb. 1994;14(5):707–20.
Wade DP, Knight BL, Soutar AK. Hormonal regulation of low-density lipoprotein (LDL) receptor activity in human hepatoma Hep G2 cells. Insulin increases LDL receptor activity and diminishes its suppression by exogenous LDL. Eur J Biochem. 1988;174(1):213–8.
Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–99.
Gerber PA, Thalhammer C, Schmied C, Spring S, Amann-Vesti B, Spinas GA, et al. Small, dense LDL particles predict changes in intima media thickness and insulin resistance in men with type 2 diabetes and prediabetes--a prospective cohort study. PLoS One. 2013;8(8):e72763.
Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32(9):2104–12.
Baynes C, Henderson AD, Anyaoku V, Richmond W, Hughes CL, Johnston DG, et al. The role of insulin insensitivity and hepatic lipase in the dyslipidaemia of type 2 diabetes. Diabet Med. 1991;8(6):560–6.
Eckel RH, Yost TJ, Jensen DR. Alterations in lipoprotein lipase in insulin resistance. Int J Obes Relat Metab Disord. 1995;19(Suppl 1):S16–21.
Yost TJ, Froyd KK, Jensen DR, Eckel RH. Change in skeletal muscle lipoprotein lipase activity in response to insulin/glucose in non-insulin-dependent diabetes mellitus. Metabolism. 1995;44(6):786–90.
Goodarzi MO, Guo X, Taylor KD, Quinones MJ, Saad MF, Yang H, et al. Lipoprotein lipase is a gene for insulin resistance in Mexican Americans. Diabetes. 2004;53(1):214–20.
Panarotto D, Remillard P, Bouffard L, Maheux P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur J Clin Investig. 2002;32(2):84–92.
Verges B, Petit JM, Duvillard L, Dautin G, Florentin E, Galland F, et al. Adiponectin is an important determinant of apoA-I catabolism. Arterioscler Thromb Vasc Biol. 2006;26(6):1364–9.
Ng TW, Watts GF, Farvid MS, Chan DC, Barrett PH. Adipocytokines and VLDL metabolism: independent regulatory effects of adiponectin, insulin resistance, and fat compartments on VLDL apolipoprotein B-100 kinetics? Diabetes. 2005;54(3):795–802.
Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57(7):1824–33.
Medina-Santillan R, Lopez-Velazquez JA, Chavez-Tapia N, Torres-Villalobos G, Uribe M. Mendez-Sanchez N. Diabetes Metab Res Rev: Hepatic manifestations of metabolic syndrome; 2013.
Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96(3):1261–8.
Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991;88(3):960–6.
• Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612–7 Thorough mini-review on IR and dyslipidemia.
Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci. 2017;38(7):649–65.
Petersen MC, Madiraju AK, Gassaway BM, Marcel M, Nasiri AR, Butrico G, et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J Clin Invest. 2016;126(11):4361–71.
Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–45.
Takayama S, White MF, Kahn CR. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988;263(7):3440–7.
Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54(12):3458–65.
Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29(11):1975–80.
Baldeweg SE, Golay A, Natali A, Balkau B, Del Prato S, Coppack SW. Insulin resistance, lipid and fatty acid concentrations in 867 healthy Europeans. European Group for the Study of insulin resistance (EGIR). Eur J Clin Investig. 2000;30(1):45–52.
Iwani NA, Jalaludin MY, Zin RM, Fuziah MZ, Hong JY, Abqariyah Y, et al. Triglyceride to HDL-C ratio is associated with insulin resistance in overweight and obese children. Sci Rep. 2017;7:40055.
Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869–74.
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26.
Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and nutrition examination survey. JAMA. 2002;287(3):356–9.
Rivellese AA, De Natale C, Di Marino L, Patti L, Iovine C, Coppola S, et al. Exogenous and endogenous postprandial lipid abnormalities in type 2 diabetic patients with optimal blood glucose control and optimal fasting triglyceride levels. J Clin Endocrinol Metab. 2004;89(5):2153–9.
• Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29 Registry data reporting dyslipidemia in youth with T1DM.
Bjornstad P, Nehus E, El Ghormli L, Bacha F, Libman IM, McKay S, et al. Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the TODAY clinical trial. Am J Kidney Dis. 2018;71(1):65–74.
Group TS. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735–41.
Consortium R. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. observations using the hyperglycemic clamp. Diabetes Care. 2018;41(8):1696–706.
Amutha A, Pradeepa R, Chella KS, Anjana RM, Unnikrishnan R, Mohan V. Lipid profile in childhood-and youth-onset type 2 diabetes and their association with microvascular complications. J Assoc Physicians India. 2017;65(6):42–7.
Paramsothy P, Knopp R, Bertoni AG, Tsai MY, Rue T, Heckbert SR. Combined hyperlipidemia in relation to race/ethnicity, obesity, and insulin resistance in the multi-ethnic study of atherosclerosis. Metabolism. 2009;58(2):212–9.
de Knijff P, van den Maagdenberg AM, Stalenhoef AF, Leuven JA, Demacker PN, Kuyt LP, et al. Familial dysbetalipoproteinemia associated with apolipoprotein E3-Leiden in an extended multigeneration pedigree. J Clin Invest. 1991;88(2):643–55.
•• Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–21 Translational studies demonstrating reduced insulin sensitivity in youth and adults with T1DM by hyperinsulinemic-euglycemic clamp technique.
Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, et al. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64(2):383–92.
•• Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60(1):306–14 Translational studies demonstrating reduced insulin sensitivity in youth and adults with T1DM by hyperinsulinemic-euglycemic clamp technique.
Cree-Green M, Stuppy JJ, Thurston J, Bergman BC, Coe GV, Baumgartner AD, et al. Youth with type 1 diabetes have adipose, hepatic and peripheral insulin resistance. J Clin Endocrinol Metab. 2018.
Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109(14):1750–5.
Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, et al. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: a RESistance to InSulin in type 1 ANd type 2 diabetes (RESISTANT) study. J Diabetes Complicat. 2016;30(6):1103–10.
Cree-Green M, Maahs DM, Ferland A, Hokanson JE, Wang H, Pyle L, et al. Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes. 2016;17(4):257–65.
Maahs DM, Hokanson JE, Wang H, Kinney GL, Snell-Bergeon JK, East A, et al. Lipoprotein subfraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes. 2010;59(7):1771–9.
Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162(2):297–301.
Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, et al. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complicat. 2016;30(4):586–90.
Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh epidemiology of diabetes complications study. Diabetes Care. 2003;26(5):1374–9.
Fredrickson DS. A physician's guide to hyperlipidemia. Mod Concepts Cardiovasc Dis. 1972;41(7):31–6.
Wadwa RP, Kinney GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, et al. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes Care. 2005;28(5):1051–6.
Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, et al. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for diabetes in youth case-control study. Diabetes Care. 2009;32(3):416–20.
Schwab KO, Doerfer J, Marg W, Schober E, Holl RW, Initiative DPVS, et al. Characterization of 33 488 children and adolescents with type 1 diabetes based on the gender-specific increase of cardiovascular risk factors. Pediatr Diabetes. 2010;11(5):357–63.
Schwab KO, Doerfer J, Hecker W, Grulich-Henn J, Wiemann D, Kordonouri O, et al. Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV). Diabetes Care. 2006;29(2):218–25.
Wood JR, Miller KM, Maahs DM, Beck RW, Dimeglio LA, Libman IM, et al. Most youth with type 1 diabetes in the T1D exchange clinic registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care. 2013;36(7):2035–7.
DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, et al. Obesity in Youth with Type 1 Diabetes in Germany, Austria, and the United States. J Pediatr. 2015;167(3):627–32 e1–4.
Maahs DM, Nadeau K, Snell-Bergeon JK, Schauer I, Bergman B, West NA, et al. Association of insulin sensitivity to lipids across the lifespan in people with type 1 diabetes. Diabet Med. 2011;28(2):148–55.
Maahs DM, Ogden LG, Dabelea D, Snell-Bergeon JK, Daniels SR, Hamman RF, et al. Association of glycaemia with lipids in adults with type 1 diabetes: modification by dyslipidaemia medication. Diabetologia. 2010;53(12):2518–25.
Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59(8):750–5.
Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The coronary artery calcification in type 1 diabetes (CACTI) study. Diabetes. 2003;52(11):2833–9.
Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972–82.
Steineck I, Cederholm J, Eliasson B, Rawshani A, Eeg-Olofsson K, Svensson AM, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. 2015;350:h3234.
Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 Suppl 1):A8 e1-526.
Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, et al. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases working group on cardiovascular complications of type 1 diabetes mellitus. Circulation. 2005;111(25):3489–93.
Vistisen D, Andersen GS, Hansen CS, Hulman A, Henriksen JE, Bech-Nielsen H, et al. Prediction of first cardiovascular disease event in type 1 diabetes mellitus: the Steno type 1 risk engine. Circulation. 2016;133(11):1058–66.
Soliman EZ, Backlund JC, Bebu I, Orchard TJ, Zinman B, Lachin JM, et al. Electrocardiographic abnormalities and cardiovascular disease risk in type 1 diabetes: the epidemiology of diabetes interventions and complications (EDIC) study. Diabetes Care. 2017;40(6):793–9.
Feitosa AC, Feitosa-Filho GS, Freitas FR, Wajchenberg BL, Maranhao RC. Lipoprotein metabolism in patients with type 1 diabetes under intensive insulin treatment. Lipids Health Dis. 2013;12:15.
Bjornstad P, Eckel RH, Pyle L, Rewers M, Maahs DM, Snell-Bergeon JK. Relation of combined non-high-density lipoprotein cholesterol and apolipoprotein B with atherosclerosis in adults with type 1 diabetes mellitus. Am J Cardiol. 2015;116(7):1057–62.
Bruckert E, Davidoff P, Grimaldi A, Truffert J, Giral P, Doumith R, et al. Increased serum levels of lipoprotein(a) in diabetes mellitus and their reduction with glycemic control. JAMA. 1990;263(1):35–6.
Kollerits B, Auinger M, Reisig V, Kastenbauer T, Lingenhel A, Irsigler K, et al. Lipoprotein(a) as a predictor of cardiovascular disease in a prospectively followed cohort of patients with type 1 diabetes. Diabetes Care. 2006;29(7):1661–3.
Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24(7):1266–71.
Mautner SL, Lin F, Roberts WC. Composition of atherosclerotic plaques in the epicardial coronary arteries in juvenile (type I) diabetes mellitus. Am J Cardiol. 1992;70(15):1264–8.
Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102(18):2180–4.
Djaberi R, Schuijf JD, Boersma E, Kroft LJ, Pereira AM, Romijn JA, et al. Differences in atherosclerotic plaque burden and morphology between type 1 and 2 diabetes as assessed by multislice computed tomography. Diabetes Care. 2009;32(8):1507–12.
Spagnoli LG, Mauriello A, Palmieri G, Santeusanio G, Amante A, Taurino M. Relationships between risk factors and morphological patterns of human carotid atherosclerotic plaques. A multivariate discriminant analysis. Atherosclerosis. 1994;108(1):39–60.
•• Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29(11):2528–38 Randomized control trial with metformin in adults with T1DM demonstrating no effect on dyslipidemia.
van Stee MF, de Graaf AA, Groen AK. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc Diabetol. 2018;17(1):94.
Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid Res. 2012;53(12):2490–514.
Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21(4):507–11.
Field FJ, Born E, Murthy S, Mathur SN. Gene expression of sterol regulatory element-binding proteins in hamster small intestine. J Lipid Res. 2001;42(1):1–8.
Kim SY, Kim HI, Kim TH, Im SS, Park SK, Lee IK, et al. SREBP-1c mediates the insulin-dependent hepatic glucokinase expression. J Biol Chem. 2004;279(29):30823–9.
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–8.
Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–6.
Sonne DP, Knop FK, Comment on Xu, et al. Effects of Metformin on Metabolite Profiles and LDL Cholesterol in Patients With Type 2 Diabetes. Diabetes Care. 2015;38:1858–67 Diabetes Care. 2015;38(12):e215.
Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55(8):1553–95.
Madsen A, Bozickovic O, Bjune JI, Mellgren G, Sagen JV. Metformin inhibits hepatocellular glucose, lipid and cholesterol biosynthetic pathways by transcriptionally suppressing steroid receptor coactivator 2 (SRC-2). Sci Rep. 2015;5:16430.
Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf). 2009;196(1):81–98.
Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):597–609.
Anderson JJA, Couper JJ, Giles LC, Leggett CE, Gent R, Coppin B, et al. Effect of metformin on vascular function in children with type 1 diabetes: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 2017;102(12):4448–56.
• Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, et al. Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2015;314(21):2241–50 Randomized control trial with metformin in youth with T1DM demonstrating no effect on dyslipidemia.
Nadeau KJ, Chow K, Alam S, Lindquist K, Campbell S, McFann K, et al. Effects of low dose metformin in adolescents with type I diabetes mellitus: a randomized, double-blinded placebo-controlled study. Pediatr Diabetes. 2015;16(3):196–203.
Bjornstad P, Schafer M, Truong U, Cree-Green M, Pyle L, Baumgarten A, et al. Metformin improves insulin sensitivity and vascular health in youth with type 1 diabetes: a randomized control trial. Circulation 2018 (in press).
•• Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–56 Randomized control trial with metformin, rosiglitazone, and lifestyle changes in youth with T2DM demonstrating no effect on dyslipidemia.
Kashi Z, Mahrooz A, Kianmehr A, Alizadeh A. The role of metformin response in lipid metabolism in patients with recent-onset type 2 diabetes: HbA1c level as a criterion for designating patients as responders or nonresponders to metformin. PLoS One. 2016;11(3):e0151543.
Xu T, Brandmaier S, Messias AC, Herder C, Draisma HH, Demirkan A, et al. Effects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetes. Diabetes Care. 2015;38(10):1858–67.
Funding
P.B. receives salary support by NIH (K23 DK116720-01), in addition to research support from Thrasher Research Fund, Juvenile Diabetes Research Foundation (JDRF), International Society of Pediatric and Adolescent Diabetes (ISPAD), Colorado Clinical and Translational Sciences Institute and Center for Women’s Health Research at University of Colorado. R.H.E. is supported by NIH (R21NS102506; P30DK48520; P50HD073063) a grant from ENDECE LLC. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Author information
Authors and Affiliations
Contributions
PB and RHE wrote, contributed to discussion, and reviewed/edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
P.B. has received speaking honoraria and research operating funding from Horizon Pharmaceuticals, and travel support from Boehringer Ingelheim.
R.H.E. serves on advisory boards for Regeneron/Sanofi, Kowa, Merck, and Novo Nordisk.
Human and Animal Rights and Informed Consent
This is a review paper and summarizes existing data. It does not contain any unpublished data in human or animal subjects performed by either of the authors.
Additional information
This article is part of the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Bjornstad, P., Eckel, R.H. Pathogenesis of Lipid Disorders in Insulin Resistance: a Brief Review. Curr Diab Rep 18, 127 (2018). https://doi.org/10.1007/s11892-018-1101-6
Published:
DOI: https://doi.org/10.1007/s11892-018-1101-6