Abstract
Purpose of Review
Guidelines for a standard second diabetes medication for the treatment of type 2 diabetes (T2DM) have yet to be established. The rapid increase in the number of newer therapies available makes the choice more difficult. Thus, we reviewed clinical trial evidence evaluating newer therapies available for treatment intensification beyond monotherapy.
Recent Findings
Head-to-head studies comparing newer therapies versus traditional (i.e., sulfonylurea) approaches consistently find lower incidence of hypoglycemia and weight gain with newer therapies. Glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose co-transporter 2 (SGLT2) inhibitors demonstrate high glycemic efficacy, while merits of dipeptidyl peptidase-4 (DPP-4) inhibitors include their tolerability. Secondary effects (weight loss, cardiovascular outcomes, renal function) are of growing interest with newer therapies.
Summary
Choices for treatment intensification in T2DM diabetes are numerous. Understanding the comparative evidence of newer treatment choices, as provided in this review, may help guide clinical decision making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
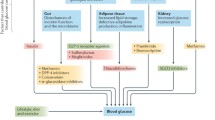
The last decade has seen an exponential increase in the number of new therapies available to treat type 2 diabetes (T2DM) (Fig. 1). Where do newer therapies fit in the treatment of T2DM? What possible advantages do they offer compared to “older” therapies once treatment intensification beyond monotherapy is required?
To appreciate the context of these contemporary questions, it is important to recognize the significant progress made with traditional therapies, as well as gaps in care highlighted through their detailed study. To start, the seminal UK Prospective Diabetes Study (UKPDS) demonstrated that intensive glycemic control with pharmacotherapy (sulfonylurea, insulin, or metformin), aiming for a fasting glucose ≤108 mg/dl, in newly diagnosed patients with type 2 diabetes prevents diabetic complications and their associated morbidity and mortality compared to dietary modification alone [1,2,3,4,5]. Even though the glycemic differential between the two treatment groups was lost in the post-trial period, long-term morbidity and mortality benefits of early intensive therapy in the UKPDS persisted [5].
Subsequent intensive glycemic control studies later in the spectrum of diabetes (VADT, ACCORD, ADVANCE) [6,7,8,9,10], called to attention several limitations in existing therapies (sulfonylureas, thiazolidinediones, insulin), which may have impacted potential benefit. These limitations included the following:
-
(a)
Despite an unlimited toolbox of therapies, including insulin in ACCORD and VADT, mean A1c treatment goals were not achieved in these studies;
-
(b)
Intensification efforts using the available therapies was associated with an unacceptable level of weight gain. In the ACCORD study, for example, 28% of subjects in the intensive treatment group gained more than 10 kg [8].
-
(c)
Intensive treatment was associated with significantly greater hypoglycemia, with hypoglycemia requiring medical assistance occurring three times and two times more often in the intensive treatment groups in ACCORD [8] and the VADT [6], respectively, compared to the standard treatment arms.
While microvascular benefit has been appreciated with treatment intensification, initial macrovascular benefit has not been established, begetting the question of whether the approaches used for treatment intensification (i.e., traditional therapies), with the above limitations, influence the potential for short- and long-term macrovascular benefit.
Hence, achieving glycemic goals with efficacious approaches safely—i.e., without hypoglycemia, weight gain, or other untoward effects—has been a prominent goal with newer therapies. This review will focus on the major classes of newer therapies of the past decade, namely glucagon-like peptide-1 receptor agonists (GLP-1 RAs), dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose co-transporter 2 (SGLT2) inhibitors, summarizing their evidence on efficacy, safety, tolerability, and a new era of outcomes data.
To focus the scope of this review, the authors reviewed trials in T2DM published in clinicaltrials.gov as of August 10, 2016 meeting the following criteria: published phase 3 or phase 4 prospective, randomized studies of current FDA-approved pharmacotherapy for the treatment of T2DM representing the aforementioned classes, USA as a site of study, and study population representing treatment intensification beyond monotherapy.
GLP-1 Receptor Agonists
Introduction and Mechanism of Action
Glucagon-like peptide-1 (GLP-1) is secreted from the small intestine in response to nutrient entry which then causes glucose-medicated insulin synthesis and secretion. There is noted slowed gastric emptying and an effect on the satiety center in the brain which results in decreased caloric intake. Post-meal glucagon secretion is also decreased [11, 12]. GLP-1 RAs have been created in subcutaneous form to prolong and increase these effects for the treatment of T2DM [13]. GLP-1RAs were first approved for use in the USA in 2005. GLP-1 RAs affect a decrease in hemoglobin A1c (HbA1c) in a glucose-dependent manner, and thus have comparatively low risk for hypoglycemia, and result in weight loss and a decrease in appetite. There are currently six GLP-RAs available in the USA (Fig. 1): exenatide twice daily, liraglutide, exenatide once weekly, albiglutide once weekly, dulaglutide once weekly, and lixisenatide. Each of these preparations has varying effects in duration of action, efficacy, and side effect profiles.
Clinical Efficacy of GLP-1 RAs in Treatment Intensification Beyond Monotherapy
GLP-1 RAs are effective at glucose lowering as monotherapy and as add-on to oral diabetes medications and insulin. Exenatide twice daily was the first GLP-1RA to be approved for use. In 30-week studies, three trials showed a mean A1c lowering of 0.78 to 0.86% with exenatide 10 μg twice daily compared to placebo, sulfonylurea, metformin, or combination of sulfonylurea/metformin. Weight loss was noted to be 1.6 to 2.8 kg [14,15,16]. Two hundred seventeen patients from these studies continued exenatide treatment for ≥3 years and showed sustained lowering of A1c of −1.0 ± 0.1% [17]. Weight loss was progressive over the 3 years with a drop in weight by mean 5.3 kg. Clinical limitations with this exenatide preparation are the twice daily subcutaneous dosing around meals and short half-life.
Liraglutide was approved for use 5 years later, offering a once daily option for GLP-1 RA therapy. A 26-week study comparing liraglutide to exenatide twice daily in patients on metformin and/or sulfonylurea highlighted the differences between short- vs long-acting GLP-1 RA. A1c lowering was 0.33% more with liraglutide, with greater reductions in FPG, while exenatide had greater reductions in postprandial glucose. Nausea was less persistent with liraglutide and weight loss was similar between the two [18].
Approved in 2012, exenatide once weekly has been compared with exenatide twice daily (DURATION-1 [19] and DURATION-5 [20]), other oral anti-hyperglycemic medications (sitagliptin, pioglitazone: DURATION-2 [21]), insulin glargine (DURATION-3 [22]), or daily liraglutide (DURATION-6 [23•]) for treatment intensification. In these studies, there was a −1.3 to −1.9% decrease in hemoglobin A1c with exenatide once weekly, significantly more than with exenatide twice daily [19, 20], other orals, or basal insulin [22]. A1c reduction was less than with liraglutide [23•], although once weekly exenatide had fewer gastrointestinal side effects than liraglutide. Weight loss was in the range of −2.3 to −3.7 kg in these studies [19,20,21,22, 23•].
Once weekly albiglutide and dulaglutide have also been compared with daily liraglutide. In the HARMONY 7 study [24], liraglutide reduced hemoglobin A1c more than albiglutide at 32 weeks by 0.21% at 32 weeks(0.99% versus 0.78; 95% CI 0.08–0.34; non-inferiority p value = 0.0846). There were more gastrointestinal side effects with liraglutide; however, weight loss was more significant in this group [24]. In a head-to-head study comparing the efficacy and safety of once weekly dulaglutide with liraglutide (AWARD-6), patients on metformin with suboptimal glycemic control had the addition of either GLP-1 RA to their regimen. Least-squares mean reduction in HbA1c was −1.42% (SE 0.05) in the dulaglutide group and −1.36% (0.05) in the liraglutide group, confirming non-inferiority between the two [25•].
Compared to DPP-4 inhibitors (see “DPP-4 Inhibitors” section below), GLP-1 RA have greater efficacy. In a 52-week study comparing the efficacy and safety of dulaglutide to sitagliptin (AWARD-5), for example, both dulaglutide doses lowered hemoglobin A1c more effectively than sitagliptin (dulaglutide 1.5 mg, −1.10%; dulaglutide 0.75 mg, −0.87%; sitagliptin, −0.39%) [26•].
Lixisenatide phase 3 studies (GLP-1 agonist AVE0010 in paTients with type 2 diabetes mellitus for Glycemic cOntrol and safety evaLuation [GetGoal] program) comprised of 11 studies. In the GetGoal with Metformin (GetGoal-M) study, 680 patients with type 2 diabetes on metformin were randomized to morning or evening doses of lixisenatide or placebo. Whether lixisenatide was provided before the morning or evening meal, there was a significant decrease in HbA1c (−0.9 and −0.8%, respectively) compared to placebo (−0.4%) over 24 weeks. Lixisenatide had a pronounced effect on postprandial glucose, with a significant 2-h post prandial glucose difference of ∼−81 mg/dl in the morning compared to placebo. Decrease in weight was similar in all groups. The main adverse events were gastrointestinal related, with nausea and vomiting occurring more frequently with lixisenatide [27, 28•].
Safety and Tolerability
The most common adverse effects with GLP-1 RAs are nausea, vomiting, and diarrhea [29]. However, this has been noted to decrease over time with continued use. In initial rodent studies with liraglutide, increased risk of thyroid C-cell focal hyperplasia, C-cell tumors, and malignant C-cell carcinomas was noted, but this has not been seen in humans. Post-marketing reports to the FDA raised a concern of a possible risk of pancreatitis with GLP-1 RAs. However, the low number of events in the clinical development programs of GLP-1 RAs, and the already increased risk of pancreatitis with T2DM, have made it difficult to establish causality [30, 31••]. Further, long-term prospective outcomes studies (Liraglutide and Cardiovascular Outcomes in Type 2 diabetes (LEADER)) [32••] have not demonstrated an increase in pancreatitis. Nevertheless, patients who initiate GLP-1 RA therapy should be instructed that severe nausea and vomiting, which is persistent and possibly accompanied by abdominal pain, should prompt discontinuation of the medication and further investigation with the clinician [30].
Cardiovascular Outcomes Studies with GLP-1 RAs
There have been few studies evaluating the cardiovascular outcomes of GLP-1 RAs. In the Evaluation of LIXisenatide in Acute coronary syndrome (ELIXA) study, 6068 patients with type 2 diabetes and acute coronary syndrome (myocardial infarction or hospitalization for unstable angina within previous 180 days) were randomly assigned to lixisenatide or placebo. There was no significant change in rate of major cardiovascular events with the addition of lixisenatide, nor were there differences in heart failure and death from any cause between both groups [33•].
The LEADER trial is the first cardiovascular outcomes trial to show long-term cardiovascular benefit with a GLP-1 RA. LEADER started in 2010 to assess the cardiovascular effect of liraglutide in patients with type 2 diabetes with A1c ≥7% with cardiovascular risk as defined by age ≥50 years with at least one cardiovascular condition or 60 years of age or older with a cardiovascular risk factor. In this randomized double-blind trial, patients received liraglutide or placebo in addition to standard of care. The primary composite outcome was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (3-point MACE). Target hemoglobin A1c level was ≤7% (or per investigator discretion) after randomization and the addition of anti-hyperglycemic agents besides GLP-1 RAs, DPP-4 inhibitors, or pramlintide was allowed. The median follow-up was 3.8 years. A significantly lower number of patients taking liraglutide (608 of 4668 [13%]) had primary composite outcome occur in comparison to patients taking placebo (694 of 4672 [14.9%] (hazard ratio, 0.87; 95% confidence interval [CI] 0.78 to 0.97; p < 0.001 for non-inferiority; p = 0.01 for superiority)). There were significantly lower number of deaths from cardiovascular causes in the liraglutide group (219 participants [4.7%]) than in the placebo group (278 [6.0%]) (hazard ratio, 0.78; 95% CI, 0.66 to 0.93; p = 0.007). There were no significant differences in hospitalization for heart failure or stroke. Of note, participants in the liraglutide group had less add-on anti-hyperglycemic medications, lipid-lowering therapies, and diuretics. Over the 36 months, there was significant weight loss of 2.3 kg more in the liraglutide group. Heart rate was also higher at 3.0 beats per minute in the liraglutide group [32••].
Differences of the cardiovascular benefit with the LEADER trial compared to the EMPA-REG OUTCOME [34••] trial (discussed in SGLT2 inhibitor section below) have been noted. The time to benefit was earlier with empagliflozin than with liraglutide, with thoughts that observed benefits in EMPA-REG may be related to hemodynamic changes, whereas benefits in LEADER may represent modified progression of atherosclerotic cardiovascular disease [32••].
In the pipeline is semaglutide, a once weekly GLP-1 RA not yet approved for use, however now noted to be the second GLP-1 RA to show cardiovascular benefit in patients with T2DM at high risk for cardiovascular disease [35•].
Clinical Recommendations with GLP-1 RAs
Current diabetes guidelines, the European Association for the Study of Diabetes (EASD)/American Diabetes Association (ADA), and American Association of Clinical Endocrinologists (AACE) emphasize the essential goal of achieving glycemic control and improvement in clinical outcomes for patients with the least amount of adverse effects as possible. GLP-1RAs have been recommended as second or third line agents after metformin or other oral agents/basal insulin by ADA/EASD [36••]. AACE recommendations include GLP-1 RAs as early as monotherapy, or as part or dual or triple therapy, or as add-on to basal insulin [37••]. GLP-1 RAs have been attractive because of the decreased risk of hypoglycemia (when not in combination with sulfonylureas and insulin) and weight loss versus the weight gain seen with other pharmacologic options, i.e., insulin or sulfonylureas. In addition, results of LEADER now suggest possible benefit of liraglutide in patients with a history of established cardiovascular disease. Decisions for when GLP-RA therapy is started for a patient with T2DM may be limited by several factors, however. These include cost, patient preference (especially with the decision for an oral vs injectable medication), and initial gastrointestinal side effects, such as nausea, vomiting, and diarrhea.
DPP-4 Inhibitors
Introduction and Mechanism of Action
DPP-4 inhibitors, represented in the USA by sitagliptin, saxagliptin, alogliptin, and linagliptin, inhibit DPP-4 degradation of active GLP-1, thereby enhancing and prolonging the action of endogenously released GLP-1 [38, 39•]. DPP-4 inhibitors efficiently inhibit plasma DPP-4 activity for a prolonged period of time, allowing once daily oral dosing [38]. Like GLP-1 RA, DPP-4 inhibitors have a glucose-dependent effect on insulin secretion, limiting potential for hypoglycemia, and reduce glucagon levels. In contrast to GLP-1 RA, DPP-4 inhibitors do not appear to affect gastric emptying, and are weight neutral overall [38].
Clinical Efficacy of DPP-4 inhibitors in Treatment Intensification Beyond Monotherapy
A detailed review of phase 3 and phase 4 prospective, randomized studies involving DPP-4 inhibitors as a treatment choice for intensification beyond monotherapy is provided in Supplemental Table 1. On a background of metformin, the different DPP-4 inhibitors have comparable HbA1c reducing effects, ranging from ∼0.6 to 0.9% (Supplemental Table 1). Higher A1c reductions of ∼1.00–1.1% are seen in studies that start off at a higher baseline level (i.e., 8.5–9.0%, Supplemental Table 1) [40,41,42,43]. In general, on a background of metformin therapy, intensification with DPP-4 inhibitors is well tolerated, without an increase in hypoglycemia or weight gain (Supplemental Table 1). In contrast, hypoglycemia is increased when used on a background of sulfonylreas, likely attributable to the presence of sulfonyulreas [44,45,46,47].
Randomized controlled trials juxtaposing DPP-4 inhibitors with other traditional (e.g., sulfonylureas) and newer (e.g., GLP-1 RA, insulin) options for treatment intensification are also of interest. Several 2-year studies comparing DPP-4 inhibitors and sulfonylureas in patients on metformin have been conducted, though conclusions are limited based on relatively low number of completers in these studies. For example, a 2-year study comparing sitagliptin 100 mg daily with glipizide demonstrated comparable A1c reductions of −0.54 and −0.51%, respectively, though this per-protocol analysis reported only on 43% of the initial population [48]. Another 2-year study comparing alogliptin (12.5 or 25 mg) to sulfonylurea (glipizide) in patients on metformin showed non-inferiority in A1c reduction between alogliptin and glipizide, with superior reduction at the higher dose of 25 mg alogliptin (though a modest final dose of glipizide of 5.2 mg). Hypoglycemia was markedly higher with glipizide (23.2%) compared with alogliptin (1–3%) [49]. Finally, a 2-year study comparing linagliptin with glimepride added to metformin showed statistical non-inferiority in A1c change (−0.16%, linagliptin, versus −0.36% with glimepiride, difference 0.20, 97.5% CI 0.09–0.30). Though these are numerically small long-term glycemic changes, linagliptin was associated with less hypoglycemia and no weight gain compared to glimepiride [50].
Compared to GLP-1 RAs, DPP-4 inhibitors have more modest glycemic efficacy, less weight loss, though appear to be better tolerated with fewer gastrointestinal side effects. In a head-to-head study of sitagliptin with liraglutide in patients requiring intensification after metformin monotherapy, A1c reductions at 26 weeks were significantly greater with either liraglutide 1.2 or 1.8 mg compared to sitagliptin (−1.24, −1.50, and −0.90%, respectively), as were FPG reductions. Weight loss was also greater with liraglutide 1.2 or 1.8 mg than with sitagliptin, with estimated mean differences of −1.90 kg (95% CI −2.61 to −1.18) and −2.42 kg (95% CI −3.14 to −1.70) versus sitagliptin. Nausea and other gastrointestinal events were greater with liraglutide, as well as heart rate, consistent with known profiles of the class. Hypoglycemia was low and comparable [51]. Similarly, as described above, in the DURATION-2 study, A1c lowering with sitagliptin was significantly lower than with exenatide once weekly over 26 weeks. Weight loss was also less with sitagliptin, while hypoglycemia was comparable [21].
Longer term studies demonstrate similar findings of modest efficacy of DPP-4 relative to GLP-1 RA, though overall better tolerability. A 2-year study comparing albiglutide (once-weekly GLP-1 RA) with glimepiride or sitagliptin or placebo, which reported a 67% completion rate, showed a −0.28% reduction in A1c with sitagliptin, significantly lower than the −0.63% seen with albiglutide [52]. Similarly, 104-week follow-up from AWARD-5, comparing dulaglutide with sitagliptin, showed a greater reduction in A1c with dulaglutide 1.5 mg (−0.99%) or 0.75 mg (−0.71%), compared to sitagliptin (−0.32%), greater weight loss with dulaglutide 1.5 mg (−2.88 kg) versus sitagliptin (−1.75 kg), with higher incidence of gastrointestinal events with dulaglutide [53].
DPP-4 inhibitors have also been compared head-to-head with basal insulin for treatment intensification in patients on metformin. In a 24-week study comparing sitagliptin with insulin glargine in patients with a mean baseline A1c of 8.5%, A1c reduction with sitagliptin was 1.13% compared to 1.72% with insulin glargine [41], and in a 26-week study comparing sitagliptin with insulin degludec, A1c reduction with sitagliptin was 1.09% compared with 1.52% with insulin degludec [42]. While A1c reductions were significantly greater with basal insulin in both studies, hypoglycemia and weight gain were also greater with basal insulin, again suggesting a comparative advantage in tolerability with DPP-4 inhibitors despite modest efficacy compared to more potent glucose-lowering strategies.
Studies also suggest a potential role for DPP-4 inhibitors in treatment intensification in populations that may be prone to hypoglycemia, including those with chronic kidney disease and the elderly. In a 26-week study comparing albiglutide to sitagliptin in renally impaired patients with T2DM, A1c reduction with sitagliptin (dose range 25–100 mg daily, depending on renal function) was −0.52%, significantly lower than the −0.83% achieved with albiglutide, as was weight loss, but better tolerated with fewer gastrointestinal adverse events than with albiglutide [54]. In a study comparing linagliptin to placebo for 12 weeks, followed by glimepiride for 40 weeks in patients with an estimated GFR <60 ml/min/1.73 m2, linagliptin was efficacious and well tolerated, with overall less hypoglycemia than glimepride [55]. Finally, a 52-week study comparing alogliptin to glipizide in elderly patients with T2DM showed comparable A1c reductions, with substantially lower hypoglycemia with alogliptin (5 vs 26%) [56].
Given their comparative advantage in tolerability and modest efficacy, there has been growing interest in the use of DPP-4 inhibitors in combination therapy. Saxagliptin has been studied in combination with dapagliflozin [57•], alogliptin in combination with pioglitazone [58], and linagliptin in combination with empagliflozin [59] in treatment intensification strategies after metformin, with combination therapy showing greater reductions in A1c, and greater likelihood of achieving target A1c goals than either therapy alone (Supplemental Table 1). Several combination drugs including DPP-4 inhibitors are now available (Fig. 1).
Cardiovascular Outcomes Studies with DPP-4 Inhibitors
Cardiovascular outcome studies with DPP-4 inhibitors confirm long-term safety and tolerability with this class. In a trial of over 14,000 patients with established cardiovascular disease (TECOS), there were no differences in cardiovascular outcomes between sitagliptin and placebo/standard of care. There were no remarkable long-term (3-year) safety signals, and differences in pancreatic events or heart failure hospitalization were absent [60•]. Similarly, SAVOR-TIMI 53 compared saxagliptin to placebo/standard of care in patients with a history of or risk factors for cardiovascular disease showed no difference in the primary composite of cardiovascular death, myocardial infarction, or ischemic stroke. Additional analyses of individual endpoints noted greater hospitalizations for heart failure (3.5 vs 2.8%, hazard ratio 1.27, 95% CI 1.07–1.51, p = 0.007) with saxagliptin, an unexpected finding that, within the context of multiple testing, warrants further study [61•]. An 18-month trial of alogliptin versus placebo in patients with recent acute coronary syndrome (EXAMINE) showed no differences in composite cardiovascular outcomes, nor any differences in hospital admission for heart failure [62•, 63]. In sum, these prospective randomized cardiovascular outcomes studies have confirmed long-term safety and tolerability for this class of agents.
SGLT2 Inhibitors
Introduction and Mechanism of Action
SGLT2 inhibitors are a novel class of antidiabetic drugs currently represented by canagliflozin, dapagliflozin, and empagliflozin. Unlike other anti-hyperglycemic drugs, SGLT2 inhibitors act in an insulin-independent fashion, helping to lower blood glucose levels by lowering the threshold at which the kidneys remove glucose from the blood [64, 65]. In the kidney, both SGLT1 and SGLT2 act in the proximal convoluted tubule to maintain glucose homeostasis. SGLT1 has a high affinity but low capacity for glucose transportation, while SGLT2 has the opposite properties, with a high capacity and low affinity for glucose. This trait contributes to SGLT2 being responsible for ∼90% of glucose reabsorption by the kidney. In a normal state, the kidneys will reabsorb nearly all glucose in the blood, resulting in negligible glucose in the urine [66, 67]. This holds true for up to approximately 180 mg/dl of circulating glucose; upon exceeding this threshold, excess glucose will spill into the urine, which is commonplace in uncontrolled diabetes [68]. SGLT2 inhibitors take advantage of this, preventing the reabsorption of circulating glucose, causing it to be expelled in the urine, thereby lowering circulating blood glucose.
Clinical Efficacy of SGLT2 Inhibitors in Treatment Intensification Beyond Monotherapy
Several randomized clinical trials have demonstrated the comparative efficacy of SGLT2 inhibitors as add-on therapy (Supplemental Table 2). In patients inadequately controlled with metformin, canagliflozin (CANA) 100 mg daily (CANA100) and 300 mg daily (CANA300) demonstrated A1c reductions ranging from 0.73–0.82 to 0.88–0.94%, respectively [69,70,71]. In addition to A1c control, canagliflozin has shown secondary improvements in blood pressure and weight. When added to metformin, CANA100 and CANA 300 decreased mean adjusted systolic blood pressure by 3.30–3.84 mmHg and 4.60–5.06 mmHg, respectively, and also significantly improved diastolic blood pressure [69,70,71,72]. These trials also showed both CANA100 and CANA300 to reduce participant body weight by a mean adjusted 3.3–3.7 and 3.7–4.2%, respectively. When compared to sitagliptin and glimepiride, CANA100 proved non-inferior and CANA300 proved superior in terms of A1c reduction. Secondary benefits discussed above held when compared to both sitagliptin and glimepiride, showing superior blood pressure and weight control [69,70,71].
When studied as an add-on to metformin or sitagliptin, dapagliflozin (DAPA) at doses of 10 mg daily (DAPA10) showed mean adjusted A1c reduction of 0.80–1.2% [57•, 73, 74]. Consistent with other members of the class, DAPA also demonstrated similar secondary benefits of blood pressure and weight reduction. DAPA10 showed mean adjusted reductions of systolic blood pressure of 1.8–4.1 mmHg [73, 74]. Diastolic blood pressure changes were minimal or not reported in the trials examined [57•, 73, 74]. Body weight was reduced by a mean adjusted 2.9–3.6% in patients taking DAPA10 and background metformin therapy [57•, 74].
Empagliflozin has shown similar efficacy when added to metformin monotherapy (Supplemental Table 2), with mean adjusted A1c reductions of 0.61–0.70 and 0.62–0.77% for empagliflozin 10 mg daily (EMPA10) and empagliflozin 25 mg daily (EMPA25), respectively [59, 75, 76]. EMPA10 and EMPA25 also reduced mean adjusted systolic blood pressure 3.3–4.5 and 2.8–5.2 mmHg, respectively, and mean diastolic blood pressure by 0.9–2.0 and 1.6–2.0 mmHg, respectively. EMPA10 and EMPA25 also resulted in weight loss, with adjusted mean decreases of 2.6–3.4 and 3.0–4.5% for EMPA10 and EMPA25, respectively [59, 75, 76]. When compared to either sitagliptin or linagliptin, EMPA10 and EMPA25 proved non-inferior in glycemic efficacy, though had greater reductions in blood pressure control and weight compared to either DPP-4 inhibitor [59, 76].
Cardiovascular Outcomes Studies, SGLT-2 Inhibitors
The EMPA-REG trial investigated the effects of empagliflozin compared to placebo in patients with established cardiovascular disease. Those with background anti-hyperglycemic medications had an A1c between 7.0 and 10.0%, inclusive; those with no background anti-hyperglycemic medications had an A1c between 7.0 and 9.0%, inclusive. The composite primary outcome was death from cardiovascular causes, nonfatal myocardial infarction (excluding silent myocardial infarction), or nonfatal stroke. Key secondary outcome measure was the composite primary outcome plus hospitalization for unstable angina. EMPA-REG showed that treatment with empagliflozin improved cardiac outcomes, reducing relative risk from death from all cardiovascular causes by 38%, hospitalization for heart failure by 35% and death from any cause by 32% when compared to placebo. However, no significant difference was seen between empagliflozin and placebo when comparing rates of myocardial infarction, stroke, or hospitalization for unstable angina. Though the mechanisms are not known, effects are likely multifaceted. Speculated mechanisms include changes in cardiac and renal function, hemodynamic status, reduction of albuminuria and uric acid, with possible contribution of class benefits such as reduction of hyperglycemia, weight, blood pressure, and visceral adiposity [34••, 77]. Results from the CANVAS and DECLARE trials are awaited to determine whether these effects are class-wide or drug-specific.
Safety and Tolerability
Although SGLT2 inhibitors have unlocked another physiologic approach to treating diabetes, the full extent of their effects, including both beneficial nonglycemic effects and adverse effects, is yet to be appreciated. Several safety considerations have emerged in their clinical study, as summarized in their clinical labels [78•, 79•, 80•, 81•], including hypotension/hypovolemia, ketoacidosis, acute kidney injury, and impairment in renal function, urinary tract infections (UTIs), urosepsis and pyelonephritis, genital mycotic infections, increased LDL-cholesterol, bladder cancer imbalance, hyperkalemia (canagliflozin), and bone fracture (canagliflozin). Clinical trial evidence surrounding select issues is discussed here.
Explicable by their primary glucose-lowering method of action, UTIs, and genital mycotic infections were seen at variable rates across several studies, both compared to placebo and versus active comparators, in all representatives of the class. Compared to placebo rates of 2.0–18.1%, UTI rates for SGLT2 inhibitors ranged from 3.5 to 18.0% (Supplemental Table 2). In trials with active comparators, rates of UTIs ranged from 3.9 to 12.7% for SGLT2 inhibitors, compared to a range of 5.0–12.5% for active comparators (Supplemental Table 2). A consistent increase in genital mycotic infections across the trials is seen, with an incidence of 1.7–14.6% with SGLT2 inhibitors compared to 0.0–5.1% in placebo, and in active comparator studies, 2.4–14.0% for SGLT2 inhibitors compared to 0.6–2.6% with active comparators. It should be noted that rates of mycotic infections across the class are significantly lower in men than in women [34,70,71,72,73,83,84,••, 57•, 59, 69–74, 82–85].
Bone fractures have emerged as a topic of recent interest for SGLT2 inhibitors, with several trials specifically investigating rates at which patients have fractures while on SGLT2 inhibitors compared to placebo or other OADs. SGLT2 inhibitors have been shown to both increase serum phosphate levels as well as affect the expression of parathyroid hormone (PTH), which may enhance fracture risk. The exact mechanism by which SGLT2 inhibitors may deplete bone density is not well understood; however, it is possible that increased phosphate reabsorption, occurring in the proximal tubule of the kidney, affects the action of PTH [86•]. In a pooled analysis of several canagliflozin trials and the CANVAS trial, canagliflozin was associated with a significant increase in fracture rate. This analysis was stratified by high risk for or pre-existing cardiovascular disease. Those patients in the cardiovascular group (n = 4327) had significantly increased risk of bone fracture when compared to placebo, with an incidence rate of 3.9, 4.0, and 2.6% for CANA100, CANA300, and placebo, respectively. In the analysis of the non-cardiovascular patients (n = 5867), fracture rates were lower overall and the difference between canagliflozin and placebo was minimal, with incidences of fracture at 1.6, 1.8, and 1.5% for CANA100, CANA300, and placebo, respectively [87]. In the EMPA-REG trial, bone fractures were comparable between the pooled empagliflozin group (3.8%) and placebo group (3.9%) [34••]. Further studies are needed to understand the relationship between SGLT2 inhibitors and fracture risk, and whether any potential effect is a class vs. drug effect.
Several studies have also shown a modest increase in low-density lipoprotein (LDL) cholesterol (∼3.6–7.3 mg/dl) [69, 70, 73, 74, 83]. Increases in HDL have also been noted, on the order of ∼3–8 mg/dl [69, 70, 73, 74]. These effects on LDL and HDL have also been demonstrated in comparison to both sitagliptin and glimepiride [69, 70, 73]. The reasons for alterations in lipid profiles and clinical implications of these observations are not fully understood and warrant further study.
Effects on Renal Function
Several studies have been conducted to determine the safety of SGLT2 inhibitors in patients with reduced renal function. Effectiveness in reduction of A1c declines in parallel with the reduction in renal function, limiting the clinical usefulness of the class in those with decreased renal function. Dapagliflozin is not indicated for patients with eGFR <60 ml/min/1.73 m2 and canagliflozin and empagliflozin are not recommended for eGFR <45 ml/min/1.73 m2 [72, 78•, 79•, 80•, 81•, 82, 88, 89].
Short- and long-term effects on renal function are of high interest. Based on post-marketing reports, the FDA has recently strengthened warnings of the possibility of acute kidney injury for canagliflozin and dapagliflozin [80•]. Yet, in a 2-year study, addition of CANA100 or CANA300 to background metformin therapy was shown to slow the annual rate of renal decline (eGFR) compared to addition of glimepiride. Additionally, in patients with baseline urinary albumin/creatinine ≥30 mg/g, urinary albumin/creatinine decreased more with CANA100 or CANA300 compared to glimepiride [88]. Though, in a smaller 2-year study in patients with moderate renal impairment, there were no long-term differences in eGFR with DAPA5, DAPA10 compared with placebo (−1.71, −3.50, −2.38 ml/min/1.73 m2, respectively). Interestingly, those on dapagliflozin saw an early decrease in eGFR at 1 week, followed by longer term stability, whereas those on placebo saw a relatively steady decline throughout. Further, those on dapagliflozin were more likely to regress to a lower urine albumin excretion category than patients receiving placebo [82].
In the EMPA-REG trial in patients with established cardiovascular disease and eGFR of at least 30 ml/min/1.73 m2, empagliflozin was associated with slower progression of kidney disease (lower incident or worsening nephropathy, reduced doubling of serum creatinine, and reduced renal replacement therapy requirement). The initial drop in eGFR seen with empagliflozin was entirely reversible after study drug discontinuation [89].
Conclusions
The choices for treatment intensification beyond monotherapy in T2DM are vast, and are continuing to grow. We now have an array of glucose-lowering therapies that do not carry the inherent risk of hypoglycemia and weight gain highlighted in previous intensification studies with older therapies. We have also entered another era of outcomes studies with these newer agents, where we are able to test whether newer glucose-lowering strategies compared to traditional approaches have benefits beyond glucose lowering, including microvascular and macrovascular benefit. These newer tools open up a whole new set of questions: How does longer term durability compare among the classes? Does earlier intervention with medications that sustain control glucose and have additional nonglycemic benefits (e.g., weight, blood pressure) impact long-term morbidity and mortality? Now that several cardiovascular outcomes studies have confirmed that glucose-lowering approaches do matter in high-risk patients, do the same benefits extend to patients earlier in the course of their disease? What is the mechanism of benefit seen in the recent cardiovascular outcomes studies—are the positive outcomes in these studies due to direct benefit of the intervention or do they rather reflect risks associated with older treatment approaches used in the placebo/standard of care arms? Advances in therapeutics will continue to allow us to address these, and many more, questions for the treatment of T2DM and its long-term impact.
Additional Data
Additional data relevant to this review [Ref 90–108] is summarized in the Supplement.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865.
UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia. 1991;34:877–90.
United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83–8.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–206.
Gerstein HC, Miller ME, Byington RP, Goff Jr DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff Jr DC, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28.
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–42.
Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35.
DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100.
Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91.
Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–86.
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39–47.
Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–50.
Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–10.
Bergenstal RM, Wysham C, MacConell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–9.
Diamant M, Van GL, Stranks S, Northrup J, Cao D, Taylor K, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–43.
• Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–24. This study compares short vs longer-acting GLP-1 RA in T2DM.
Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289–97.
• Dungan KM, Povedano ST, Forst T, Gonzalez JG, Atisso C, Sealls W, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–57. This study compares once weekly GLP-1RA vs daily GLP-1RA in metformin-treated patients with T2DM.
• Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37:2149–58. This study compares once weekly GLP-1RA vs DPP-4 inhibitor in T2DM.
Ahren B, Leguizamo DA, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care. 2013;36:2543–50.
• Bain SC. The clinical development program of lixisenatide: a once-daily glucagon-like peptide-1 receptor agonist. Diabetes Ther. 2014;5:367–83. This summarizes the lixisenatide phase 3 program.
Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206.
Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362:774–7.
•• Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med. 2014;370:794–7. This provides a detailed review of evidence and recommendations surrounding risk of pancreatitis with GLP-1RA.
•• Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. This is the first positive cardiovascular outcomes study with GLP-1 RA.
• Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. This study shows cardiovascular safety with a GLP-1RA in patients with recent acute coronary syndrome.
•• Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. This is the first positive cardiovascular outcomes trial, showing reduction in cardiovascular outcomes, cardiovascular death, and death from any cause with empagliflozin in the treatment of T2DM with established cardiovascular disease.
• Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016. This is a positive cardiovascular outcomes study with a once weekly investigational GLP-1 RA.
•• Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–42. This summarizes the ADA/EASD guidance on treatment of T2DM.
•• Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract. 2016;22:84–113. This provides a review of AACE guidance for the treatment of T2DM.
Ahren B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007;30:1344–50.
• Aroda VR, Henry RR, Han J, Huang W, DeYoung MB, Darsow T, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247–58. This is a systematic review and meta-analysis of efficacy of GLP-1 receptor agonists and DPP-4 inhibitors.
Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008;24:537–50.
Aschner P, Chan J, Owens DR, Picard S, Wang E, Dain MP, et al. Insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet. 2012;379:2262–9.
Philis-Tsimikas A, Del PS, Satman I, Bhargava A, Dharmalingam M, Skjoth TV, et al. Effect of insulin degludec versus sitagliptin in patients with type 2 diabetes uncontrolled on oral antidiabetic agents. Diabetes Obes Metab. 2013;15:760–6.
Henry RR, Staels B, Fonseca VA, Chou MZ, Teng R, Golm GT, et al. Efficacy and safety of initial combination treatment with sitagliptin and pioglitazone—a factorial study. Diabetes Obes Metab. 2014;16:223–30.
Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395–406.
Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167–76.
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–45.
Chacra AR, Tan GH, Ravichandran S, List J, Chen R. Safety and efficacy of saxagliptin in combination with submaximal sulphonylurea versus up-titrated sulphonylurea over 76 weeks. Diab Vasc Dis Res. 2011;8:150–9.
Seck T, Nauck M, Sheng D, Sunga S, Davies MJ, Stein PP, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract. 2010;64:562–76.
Del PS, Camisasca R, Wilson C, Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes Obes Metab. 2014;16:1239–46.
Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Maximilian E, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475–83.
Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56.
Ahren B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–8.
Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17:849–58.
Leiter LA, Carr MC, Stewart M, Jones-Leone A, Scott R, Yang F, et al. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care. 2014;37:2723–30.
Laakso M, Rosenstock J, Groop PH, Barnett AH, Gallwitz B, Hehnke U, et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care. 2015;38:e15–7.
Rosenstock J, Wilson C, Fleck P. Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year study. Diabetes Obes Metab. 2013;15:906–14.
• Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376–83. This study reports on combination DPP-4 inhibitor with SGLT2 inhibitor versus individual components in treatment of T2DM.
DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Efficacy and tolerability of the DPP-4 inhibitor alogliptin combined with pioglitazone, in metformin-treated patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1615–22.
DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384–93.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42. This reports on cardiovascular safety and outcomes with sitagliptin in T2DM.
• Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. This reports on cardiovascular safety and outcomes with saxagliptin in T2DM.
• White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. This reports on cardiovascular safety and outcomes with alogliptin in T2DM.
Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–76.
Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31.
List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl. 2011;S20–S27.
Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–7.
Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Investig. 1994;93:397–404.
DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36:3169–76.
Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–92.
Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–50.
Bhatia J, Gamad N, Bharti S, Arya DS. Canagliflozin-current status in the treatment of type 2 diabetes mellitus with focus on clinical trial data. World J Diabetes. 2014;5:399–406.
Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73.
Jabbour SA, Hardy E, Sugg J, Parikh S. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014;37:740–50.
Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11:43.
Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–9.
Ferrannini E, Berk A, Hantel S, Pinnetti S, Hach T, Woerle HJ, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015–21.
Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100.
• U.S. Food & Drug Administration. Highlights of prescribing information: Invokana. U.S. Food & Drug Administration. 2016. 11-2-2016. Ref Type: Electronic Citation. This reviews prescribing information for an SGLT2 inhibitor, including safety data and precautions.
• U.S. Food & Drug Administration. Highlights of prescribing information: Farxiga. U.S. Food & Drug Administration. 2016. 11-2-2016. Ref Type: Electronic Citation. This reviews prescribing information for an SGLT2 inhibitor, including safety data and precautions.
• U.S. Food & Drug Administration. Canaglilfozin (Invokana, Invokamet) and Dapagliflozin (Farxiga, Xigduo XR): Drug Safety Communication - Strengthened Kidney Warnings. U.S. Food & Drug Administration. 2016. 11-2-2016. Ref Type: Electronic Citation. This reviews recent updates in warnings and precautions with select SGLT2 inhibitors.
• U.S. Food & Drug Administration. Highlights of prescribing information: Jardiance. U.S. Food & Drug Administration . 2014. 11-22-0016. Ref Type: Electronic Citation. This reviews prescribing information for an SGLT2 inhibitor, including safety data and precautions.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71.
Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. 2015;38:1218–27.
Lewin AJ, Arvay L, Liu D, Patel S, von Maximilian E, Woerle HJ. Efficacy and tolerability of linagliptin added to a sulfonylurea regimen in patients with inadequately controlled type 2 diabetes mellitus: an 18-week, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2012;34:1909–19.
Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–8.
• Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3:8–10. This reviews possible adverse effects of SGLT2 inhibitors on bone.
Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–66.
Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2016.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Maximilian E, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–43.
Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–68.
Rigby SP, Handelsman Y, Lai YL, Abby SL, Tao B, Jones MR. Effects of colesevelam, rosiglitazone, or sitagliptin on glycemic control and lipid profile in patients with type 2 diabetes mellitus inadequately controlled by metformin monotherapy. Endocr Pract. 2010;16:53–63.
DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–52.
Berg JK, Shenouda SK, Heilmann CR, Gray AL, Holcombe JH. Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: a randomized, double-blind, crossover study. Diabetes Obes Metab. 2011;13:982–9.
Charbonnel B, Steinberg H, Eymard E, Xu L, Thakkar P, Prabhu V, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56:1503–11.
Hollander P, Raslova K, Skjoth TV, Rastam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the TRANSITION randomized controlled trial. Diabetes Obes Metab. 2011;13:268–75.
White JL, Buchanan P, Li J, Frederich R. A randomized controlled trial of the efficacy and safety of twice-daily saxagliptin plus metformin combination therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy. BMC Endocr Disord. 2014;14:17.
DeFronzo RA, Hissa MN, Garber AJ, Luiz GJ, Yuyan DR, Ravichandran S, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649–55.
Rosenstock J, Gross JL, Aguilar-Salinas C, Hissa M, Berglind N, Ravichandran S, et al. Long-term 4-year safety of saxagliptin in drug-naive and metformin-treated patients with type 2 diabetes. Diabet Med. 2013;30:1472–6.
Stenlof K, Raz I, Neutel J, Ravichandran S, Berglind N, Chen R. Saxagliptin and metformin XR combination therapy provides glycemic control over 24 hours in patients with T2DM inadequately controlled with metformin. Curr Med Res Opin. 2010;26:2355–63.
Fonseca V, Zhu T, Karyekar C, Hirshberg B. Adding saxagliptin to extended-release metformin vs. uptitrating metformin dosage. Diabetes Obe Metab. 2012;14:365–71.
Neutel JM, Zhao C, Karyekar CS. Adding saxagliptin to metformin extended release (XR) or uptitration of metformin XR: efficacy on daily glucose measures. Diabetes Ther. 2013;4:269–83.
Hollander P, Li J, Allen E, Chen R. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94:4810–9.
Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46–55.
Pratley RE, Reusch JE, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2009;25:2361–71.
Taskinen MR, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:65–74.
Thrasher J, Daniels K, Patel S, Whetteckey J, Woerle HJ. Efficacy and safety of linagliptin in black/African American patients with type 2 diabetes: a 6-month, randomized, double-blind, placebo-controlled study. Endocr Pract. 2014;20:412–20.
Barnett AH, Patel S, Harper R, Toorawa R, Thiemann S, von Maximilian E, et al. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes Metab. 2012;14:1145–54.
Acknowledgments
The authors would like to thank Sameer Desale, MS, MedStar Health Research Institute, for assistance in creating Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alexander Kuhn declares no conflict of interest.
Jean Park has served as an investigator on clinical trials (Janssen) through her employer.
Adline Ghazi declares no conflict of interest.
Vanita R. Aroda has served as an investigator on clinical trials (Astra Zeneca/BMS, Calibra, Eisai, Elcelyx, Janssen, Novo Nordisk, Sanofi, Theracos) and has served as a consultant (Adocia, Astra Zeneca, Janssen, Medscape, Novo Nordisk, Sanofi), all activities through her employer.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Diabetes and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Kuhn, A., Park, J., Ghazi, A. et al. Intensifying Treatment Beyond Monotherapy in Type 2 Diabetes Mellitus: Where Do Newer Therapies Fit?. Curr Cardiol Rep 19, 25 (2017). https://doi.org/10.1007/s11886-017-0832-3
Published:
DOI: https://doi.org/10.1007/s11886-017-0832-3