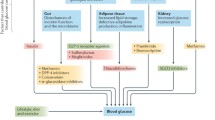
Abstract
Purpose of Review
Insulin therapy alone fails to achieve target glycemic control in the majority of individuals with type 1 diabetes (T1D), motivating the investigation of additive medications. This review focuses on the recent findings on the use of adjunctive pharmacotherapy in T1D.
Recent Findings
Metformin and glucagon-like peptide-1 receptor agonists have been associated with weight reduction and decrease in daily insulin requirements without sustainable improvement in glycemic control. Sodium-glucose cotransporter (SGLT)-2 inhibitors, dual SGLT-1/2 inhibitors, and pramlintide have been shown to reduce hemoglobin A1c, induce weight loss, and lower insulin dose. The benefits of dipeptidyl peptidase-4 inhibitors, thiazolidinediones, and alpha glucosidase inhibitors appear to be more limited. Gastrointestinal symptoms and increased hypoglycemia are adverse effects of certain classes.
Summary
Although not devoid of side effects, additive pharmacotherapies in T1D can improve glycemic control and lower body weight and insulin requirement. Longer studies are needed before consideration for widespread clinical care.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The mainstay of treatment for type 1 diabetes (T1D) has not changed since the first use of exogenous insulin in 1922. The landmark Diabetes Control and Complications Trial in 1993 set the stage for widespread use of intensive insulin management in individuals with T1D to reduce the onset and progression of microvascular complications [1]. The follow-up observational Epidemiology of Diabetes Interventions and Complications Study showed an additional cardiovascular disease benefit with intensive insulin management [2]. The results of these important studies formed the basis of the American Diabetes Association’s recommendations for target hemoglobin A1c (HbA1c) of < 7.5% in children and < 7% in adults with T1D [3, 4].
Despite the importance of glycemic control in mitigating the risk of diabetes-associated complications, only approximately 20% of the children and 25% of the adults in the large T1D Exchange Clinic Registry (16,061 participants) achieved target HbA1c [5•]. Part of the difficulty in reaching glycemic target stems from the inability of current insulin therapy to address pathophysiological disturbances in T1D apart from endogenous insulin deficiency including alpha-cell dysfunction [6, 7] and insulin resistance secondary to overweight/obesity [8, 9, 10••]. Thus, additive treatments to insulin may improve glycemic control by targeting different disease processes beyond what is achieved by insulin replacement therapy. This review focuses on adjunctive pharmacotherapy options to insulin in the treatment of T1D.
Search Strategy and Selection Criteria
We searched the PubMed using the terms “adjuvant therapy,” “metformin,” “glucagon-like peptide-1 receptor agonists,” “GLP-1 receptor agonists,” “dipeptidyl peptidase-4 inhibitors,” “DPP-4 inhibitors,” “SGLT-2 inhibitors,” “SGLT-1/2 inhibitors,” “amylin receptor agonist,” “thiazolidinediones,” and “alpha glucosidase inhibitors” combined with the terms “type 1 diabetes” to identify the reference articles published in English during the last 5 years. We also reviewed authors’ bibliographies for relevant past studies.
Additive Treatments to Insulin
Metformin
Metformin is the preferred initial medication in individuals with type 2 diabetes (T2D) [3, 11, 12]. It is approved by the Food and Drug Administration (FDA) for treatment of T2D in individuals 10 years of age and older, but not for T1D. Metformin works primarily by decreasing hepatic glucose production, improving peripheral insulin sensitivity, decreasing intestinal glucose absorption, and increasing postprandial glucagon-like-peptide-1 levels [13•]. It generally is well tolerated [12]. Gastrointestinal side effects are common but usually mild. Because of its easy tolerability, affordability, and mechanism of actions, metformin has been of clinical interest as an adjunctive treatment of T1D.
Although earlier randomized controlled trials (RCTs) of shorter durations showed improvement in HbA1c with addition of metformin, more recent and larger RCTs failed to show this benefit. In a meta-analysis of four RCTs and a prospective controlled study with durations of 3–6 months (n = 149) in adults and children, Pang et al. found a small but statistically significant reduction in levels of HbA1c (~ 0.3%) and a moderate reduction in total daily insulin dose without significant increase in adverse events or change in weight [14]. A systematic review of five RCTs with combined effects estimated by Vella et al. showed that metformin was associated with decreased daily insulin dose (6.6 units/day, p < 0.001) and weight reduction in some studies but no significant HbA1c reduction (~ 0.1%, p = 0.42) [15]. Metformin was well tolerated, with minimal gastrointestinal side effects, but there was a trend towards development of hypoglycemia. Codner et al. evaluated the effect of metformin on 24 adolescent girls with T1D and hyperandrogenism in a RCT for 9 months [16]. Assessment of secondary outcomes revealed no difference in levels of HbA1c or daily insulin doses. Two other RCTs that included adolescents with T1D failed to show statistically significant reduction in levels of HbA1c, although a decrease of 0.4% in the level of HbA1c occurred at 6–9 months [17, 18]. However, Nadeau et al. found significant reduction in insulin dose, BMI z-score, and waist circumference with low-dose metformin (500 mg twice a day) at 6 months compared to baseline (within group comparison) in adolescents, but these findings were not significantly different from those individuals given a placebo. An analysis from the German/Austrian Diabetes Patienten Verlaufsdokumentation (DPV) registry in Germany and Austria that evaluated the effects of metformin in addition to insulin in pediatric individuals with T1D showed a slight reduction of BMI standard deviation score (BMI-SDS) but no improvement in HbA1c or insulin requirement during an average treatment period of 1.4 years [19•]. A multicenter RCT conducted by Libman et al. involving 140 adolescents in T1D Exchange Clinic Registry revealed no difference in HbA1c at 26-week follow-up with metformin compared to the placebo group [20•]. However, a higher percentage of participants in the metformin group had ≥ 25% reduction in total daily insulin/kg of body weight (23% vs 1% in placebo group) and ≥ 10% reduction in BMI z-score (24% vs 7% in placebo group). Not surprisingly, there were more participants with gastrointestinal side effects in the metformin group than in the placebo group. Liu et al. reported similar findings in a meta-analysis including eight RCTs with 300 participants [21]. There were reductions in weight, total daily insulin dose, and total and low density lipoprotein (LDL) cholesterol but no effect on HbA1c or fasting plasma glucose with metformin use. Although an increase in gastrointestinal side effects occurred, metformin did not increase the risk of severe hypoglycemia. A systematic review and meta-analysis of six RCTs with 325 children with T1D showed consistent results, with decreased total daily insulin dose, BMI and BMI z-score, but similar levels of HbA1c [22•]. In contrast to these findings, in a 10-year retrospective study, Staels et al. showed that adjuvant metformin added to intensive insulin therapy in individuals with T1D had no long-term beneficial effects on BMI, HbA1c, or insulin dose, although small but non-significant decreases were seen in BMI and insulin dose in the first years of metformin therapy [23]. The REMOVAL trial, the largest and longest RCT of metformin in T1D for 3-years duration, studied 428 adults with T1D older than 40 years with at least 5 years of diabetes and at least 3 of 10 cardiovascular risk factors [24••]. The addition of metformin did not significantly change glycemic control or mean carotid artery intima media thickness, a surrogate of atherosclerosis progression. However, metformin use was associated with reductions in body weight, LDL cholesterol, and insulin dose requirement. Although gastrointestinal adverse effects and vitamin B12 deficiency were increased in prevalence in the metformin group, risk of hypoglycemia did not change. Most recently, in a meta-analysis of 13 RCTs with 1183 individuals with T1D, Meng et al. found reductions in BMI, insulin requirements, total cholesterol, and LDL cholesterol but not in levels of HbA1c with use of metformin. Additionally, metformin was associated with a slight increase in risk of severe hypoglycemia and gastrointestinal side effects [25]. Findings from recent RCT are summarized in Table 1.
Overall, available data do not show evidence of sustainable improvement in glycemic control with use of adjunctive metformin in individuals with T1D. However, metformin appears to have other benefits including weight reduction, improvement in lipid profile, and decrease in total daily insulin requirement which may translate to decreased risk of developing cardiovascular disease. Gastrointestinal adverse effects have been reported consistently, whereas metformin does not appear to increase the risk of hypoglycemia.
GLP-1 Receptor Agonists and DPP-4 Inhibitors
Glucagon-like peptide-1 (GLP-1) is an incretin produced by intestinal cells in response to a meal stimulus [26]. It stimulates insulin secretion in a glucose-dependent manner, inhibits secretion of glucagon, slows gastric emptying, and reduces food intake by inducing satiety [27]. Additionally, it improves proliferation/survival of beta cells, peripheral insulin sensitivity, and cardiac output, and decreases hepatic glucose production [28]. After its secretion, GLP-1 is rapidly (in minutes) degraded by dipeptidyl peptidase-4 (DPP-4). Two classes of pharmacological agents target this incretin-mediated physiological process. Whereas GLP-1 receptor agonists are resistant to degradation by DPP-4, DPP-4 inhibitors enhance the action of endogenous incretins by inhibiting their degradation. Although GLP-1 receptor agonists may cause gastrointestinal side effects, DPP-4 inhibitors usually are well tolerated. Currently, six GLP-1 receptor agonists (i.e., exenatide, liraglutide, albiglutide, dulaglutide, lixisenatide, and semaglutide) and four DPP-4 inhibitors (i.e., sitagliptin, saxagliptin, linagliptin, and alogliptin) are approved by the FDA for treatment of T2D but not T1D [12, 29].
Wang et al. examined seven RCTs on the effects of additive GLP-1 receptor agonists in T1D through a systematic review and meta-analysis [30•]. A total of 206 adults were included in the analysis with study durations from 12 weeks to 15 months. They found that adjunctive GLP-1 receptor agonists were associated with a statistically significant but small reduction in levels of HbA1c (0.2%), decrease in body weight, and weight-adjusted-bolus insulin doses. An in-depth review of individual studies shows that use of GLP-1 receptor agonists resulted in generally 0–0.2% statistically non-significant difference in HbA1c reduction, approximately 3.0–6.8-kg weight loss, and 5–8-units decrease in total daily insulin dose, driven mainly by the decrease in bolus insulin requirement with GLP-1 receptor agonist use. Its use was associated with increased gastrointestinal side effects consistently but not with hypoglycemia [31,32,33, 34•].
Another recent systematic review and meta-analysis studied the effects of DPP-4 inhibitors in the treatment of T1D in adults and youth [35•]. Five RCTs were eligible for analysis (n = 253). Overall, DPP-4 inhibitors did not significantly affect HbA1c, weight, daily insulin requirement, or incidence of hypoglycemia. However, they were associated with reduced levels of glucagon during hyperglycemia [36], improved beta-cell function [37], and post-meal rise in levels of endogenous GLP-1 [38] in individual studies. In contrast, Guo et al. found in a meta-analysis of six RCTs that DPP-4 inhibitors decreased daily insulin requirements significantly (2.4 units/day) without any significant change in HbA1c or incidence of hypoglycemia [39•]. Lastly, both human and rat studies suggest that DPP-4 inhibitors may be beneficial for diabetic nephropathy [40, 41]. On the other side, there are concerns for association between DPP-4 inhibitor use and pancreatic and thyroid cancer [42]. However, in a recent systematic review and meta-analysis assessing 12 RCTs and 13 observational studies, Overbeek et al. reported that there was no statistically significant risk for pancreatic or thyroid cancer [43].
Overall, neither adjunctive GLP-1 receptor agonists nor DPP-4 inhibitors provide any substantial benefit for glycemic control in individuals with T1D. However, GLP-1 receptor agonists were associated consistently with weight loss and decreased daily insulin requirements at an expense of increased gastrointestinal side effects. The benefits of DPP-4 inhibitors in individuals with T1D appear to be more limited based on available data.
SGLT-2 and SGLT-1/2 Inhibitors
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors work primarily by increasing excretion of glucose in the urine secondary to blocking reabsorption of glucose in the proximal renal tubule [44]. In comparison, dual SGLT-1/2 inhibitor additionally blocks SGLT-1, a major transporter of glucose in the intestines, and decreases intestinal reabsorption of glucose as well [45]. Three SGLT-2 inhibitors (i.e., canagliflozin, dapagliflozin, and empagliflozin) are FDA-approved for the treatment of T2D but not for T1D. The dual SGLT-1/2 inhibitor sotagliflozin is still an investigational drug, for which the FDA has just recently accepted its marketing application for review as an adjunct therapy in T1D [46]. The FDA issued a warning with regard to euglycemic diabetic ketoacidosis with SGLT-2 inhibitors in individuals with T1D and T2D, based on reported cases [47•].
We identified three RCTs assessing adjunct SGLT-2 inhibitors in T1D with study durations of 2–18 weeks. In a 4-week RCT involving 75 individuals, Pieber et al. found that additive empagliflozin was associated with 0.5% reduction in levels of HbA1c and 1.9-kg weight loss without increased risk of symptomatic hypoglycemia [48•]. Further analysis also showed decreased glycemic variability and increased time spent in target glycemic range without increasing time spent in hypoglycemia [49•]. Similarly, in a RCT involving 351 participants who were followed for 18 weeks, Henry et al. reported that with adjunct canagliflozin, compared to placebo group, there were ≥ 0.4% reduction in HbA1c in 41% of individuals, average weight loss of 4.4 kg, and decrease in total daily insulin requirement by 7.6 units but increased incidence of ketone-related adverse events (9.4% vs 0% in placebo group) including diabetic ketoacidosis (DKA; 6% vs 0% in placebo group). No difference in incidence of hypoglycemia was noted among the groups [50•]. It also improved time spent in target glycemic range and reduced time spent in hyperglycemia [51]. In a 2-week pilot RCT with dapagliflozin, Henry et al. found a reduction in average daily glucose level by 41 mg/dL and total daily insulin dose by 16% without any incident of DKA [52]. Furthermore, empagliflozin may preserve beta-cell mass in a rat model of T1D [53•].
The dual SGLT-1/2 inhibitor sotagliflozin is emerging as a potential adjunct treatment in T1D. In a RCT involving 33 participants with a treatment duration of 1 month, Sands et al. showed that dual SGLT-1/2 inhibition decreased bolus insulin dose by 32% and total daily insulin dose by 15%, reduced levels of HbA1c by 0.5%, decreased body weight (1.7 kg), and increased time in target glycemic range by 11% [54•]. The participants had increased incidence of gastrointestinal symptoms with SGLT-1/2 inhibitor compared with placebo (50% vs 18%). There were two incidents of DKA in the SGLT-1/2 inhibitor group, although both were attributed to pump-related issues. Consistent with these findings, in a multicenter, double-blind, phase 3 study over 24 weeks with 1402 participants with T1D, Garg et al. found that the SGLT-1/2 inhibitor arm compared to controls had reductions of HbA1c (~ 0.5%), weight (~ 3 kg), and daily dose of insulin (~ 5 units/day) but the rate of diabetic ketoacidosis was higher (3% vs 0.6% in placebo) [55•]. Furthermore, a significantly larger percentage of participants achieved HbA1c < 7% without severe hypoglycemia or DKA in SGLT-1/2 inhibitors than placebo (28.6% vs 15.3%).
In summary, SGLT-2 and dual SGLT-1/2 inhibitors are potential adjunctive therapies to insulin in individuals with T1D. While there is a paucity of studies, available data show benefit in HbA1c, weight loss, and reduction in insulin dose although increased incidence of ketone-related adverse events including DKA.
Amylin Receptor Agonists
Amylin is a neuroendocrine hormone co-secreted with insulin from pancreatic beta cells [56]. It stimulates the satiety center, decreases secretion of glucagon postprandially, and delays gastric emptying. Thus, it reduces postprandial glucose excursions. Pramlintide, an amylin receptor agonist, is the only non-insulin agent approved by the FDA as an adjunct therapy to insulin in T1D. It requires injections three times per day, which may add to the burden of T1D management.
The effects of pramlintide as an adjunct therapy in T1D were studied in three main RCTs with durations of 29–52 weeks. In a RCT with 480 participants for 52 weeks, Whitehouse et al. found that reduction of levels of HbA1c was significantly greater in individuals taking pramlintide than in the placebo group throughout the study (0.5%, 0.4%, and 0.3% difference at 13, 26, and 52 weeks, respectively) [57]. At the end of the study, the pramlintide group had a smaller increase in total daily insulin dose (2.3% vs 10.3%), and weight loss (0.5-kg weight loss vs 1-kg weight gain in placebo). Although there was no difference in the rate of severe hypoglycemia events, the rate of adverse events, primarily nausea, was higher in the pramlintide group (47% vs 22%). In a multicenter RCT, Ratner et al. showed that pramlintide was associated with significant reductions in levels of HbA1c (~ 0.3%) and body weight (0.4-kg loss vs 0.8-kg gain in placebo) over 52 weeks. Mild-to-moderate nausea was the most common adverse event with pramlintide [58]. In contrast to these two RCTs, Edelman et al. showed no difference in HbA1c between the pramlintide and placebo groups at 29 weeks [59]. However, pramlintide significantly decreased postprandial glucose excursions, weight (1.3-kg weight loss vs 1.2-kg weight gain), and insulin dose (reduction by 28% vs 4%). There were higher incidence of nausea, severe hypoglycemia, and decreased appetite in the pramlintide group compared to the placebo group. The authors postulated that lack of a difference in HbA1c was anticipated because of the study design, in which insulin doses were adjusted for pre-specified glycemic targets in both groups.
Overall, pramlintide may be beneficial in glycemic control, weight loss, and reduction of insulin doses, but it comes with an increased risk of gastrointestinal side effects and hypoglycemia.
Thiazolidinediones
Thiazolidinediones are peroxisome proliferator-activated receptor-γ agonists that improve insulin sensitivity mainly in peripheral tissues and reduce lipolysis [60]. Pioglitazone and rosiglitazone are the only current FDA-approved thiazolidinediones for treatment of adults with T2D but not in T1D [61]. There are FDA warnings for possible exacerbation of congestive heart failure, myocardial infarction, bladder cancer, fatal hepatic failure, and higher risk of developing hypoglycemia, fractures (in females), fluid retention, edema, weight gain, and anemia [61].
In four RCTs assessing the efficacy of pioglitazone or rosiglitazone in individuals with T1D, there were 0–0.2% reduction in placebo-adjusted HbA1c and mixed results in other parameters [62,63,64,65]. Whereas Strowig et al. and Stone et al. reported decreased insulin requirements compared to placebo; no difference was found between the groups in the studies conducted by Zdravkovic et al. and Bhat et al. Increased BMI was reported in only one of these studies [64]. Neither rosiglitazone nor pioglitazone was found to be associated with increased risk of hypoglycemia.
Overall, the potential benefits of thiazolidinediones do not seem to justify the possible risks associated with them in individuals with T1D.
Alpha Glucosidase Inhibitors
Alpha glucosidase, an enzyme in the proximal small intestine, plays an important role in carbohydrate absorption due to its role in breaking down disaccharides and more complex carbohydrates [60]. Alpha glucosidase inhibitors delay intestinal absorption of carbohydrates and reduce postprandial glucose excursions by inhibiting this enzyme. Acarbose and miglitol are the available FDA-approved alpha glucosidase inhibitors in the treatment of adults with T2D but not in those with T1D [61]. The main side effects are flatulence and diarrhea.
There are no recent RCTs assessing alpha glucosidase inhibitors in T1D. In older studies, Hollander et al. reported that additive treatment with acarbose was associated with reduction in mean postprandial glucose levels by 59 mg/dL and HbA1c by 0.48% in participants with T1D in a 36-week multicenter RCT [66]. In contrast, Riccardi et al. showed that acarbose had no significant effect on HbA1c but reduced 2-h postprandial glucose levels in a 24-week multicenter RCT [67]. Gastrointestinal side effects were more common with acarbose, but it was not associated with hypoglycemia in both of these studies. In a 12-week, open-label study of participants with T1D receiving intensive insulin therapy, Nagai et al. showed a significant reduction in levels of HbA1c (0.5%), postprandial glucose, BMI, and daily bolus insulin dose but increase in postprandial GLP-1 levels [68].
Overall, alpha glucosidase inhibitors decrease postprandial glucose levels with potentially small HbA1c benefit without increased risk of hypoglycemia in individuals with T1D. Gastrointestinal side effects may limit its use.
Conclusions
Although only one agent (i.e., pramlintide) is currently approved by the FDA as an additive treatment in individuals with T1D, other agents used in T2D have potential benefits in individuals with T1D for glycemic and non-glycemic therapeutic goals.
Metformin has not been shown to provide sustainable improvement in glycemic control, but it has been shown to be helpful with weight reduction, improvement in lipid profiles, and decrease in insulin requirements with potential cardiovascular benefits. Studies to date have not shown any significant glycemic benefit of GLP-1 receptor agonist and DPP-4 inhibitors in individuals with T1D. However, GLP-1 receptor agonists have been associated with weight loss and decreased daily insulin requirement, but also with gastrointestinal side effects. The benefits of DPP-4 inhibitors appear to be more limited compared to GLP-1 receptor agonists. Both SGLT-2 and dual SGLT-1/2 inhibitors are promising adjunctive therapies to insulin in individuals with T1D, despite the paucity of studies. Available data showed reduction in HbA1c, weight, and insulin dose. However, the increased incidence of ketone-related adverse effects, including diabetic ketoacidosis, is an important limitation for its use. Similarly, pramlintide may be beneficial for glycemic control, weight loss, and reduction of insulin doses, but it has an increased risk of gastrointestinal side effects and hypoglycemia. The potential benefits of thiazolidinediones do not seem to justify the possible risks associated with them in individuals with T1D. Alpha glucosidase inhibitors decrease postprandial glucose levels with potential HbA1c benefit without increased risk of hypoglycemia in individuals with T1D.
In conclusion, clinicians considering an additive pharmacologic agent to insulin in their patients with T1D should consider FDA approval status and assess individualized risk-benefit ratios. Further studies with longer follow-up durations are needed before recommending their widespread use in clinical practice.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The Diabetes Control and Complications Trial Research Group. Effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53.
American Diabetes A. 12. Children and adolescents: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S126–S36.
American Diabetes A. 6. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S55–64.
• Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes care. 2015;38(6):971–8. Study showing the pressing problem of achieving target glycemic control in type 1 diabetes in the US.
Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14–6.
Muller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–15.
Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for diabetes in youth study. Pediatr Diabetes. 2010;11(1):4–11.
• DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, et al. Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr. 2015;167(3):627–32 e1-4. A study showing the extent of obesity in children with T1D in two large clinical registries in US and Europe, and that BMI-z score is associated with higher HbA1c and more frequent hypoglycemia.
•• Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. Jama. 2018;319(16):1723–5. A study evalauting the prevalence of obesity and its trend in US children and adults over the last decade, showing the urgency of the growing obesity problem.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9.
American Diabetes A. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S73–85.
• Thomas I, Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatr Diabetes. 2017;18(1):10–6. A recent review of use of metformin in children with diabetes.
Pang TT, Narendran P. Addressing insulin resistance in type 1 diabetes. Diabet Med. 2008;25(9):1015–24.
Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia. 2010;53(5):809–20.
Codner E, Iniguez G, Lopez P, Mujica V, Eyzaguirre FC, Asenjo S, et al. Metformin for the treatment of hyperandrogenism in adolescents with type 1 diabetes mellitus. Horm Res Paediatr. 2013;80(5):343–9.
Nadeau KJ, Chow K, Alam S, Lindquist K, Campbell S, McFann K, et al. Effects of low dose metformin in adolescents with type I diabetes mellitus: a randomized, double-blinded placebo-controlled study. Pediatr Diabetes. 2015;16(3):196–203.
Nwosu BU, Maranda L, Cullen K, Greenman L, Fleshman J, McShea N, et al. A randomized, double-blind, placebo-controlled trial of adjunctive metformin therapy in overweight/obese youth with type 1 diabetes. PLoS One. 2015;10(9):e0137525.
• Konrad K, Datz N, Engelsberger I, Grulich-Henn J, Hoertenhuber T, Knauth B, et al. Current use of metformin in addition to insulin in pediatric patients with type 1 diabetes mellitus: an analysis based on a large diabetes registry in Germany and Austria. Pediatr Diabetes. 2015;16(7):529–37. A study from a large diabetes registry in Europe showing a slight reduction in BMI-SDS without improvement in HbA1c and daily insulin requirement with use of additive metformin in children with T1D.
• Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, et al. Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. Jama. 2015;314(21):2241–50. A multicenter RCT from T1D Exchange Clinic Registry showing benefits of additive metformin treatment in youth with T1D on BMI-z score and daily insulin requirement without improvement in HbA1c at 26-weeks.
Liu C, Wu D, Zheng X, Li P, Li L. Efficacy and safety of metformin for patients with type 1 diabetes mellitus: a meta-analysis. Diabetes Technol Ther. 2015 Feb;17(2):142–8.
• Al Khalifah RA, Alnhdi A, Alghar H, Alanazi M, Florez ID. The effect of adding metformin to insulin therapy for type 1 diabetes mellitus children: a systematic review and meta-analysis. Pediatr Diabetes. 2017;18(7):664–73. A systematic review and meta-analysis of six RCTs showing decreased total daily insulin dose, BMI, BMI-z score but similar HbA1c with use of additive metformin in children with T1D.
Staels F, Moyson C, Mathieu C. Metformin as add-on to intensive insulin therapy in type 1 diabetes mellitus. Diabetes Obes Metab. 2017;19(10):1463–7.
•• Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):597–609. The largest and longest RCT on metformin use in adults with T1D showing reductions in body weight, LDL cholesterol, and insulin dose requirement but no significant change in glycemic control and carotid artery intima media thickness with metformin use.
Meng H, Zhang A, Liang Y, Hao J, Zhang X, Lu J. Effect of metformin on glycaemic control in patients with type 1 diabetes: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2018;34(4):e2983.
Unger J. Rationale use of GLP-1 receptor agonists in patients with type 1 diabetes. Curr diab Rep. 2013;13(5):663–8.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705.
Drucker DJ. The biology of incretin hormones. Cell Metab. 2006 Mar;3(3):153–65.
Tran KL, Park YI, Pandya S, Muliyil NJ, Jensen BD, Huynh K, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178–88.
• Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2017;8(4):727–38. A sytematic review and meta-analysis showing small reduction in HbA1c, decrease in body weight and weight-adjusted bolus insulin doses in individuals treated with GLP-1 receptor agonists.
Abstracts of the 48th EASD (European Association for the Study of Diabetes) Annual Meeting of the European Association for the Study of Diabetes. October 1–5, 2012. Berlin, Germany. Diabetologia. 2012;55 Suppl 1:S7–537.
• Kuhadiya ND, Dhindsa S, Ghanim H, Mehta A, Makdissi A, Batra M, et al. Addition of liraglutide to insulin in patients with type 1 diabetes: a randomized placebo-controlled clinical trial of 12 weeks. Diabetes Care. 2016;39(6):1027–35. A RCT of 12 weeks duration showing that addition of liraglutide was associated with modest reduction in weekly mean glucose levels with significant weight loss, small insulin dose reductions and frequent gastrointestinal side effects in overweight/obese inidividuals with T1D.
• Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care. 2015;38(12):2250–7. A RCT showing reduced body weight and insulin requirement without an improvement in HbA1c with the addition of liraglutide in normal-weight individuals with T1D.
• Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4(3):221–32. A RCT in overweight adult individuals with T1D showing that addition of liraglutide was associated with reductions in body weight, insulin doses, and hypoglycemic events but no improvement in HbA1c.
• Wang Q, Long M, Qu H, Shen R, Zhang R, Xu J, et al. DPP-4 inhibitors as treatments for type 1 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2018;2018:5308582. A systematic review and meta-analysis showing no significant difference in HbA1c, weight, daily insulin requirement and incidence of hypoglycemia with addition of DPP-4 inhibitors in individuals with T1D.
Farngren J, Persson M, Schweizer A, Foley JE, Ahren B. Vildagliptin reduces glucagon during hyperglycemia and sustains glucagon counterregulation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab. 2012;97(10):3799–806.
Zhao Y, Yang L, Xiang Y, Liu L, Huang G, Long Z, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin maintains beta-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab. 2014;99(5):E876–80.
Garg SK, Moser EG, Bode BW, Klaff LJ, Hiatt WR, Beatson C, et al. Effect of sitagliptin on post-prandial glucagon and GLP-1 levels in patients with type 1 diabetes: investigator-initiated, double-blind, randomized, placebo-controlled trial. Endocr Pract. 2013;19(1):19–28.
• Guo H, Fang C, Huang Y, Pei Y, Chen L, Hu J. The efficacy and safety of DPP4 inhibitors in patients with type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;121:184–91. A systematic review and meta-analysis showing decreased daily insulin requirement without significant change in HbA1c or incidence of hypoglycemia with DPP-4 inhibitors in individuals with T1D.
Kodera R, Shikata K, Takatsuka T, Oda K, Miyamoto S, Kajitani N, et al. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun. 2014;443(3):828–33.
Duvnjak L, Perkovic MN, Blaslov K. Dipeptidyl peptidase-4 activity is associated with urine albumin excretion in type 1 diabetes. J Diabetes Complicat. 2017;31(1):218–22.
Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013;36(7):2126–32.
Overbeek JA, Bakker M, van der Heijden AAWA, van Herk-Sukel MPP, Herings RMC, Nijpels G. Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2018;23:e3004. https://doi.org/10.1002/dmrr.3004.
Ahmed-Sarwar N, Nagel AK, Leistman S, Heacock K. SGLT-2 inhibitors: is there a role in type 1 diabetes mellitus management? Ann Pharmacother. 2017;51(9):791–6.
Subramanian S, Baidal D, Skyler JS, Hirsch IB. The management of type 1 diabetes. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editors. Endotext. South Dartmouth (MA) 2000.
Sanofi. FDA to review Zynquista™ (sotagliflozin) as potential treatment for type 1 diabetes. Press Release. 2018. http://www.news.sanofi.us/2018-05-22-FDA-to-review-Zynquista-TM-sotagliflozin-as-potential-treatment-for-type-1-diabetes. Last accessed 6/6/18.
• Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–93. A case series of euglycemic diabetic ketoacidosis associated with SGLT-2 inhibitors in patients with T1D and T2D pointing out a potentially life threatening complication of SGLT-2 inhibitors.
• Pieber TR, Famulla S, Eilbracht J, Cescutti J, Soleymanlou N, Johansen OE, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab. 2015;17(10):928–35. A RCT of 4-week duration showing reduction in HbA1c and weight loss without increased risk of hypoglycemia in individuals with T1D using additive empagliflozin.
• Famulla S, Pieber TR, Eilbracht J, Neubacher D, Soleymanlou N, Woerle HJ, et al. Glucose exposure and variability with empagliflozin as adjunct to insulin in patients with type 1 diabetes: continuous glucose monitoring data from a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Technol Ther. 2017;19(1):49–60. A RCT of 4-week duration showing decreased glycemic variability, increased time spent in target glycemic range without increasing hypoglycemia in individuals with T1D using additive empagliflozin.
• Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38(12):2258–65. A RCT of 18 week duration showing benefits of canagliflozin on improvement in HbA1c, weight loss, and reduction in total daily insulin requirement but with increased incidence of ketone-related adverse events.
Rodbard HW, Peters AL, Slee A, Cao A, Traina SB, Alba M. The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care. 2017;40(2):171–80.
Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38(3):412–9.
• Cheng ST, Chen L, Li SY, Mayoux E, Leung PS. The effects of empagliflozin, an SGLT2 inhibitor, on pancreatic beta-cell mass and glucose homeostasis in type 1 diabetes. PloS one. 2016;11(1):e0147391. A study showing preservation of beta cell mass in type 1 diabetic rats with empagliflozin.
• Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care. 2015;38(7):1181–8. A RCT of 1-month duration showing decreased daily insulin requirement, reduced HbA1c, decreased body weight and increased time in range glucose in individuals with T1D using sotagliflozin.
• Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377(24):2337–48. A multicenter, double-blind, phase 3 study of 24-weeks duration showing reduction in HbA1c, body weight, and daily insulin requirement with sotagliflozin in individuals with T1D.
Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev. 2015;67(3):564–600.
Whitehouse F, Kruger DF, Fineman M, Shen L, Ruggles JA, Maggs DG, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25(4):724–30.
Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004;21(11):1204–12.
Edelman S, Garg S, Frias J, Maggs D, Wang Y, Zhang B, et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006;29(10):2189–95.
Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287(3):360–72.
Gourgari E, Wilhelm EE, Hassanzadeh H, Aroda VR, Shoulson I. A comprehensive review of the FDA-approved labels of diabetes drugs: indications, safety, and emerging cardiovascular safety data. J Diabetes Complicat. 2017;31(12):1719–27.
Strowig SM, Raskin P. The effect of rosiglitazone on overweight subjects with type 1 diabetes. Diabetes Care. 2005;28(7):1562–7.
Bhat R, Bhansali A, Bhadada S, Sialy R. Effect of pioglitazone therapy in lean type 1 diabetes mellitus. Diabetes Res Clin Pract. 2007;78(3):349–54.
Zdravkovic V, Hamilton JK, Daneman D, Cummings EA. Pioglitazone as adjunctive therapy in adolescents with type 1 diabetes. J Pediatr. 2006;149(6):845–9.
Stone ML, Walker JL, Chisholm D, Craig ME, Donaghue KC, Crock P, et al. The addition of rosiglitazone to insulin in adolescents with type 1 diabetes and poor glycaemic control: a randomized-controlled trial. Pediatr Diabetes. 2008;9(4 Pt 1):326–34.
Hollander P, Pi-Sunyer X, Coniff RF. Acarbose in the treatment of type I diabetes. Diabetes Care. 1997;20(3):248–53.
Riccardi G, Giacco R, Parillo M, Turco S, Rivellese AA, Ventura MR, et al. Efficacy and safety of acarbose in the treatment of type 1 diabetes mellitus: a placebo-controlled, double-blind, multicentre study. Diabet Med. 1999;16(3):228–32.
Nagai E, Katsuno T, Miyagawa J, Konishi K, Miuchi M, Ochi F, et al. Effects of miglitol in combination with intensive insulin therapy on blood glucose control with special reference to incretin responses in type 1 diabetes mellitus. Endocr J. 2011;58(10):869–77.
Acknowledgments
The authors thank Dr. B. Lee Ligon of the Department of Pediatrics Center for Research, Innovation and Scholarship, Baylor College of Medicine, for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Tosur, M., Redondo, M.J. & Lyons, S.K. Adjuvant Pharmacotherapies to Insulin for the Treatment of Type 1 Diabetes. Curr Diab Rep 18, 79 (2018). https://doi.org/10.1007/s11892-018-1041-1
Published:
DOI: https://doi.org/10.1007/s11892-018-1041-1