Abstract
Diet plays an important role in the development of colorectal cancer. Emerging data have implicated the gut microbiota in colorectal cancer. Diet is a major determinant for the gut microbial structure and function. Therefore, it has been hypothesized that alterations in gut microbes and their metabolites may contribute to the influence of diet on the development of colorectal cancer. We review several major dietary factors that have been linked to gut microbiota and colorectal cancer, including major dietary patterns, fiber, red meat and sulfur, and obesity. Most of the epidemiologic evidence derives from cross-sectional or short-term, highly controlled feeding studies that are limited in size. Therefore, high-quality large-scale prospective studies with dietary data collected over the life course and comprehensive gut microbial composition and function assessed well prior to neoplastic occurrence are critically needed to identify microbiome-based interventions that may complement or optimize current diet-based strategies for colorectal cancer prevention and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
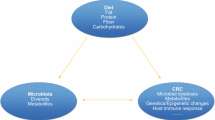
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the fourth leading cause of cancer death in the world [1]. Over the past few decades, numerous epidemiologic studies have identified a range of dietary factors that may potentially promote or prevent CRC [2 ••]. Likewise, increasing evidence has implicated the gut microbiota in CRC development (Table 1) [3,4,5,6,7,8,9,10,11,12,13,14]. Biological plausibility is supported by habitation of numerous gut microbes in the large intestine and the functional importance of the gut microbiota in maintenance of the gut barrier integrity and immune homeostasis, the disruptions of which are among the most important mechanisms in colorectal carcinogenesis [15 •]. Given the critical role of diet in the configurations of gut microbial communities and production of bacterial metabolites, it has been proposed that diet may influence CRC risk through modulation of the gut microbial composition and metabolism that in turn shape the immune response during tumor development.
Overall, the gut microbiome exhibits substantial inter-individual variation but high overall temporal stability within individuals [16,17,18,19,20,21]. Although gut bacterial abundance may respond rapidly to extreme changes in diet [22 •], predominant microbial community membership is primarily determined by long-term diet, and substantial inter-individual variation persists despite short-term dietary change [17, 23,24,25, 26 ••]. Recent data suggest that such high inter-individual variability may to a large extent determine the differences in the metabolic response to dietary intervention [27 ••], highlighting the importance for microbiome-based personalized nutrition in disease prevention and treatment [28].
Herein, we review several major dietary factors that have been linked to gut microbiota and CRC, summarizing the most recent epidemiologic and experimental evidence, with a focus on potential immune mechanisms. Overall, most of the epidemiologic evidence derives from cross-sectional or short-term, highly controlled feeding studies that are limited in size. Thus, this review focuses on the dietary factors that have strong mechanistic support, including dietary pattern, fiber, red meat and sulfur, and omega-3 fatty acid. Given the close link between diet and obesity and the predominant role of obesity in CRC as well as the substantial data linking the gut microbiome to obesity, we also include obesity at the end of the review.
Dietary Patterns
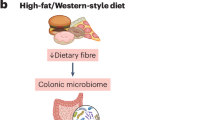
Convincing data indicate that a “Western dietary pattern,” characterized by high intake of red or processed meat, sweets, and refined grains, is associated with higher risk of colorectal neoplasia; in contrast, diets that are rich in fruits, vegetables, and whole grains (“prudent pattern diet”) are associated with lower risk of CRC [29, 30]. Western diets are associated with gut dysbiosis (microbial imbalance) [31, 32], loss of gut barrier integrity [31, 32], increased levels of inflammatory proteins [33,34,35,36,37], and dysregulated immune signatures [38,39,40]. A potential role of the gut microbiota in mediating the dietary associations with CRC risk is suggested by the dramatic difference of the gut microbial structures between populations consuming different diets. Rural Africans, whose diet is high in fiber and low in fat, have a strikingly different gut microbial composition than urban Europeans or African Americans consuming a Western diet, which parallels the lower CRC rates in Africa than Western countries [26, 41, 42]. For example, the African gut microbiota is characterized by a predominance of Prevotella genus that are involved in starch, hemicellulose, and xylan degradation, whereas the American microbiota is predominated by Bacteroides genus with a higher abundance of potentially pathogenic proteobacteria, such as Escherichia and Acinetobacter [42]. Fecal short-chain fatty acids (SCFAs) are higher in native Africans, whereas secondary bile acids are higher in African Americans. Notably, SCFAs (described in detail as follows) and secondary bile acids have been suggested to mediate the anti- and pro-cancer effect of fiber and fat on CRC, respectively. Moreover, a crossover study indicates that switching African Americans to a high-fiber, low-fat diet for 2 weeks increases production of SCFAs, suppresses secondary bile acid synthesis, and reduces colonic mucosal inflammation and proliferation biomarkers of cancer risk [26 ••].
Recently, we have shown that “prudent dietary pattern” was more strongly associated with lower risk of CRC subgroups enriched with tissue Fusobacterium nucleatum (F. nucleatum) [43], suggesting a potential role for intestinal microbiota in mediating the diet-CRC relationship. F. nucleatum is a core member of the human oral microbiome and localizes to CRC tissue through binding to a protein overexpressed in CRC [44]. Numerous studies have shown an enrichment of F. nucleatum in CRC tissue relative to normal adjacent colonic tissue and in stools from individuals with CRC compared to those without cancer [6, 9, 45,46,47,48,49,50,51]. High abundance of F. nucleatum in tumor tissue has also been associated with poor survival of CRC patients [52 •]. Experimental evidence supports that F. nucleatum may promote CRC development and worsen cancer survival by activating β-catenin pathway and potentiating tumoral immune evasion through recruitment of tumor-infiltrating myeloid cells and inhibition of natural killer (NK) cell function [53,54,55]. In support of the hypothesis that diet may influence CRC risk by modulating F. nucleatum abundance, a dietary intervention study noted a marked increase in stool F. nucleatum levels after individuals were switched to a low-fiber, high-fat diet [26 ••]. Further studies are needed to identify the major dietary factors that influence F. nucleatum localization in the gut and elucidate the underlying mechanisms.
Fiber
Numerous prospective studies have linked higher fiber intake to lower risk of CRC [2 ••]. The most recent expert report from the World Cancer Research Fund and the American Institute for Cancer Research in 2011 concludes that evidence that consumption of foods containing dietary fiber protects against CRC is convincing [56]. Besides systemic benefits for insulin sensitivity and metabolic regulation [57], which have been implicated in colorectal carcinogenesis [58,59,60], fiber possesses gut-specific activities, such as diluting fecal content, decreasing transit time, and increasing stool weight, thereby minimizing exposure to intestinal carcinogens [61].
Moreover, soluble fiber can be fermented by bacteria in the lumen of the colon into SCFAs, including butyrate, acetate, and propionate. Higher fiber intake has been shown to enrich butyrate-producing bacteria in the gut, such as Clostridium, Anaerostipes, Eubacterium, and Roseburia species, and increase production of SCFAs [26, 62]. SCFAs have been suggested as the key metabolites linking the gut microbes to various health conditions, especially CRC. Butyrate is a major energy source for colonocytes and plays an important role in energy homeostasis in the colon tissue [63]. In cancer cells, however, butyrate is metabolized to a lesser extent due to the Warburg effect (the enhanced conversion of glucose to lactate by tumor cells even in the presence of normal levels of oxygen) and accumulates in the nucleus of cancerous colonocytes, whereby it functions as an inhibitor of histone deacetylase to epigenetically downregulate expression of numerous genes responsible for tumor growth (e.g., MYC, BAX, and NRAS), angiogenesis (vascular endothelial growth factor family), migration (matrix metalloproteinase family, the plasminogen-plasmin system), and chemoresistance (P-glycoprotein) [64]. Studies using gnotobiotic (germ-free) mouse models have provided compelling data that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner via histone deacetylase inhibition [65 ••].
In addition to suppression of histone deacetylase, butyrate can also function at the colonic epithelial cell surface as an agonist for certain G protein-coupled receptors (GPRs), such as GPR43 [66] and GPR109a [67, 68], thereby inhibiting intestinal inflammation and possibly carcinogenesis. Recently, several studies have demonstrated the crucial role of SCFAs in intestinal immune homeostasis through modulation of regulatory T cells. As a T cell subset with immunosuppressive functions, regulatory T cells play a central role in the suppression of inflammatory and allergic responses by limiting proliferation of effector CD4+ T cells. Butyrate and propionate have been shown to induce extrathymic generation and functional differentiation of regulatory T cells and protect against colitis [67, 69,70,71]. Possible mechanisms include histone deacetylase inhibition, enhancement of anti-inflammatory phenotype in colonic macrophages and dendritic cells via activation of GPR109a, and a T cell intrinsic epigenetic upregulation of the Foxp3 gene, a prerequisite transcription factor for regulatory T cells. Moreover, butyrate may modulate the function of intestinal macrophages by histone deacetylase inhibition and downregulate lipopolysaccharide-induced pro-inflammatory mediators, thereby facilitating host tolerance to intestinal microbiota [72].
In agreement with these mechanistic data, resistant starch (a starch that resists digestion in the small intestine and undergoes bacterial fermentation in the large intestine to produce SCFAs) has been shown to have chemopreventive effects against colitis-associated CRC [73]. Moreover, preclinical studies have indicated the potential of butyrate and its analogs as chemotherapeutic agents in several tumor models [74, 75], including CRC [76]. Based on these reports, further translational studies are expected to provide more data about the clinical effectiveness of fiber or butyrate in CRC prevention and treatment.
Interestingly, the beneficial effect of butyrate may depend on the host genetic background. A recent study reported that butyrate fuels hyperproliferation of colon epithelial cells and induces CRC in APCMin/+MSH2−/− mice [77 •], a model system of defective DNA mismatch repair which underlies the aggressive and rapid development of adenoma and CRC with microsatellite instability in hereditary nonpolyposis CRC (Lynch syndrome) [78]. Future studies are needed to investigate whether these findings can be generalizable to human by assessing the fiber-CRC association according to microsatellite instability status.
Red Meat and Sulfur
There is convincing evidence that red and processed meats are associated with increased risk of CRC [79]. Recently, the International Agency for Research on Cancer has classified processed meat as a carcinogen to humans. Mechanisms underlying the pro-cancer effects of red or processed meats include heme iron, N-nitroso compounds, or heterocyclic amines [80, 81], and hydrogen sulfide production [82]. Hydrogen sulfide has been implicated in inflammatory disorders associated with risk of CRC, such as ulcerative colitis [83,84,85], and directly with CRC [86,87,88,89,90,91,92]. In the colon, excess chronic hydrogen sulfide exposure is associated with key drivers of carcinogenesis, including impaired colonocyte nutrition, DNA damage, epithelial hyperproliferation, inflammation, and alterations in immune cell populations and function [88, 93,94,95,96]. Hydrogen sulfide is also emerging as a modulator of T cell survival and proliferation; cysteine intake and hydrogen sulfide production influence gut T cell responses [97]. Hydrogen sulfide-high environments may favor regulatory T cells that in turn suppress the activation and proliferation of effector T cells, leading to impaired anti-tumor immunity.
Gut luminal hydrogen sulfide production appears to be fundamentally dependent on the action of sulfur-reducing bacteria, which metabolize dietary sulfur [82]. Dietary sulfur in turn modifies the abundance of sulfur-reducing bacteria in the colon [88, 89]. Meat is a rich source of sulfur-containing amino acids such as cysteine and methionine, and processed meat typically contains inorganic sulfur (sulfate and sulfite) routinely used as a preservative [82]. Thus, the consistent association between meat, particularly processed meat, and CRC may at least in part be due to the influence of meat on the abundance of sulfur-reducing bacteria. The sulfur content of foods alone is likely not the only determinant of the abundance of sulfur-reducing bacteria or hydrogen sulfide production. Macronutrients such as specific fats consumed with sulfur-containing amino acids might modulate this association [98]. Furthermore, meat-based sources of sulfur are distinct from vegetable-based sulfur, particularly glucosinolates abundant in cruciferous vegetables. A core of gut microbes distinct from sulfur-reducing bacteria appears to hydrolyze the sulfur-containing glucosinolates into isothiocyanates, which, in contrast with hydrogen sulfide, are associated with cancer preventative properties [2 ••, 99].
As a member of sulfur-reducing bacteria, F. nucleatum has been implicated in CRC development (see “Dietary Patterns” section). Besides its immunomodulatory effects, F. nucleatum may also promote genotoxicity by its ability to convert cysteine to hydrogen sulfide [100]. Limited data have also shown an association between other sulfur-reducing bacteria and CRC. In two case-control studies, the stool or luminal microbiota in colon cancer patients were enriched with bacteria producing hydrogen sulfide, such as Porphyromonas, or bacteria from the Prevotellaceae family [6, 48]. However, the retrospective design makes these studies unable to dissect whether sulfur-reducing bacteria is a cause or effect of colorectal carcinogenesis. Further prospective studies are needed to examine sulfur-reducing bacteria in relation to CRC risk and better understand how diet may influence CRC by altering the abundance and function of sulfur-reducing bacteria.
Omega-3 Fatty Acid
Marine omega-3 polyunsaturated fatty acid, including eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid, possesses potent anti-inflammatory activity and may protect against CRC [101,102,103, 104 ••]. Fish oil, a rich source of omega-3 fatty acid, is the most popular natural product used by US adults [105]. Substantial data support the beneficial effect of omega-3 fatty acid on CRC prevention and treatment [102]. In randomized controlled trials, omega-3 fatty acid supplement reduces the number and size of polyps in patients with familial adenomatous polyposis and improves survival of CRC patients with liver metastasis [104, 106]. The anti-cancer effect of omega-3 fatty acid may be related to its multifaceted anti-inflammatory activity mediated by alterations in lipid raft structure and changes in fatty acid composition of cell membranes. These changes modify downstream metabolite production, including a decrease in inflammatory eicosanoids (e.g., prostaglandin E2), and an increase in pro-resolving lipid mediators (e.g., resolvin and lipoxin) [107,108,109,110,111,112]. Our recent study showed that omega-3 fatty acid was primarily associated with lower risk of CRC subsets infiltrated with high density of FOXP3+ T cells and might protect against CRC by downregulation of the immunosuppressive activity of regulatory T cells [113 •]. These findings suggest a potential interaction of omega-3 fatty acid with tumor immunity in prevention of CRC.
Dietary fat composition is a major driver of the gut microbial community structure [114,115,116,117,118,119]. Compared to other types of fat, omega-3 fatty acid have been associated with higher intestinal microbiota diversity and omega-3 fatty acid-rich diet ameliorates the gut dysbiosis induced by omega-6 polyunsaturated fatty acid or antibiotics [116, 117, 120, 121]. Animal studies indicate that omega-3 fatty acid supplements increase the abundance of anti-inflammatory bacteria, such as lactic acid-producing bacteria (mainly Lactobacillus and Bifidobacteria), and decrease the abundance of immunosuppressive and pro-inflammatory bacteria, such as F. nucleatum, lipopolysaccharide-producing bacteria (e.g., Escherichia coli) and Akkermansia [114,115,116,117,118,119, 121].
Some species from Lactobacillus and Bifidobacteria genera support the host immunoprotective system [122, 123], promote anti-tumor immunity, and facilitate cancer immunotherapy [124, 125 •, 126]. Anaerobic gut bacteria, including some species of Lactobacillus, have been implicated in the saturation of polyunsaturated fatty acid, a detoxifying mechanism that transforms bacterial growth-inhibiting polyunsaturated fatty acid into less toxic fatty acid, such as hydroxyl fatty acid [127,128,129,130,131,132,133]. These microbial metabolites may help preserve intestinal barrier integrity, reduce oxidative stress, and lower inflammation [134, 135]. Given that Lactobacillus is selectively enriched by omega-3 fatty acid, there may exist a reciprocal mechanism by which gut microbes adapt to host dietary change with functional consequences for host health. Moreover, a cross-feeding effect has been noted between the human Bifidobacterium, which produces lactate and acetate, and the butyrate-producing species, such as Eubacterium rectale, which convert lactate to butyrate [136,137,138]. Butyrate, a short-chain fatty acid, has potent anti-inflammatory [139] and potential anti-CRC properties [65, 140] (see “Fiber” section). On the other hand, higher serum levels of lipopolysaccharide antibodies have been associated with increased CRC risk in men [141], and higher abundance of F. nucleatum has been linked to higher CRC risk and shorter survival [6, 9, 45, 46, 49, 52]. Taken together, these findings support the hypothesis that omega-3 fatty acid may preserve colonic immune homeostasis and suppress CRC through modulation of the gut microbiota.
Several potential pathways may contribute to the microbe-modifying effect of omega-3 fatty acid. A recent study showed that high omega-3 fatty acid might alter the production of microbiota regulators in colonic tissue[142 ••]. Omega-3 fatty acid metabolite resolvin stimulates host epithelial expression of a transmissible factor, intestinal alkaline phosphatase [143], whose lipopolysaccharide-detoxifying activity leads to decreased abundance of lipopolysaccharide-producing and/or pro-inflammatory bacterial groups and increased abundance of lipopolysaccharide-suppressing and/or anti-inflammatory bacteria [142 ••]. Moreover, luminal unabsorbed omega-3 fatty acid may alter the gut environmental conditions and changes in immune response due to omega-3 fatty acid may in turn confer selective pressure on the gut microbial community [127, 144, 145]. Given the sparse data, further investigations are needed to better understand the interaction network between omega-3 fatty acid, gut microbiota, and the immune system. This may lead to novel prevention strategies based upon dietary modification, manipulation of microbial ecology, or development of microbiome and immune profiling as a biomarker of chemopreventive efficacy.
Obesity
Since the 1970–1980s, the prevalence of obesity has markedly increased worldwide [146]. The obesity epidemic is believed to be largely driven by global westernization characterized by overconsumption of easily accessible and energy-dense food and a sedentary lifestyle [147, 148]. Obesity is an established risk factor for CRC and several other cancers [149]. Possible mechanisms include increased insulin levels and bioavailability of insulin-like growth factor 1, altered secretion of adipokines and inflammatory cytokines, and changes in sex hormone levels [150, 151].
Emerging evidence suggests a bidirectional relationship between obesity and the gut microbiota. On the one hand, obese individuals are more likely to demonstrate dysbiosis than lean individuals. Specifically, a decrease in the phylum Bacteroidetes and an increase in Firmicutes associated with obesity was observed in some [152,153,154] but not all [155] studies. Moreover, the relative abundance of Bacteroidetes increases as obese individuals lose weight on either a fat- or a carbohydrate-restricted low-calorie diet and the increase in Bacteroidetes is significantly correlated to weight loss [152]. On the other hand, these microbial changes are likely not a mere consequence of obesity, because the obese phenotype can be transmitted by transplantation of the obesity-associated gut microbiota in mice. When colonized with a conventional mouse microbiota, gnotobiotic (germ-free) mice that are normally lean and resistant to diet-induced obesity accumulate more adipose tissue mass and develop insulin resistance despite an associated decrease in food consumption [156, 157]. Similarly, the gut microbiota transplanted from mice with diet-induced obesity to germ-free recipients promotes greater fat deposition than transplants from lean donors [158]. It has been hypothesized that antibiotic use in early life, a critical window for metabolic development, increases risk of childhood obesity by disrupting the composition and metabolic activity of the gut microbiota that can exert long-lasting effects on body weight in adulthood [159, 160 •]. Interestingly, antibiotic use, especially during early life, has been linked to increased risk of CRC and colorectal adenoma in a few studies [161,162,163,164]. While this association needs to be confirmed by further studies, it remains unclear whether increased adiposity plays any mediating role in this association.
Mechanistic data suggest that the gut microbiota may influence energy homeostasis and obesity pathogenesis through several pathways, including peripheral control of energy harvest, central regulation of food intake via the gut-brain neural communication, and inflammation and impaired gut barrier through activation of pattern recognition receptors [165,166,167]. Taken together, these data support that while the gut microbial profile may change due to changes in body weight accompanied by systemic metabolic alterations, the composition of the gut microbiota can also predispose to the development of obesity. However, because most evidence is from animal or small human studies with short-term intervention, it remains to be characterized how the community structure and function of the gut microbiota varies with host adiposity over a long-term period, which is more relevant to cancer development [168].
Given the link between obesity and gut microbiota and the role of the gut microbiota in cancer development, it has been proposed that changes in the gut microbiota may contribute to obesity-associated carcinogenesis. Indeed, studies in liver cancer have suggested that increased enterohepatic circulation of the obesity-induced gram-positive gut microbial metabolite deoxycholic acid facilitates hepatocellular carcinoma development by inducing cellular senescence and the senescence-associated secretory phenotype in the tumor microenvironment [169, 170]. Besides deoxycholic acid, another gut microbial component, lipoteichoic acid, may also contribute to obesity-induced liver cancer by enhancing senescence-associated secretory phenotype and upregulating the expression of prostaglandin-endoperoxide synthase 2 [171 ••]. As a critical enzyme in inflammation, prostaglandin-endoperoxide synthase 2 mediates production of prostaglandin E2, which governs tumor-mediated immune dysfunction and contributes to a shift in the tumor microenvironment from anti-tumor responses to immunosuppressive responses [172]. Given the potential role of prostaglandin E2 [173] and secondary bile acid [174] in promoting CRC, further studies are needed to investigate whether microbial imbalance-induced metabolic change also mediates obesity-related tumor promotion in the colon.
Conclusion
CRC is one of the cancers that are the most closely associated with diet. Human intestinal tract is colonized by ~ 100 trillion microbes, the vast majority of which resides in the large intestine and is integral to host genomic stability, immune homeostasis, and metabolism. A growing body of evidence indicates a complex interrelation between diet, gut microbiota, and CRC. However, most of the evidence derives from cross-sectional or short-term, highly controlled feeding studies that are limited in size. Given the multistage process and long latency of colorectal carcinogenesis, high-quality prospective studies with dietary data collected over the life course and gut microbial composition and function assessed well prior to neoplastic occurrence are critically needed. To make these studies possible, further investments are needed for stool collection in the existing, preferably younger epidemiologic cohort, standardization of the microbiome study pipeline, and development of novel user-friendly statistical tools to link the high-dimensional omics data to longitudinal epidemiologic data (including diet). These investigations will provide essential data to identify microbiome-based interventions that may complement or optimize the current diet-based strategies for CRC prevention.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. GLOBOCAN 2012 v1.0. Lyon: International Agency for Research on Cancer; 2013.
•• Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–1260 e16. A comprehensive review of epidemiologic and mechanistic evidence supporting the importance of nutritional factors in colorectal cancer prevention
Scanlan PD, Shanahan F, Clune Y, et al. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ Microbiol. 2008;10:789–98.
Sobhani I, Tap J, Roudot-Thoraval F, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393.
Wang T, Cai G, Qiu Y, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–9.
Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–11.
Zackular JP, Rogers MA, Ruffin MT, et al. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila). 2014;7:1112–21.
Zeller G, Tap J, Voigt AY, et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol. 2014;10:766.
Feng Q, Liang S, Jia H, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528.
Vogtmann E, Hua X, Zeller G, et al. Colorectal cancer and the human gut microbiome: reproducibility with whole-genome shotgun sequencing. PLoS One. 2016;11:e0155362.
Yu J, Feng Q, Wong SH, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70–8.
Shah MS, DeSantis TZ, Weinmaier T, et al. Leveraging sequence-based faecal microbial community survey data to identify a composite biomarker for colorectal cancer. Gut. 2017.
Liang Q, Chiu J, Chen Y, et al. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin Cancer Res. 2017;23:2061–70.
Flemer B, Lynch DB, Brown JM, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66:633–43.
• Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230–40. An updated review of the inmportance of inflammatory components in colorectal cancer
Claesson MJ, Cusack S, O'Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–91.
Faith JJ, Guruge JL, Charbonneau M, et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439.
Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8.
Costello EK, Lauber CL, Hamady M, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–7.
Jalanka-Tuovinen J, Salonen A, Nikkila J, et al. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One. 2011;6:e23035.
Rajilic-Stojanovic M, Heilig HG, Tims S, et al. Long-term monitoring of the human intestinal microbiota composition. Environ Microbiol. 2012;
• David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. A landmark study indicating that the gut microbiome can rapidly respond to altered diet
Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8.
Lahti L, Salojarvi J, Salonen A, et al. Tipping elements in the human intestinal ecosystem. Nat Commun. 2014;5:4344.
Walter J. Murine gut microbiota-diet trumps genes. Cell Host Microbe. 2015;17:3–5.
•• O'Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. The study provides strong eivdence for the role of the gut microbiome in mediating the relationship between dietary factors and cancer risk
•• Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–94. The study suggests that the gut microbiome is an important determinant for the inter-individual variation in the metabolic response to dietary intervention
Zmora N, Zeevi D, Korem T, et al. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe. 2016;19:12–20.
Miller PE, Lesko SM, Muscat JE, et al. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62:413–24.
Magalhaes B, Peleteiro B, Lunet N. Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur J Cancer Prev. 2012;21:15–23.
Serino M, Luche E, Gres S, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–53.
Martinez-Medina M, Denizot J, Dreux N, et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116–24.
Ley SH, Sun Q, Willett WC, et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99:352–60.
Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–84. quiz 714-5
Montonen J, Boeing H, Fritsche A, et al. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2013;52:337–45.
Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–8.
Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–35.
Brown K, DeCoffe D, Molcan E, et al. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–119.
Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J. 2014;13:61.
Kramer CD, Weinberg EO, Gower AC, et al. Distinct gene signatures in aortic tissue from ApoE−/− mice exposed to pathogens or Western diet. BMC Genomics. 2014;15:1176.
De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6.
Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–20.
Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;
Abed J, Emgard JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed gal-GalNAc. Cell Host Microbe. 2016;20:215–25.
Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306.
Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8.
Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311–8.
Chen W, Liu F, Ling Z, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743.
McCoy AN, Araujo-Perez F, Azcarate-Peril A, et al. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653.
Allali I, Delgado S, Marron PI, et al. Gut microbiome compositional and functional differences between tumor and non-tumor adjacent tissues from cohorts from the US and Spain. Gut Microbes. 2015:0.
Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727.
• Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–80. The study indicates that high abundance of Fusobacterium nucleatum in the tumor tissue is associated with worse survival of colorectal cancer, providing further support for the pro-colorectal cancer effect of this bacteria.
Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–15.
Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–55.
Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206.
World Cancer Research Fund / American Institute for Cancer Research. Continuous Update Project report: food, nutrition, physical activity, and the prevention of colorectal cancer. 2011. http://www.wcrf.org/sites/default/files/Colorectal-Cancer-2011-Report.pdf.
Anderson JW, Baird P, Davis RH Jr, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205.
Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69.
Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–25.
Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21.
Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28:3–13.
Chen HM, Yu YN, Wang JL, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013;97:1044–52.
Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–26.
Encarnacao JC, Abrantes AM, Pires AS, et al. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34:465–78.
•• Donohoe DR, Holley D, Collins LB, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–97. The study suggests a model of mechanisms by which dietary fiber may protect against colorectal cancer in a gut microbiota- and butyrate-dependent manner, and provides a potential explanation for inconsistent findings about the relationship of fiber intake and colorectal cancer risk reported in epidemiologic studies.
Tang Y, Chen Y, Jiang H, et al. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2011;128:847–56.
Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–39.
Thangaraju M, Cresci GA, Liu K, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–32.
Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5.
Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73.
Chang PV, Hao L, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–52.
Hu Y, Le Leu RK, Christophersen CT, et al. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis. 2016;37:366–75.
Entin-Meer M, Rephaeli A, Yang X, et al. Butyric acid prodrugs are histone deacetylase inhibitors that show antineoplastic activity and radiosensitizing capacity in the treatment of malignant gliomas. Mol Cancer Ther. 2005;4:1952–61.
Kuefer R, Hofer MD, Altug V, et al. Sodium butyrate and tributyrin induce in vivo growth inhibition and apoptosis in human prostate cancer. Br J Cancer. 2004;90:535–41.
Bras-Goncalves RA, Pocard M, Formento JL, et al. Synergistic efficacy of 3n-butyrate and 5-fluorouracil in human colorectal cancer xenografts via modulation of DNA synthesis. Gastroenterology. 2001;120:874–88.
• Belcheva A, Irrazabal T, Robertson SJ, et al. Gut microbial metabolism drives transformation of Msh2-deficient colon epithelial cells. Cell. 2014;158:288–99. The study suggests that the effect of fiber on colorectal cancer depends on the host genetic background, with a procancer effect in the context of MSH2 −/− .
Reitmair AH, Cai JC, Bjerknes M, et al. MSH2 deficiency contributes to accelerated APC-mediated intestinal tumorigenesis. Cancer Res. 1996;56:2922–6.
World Cancer Research Fund / American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007.
Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–14.
Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–43. e10
Magee EA, Richardson CJ, Hughes R, et al. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000;72:1488–94.
Tilg H, Kaser A. Diet and relapsing ulcerative colitis: take off the meat? Gut. 2004;53:1399–401.
Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571–9.
Rowan FE, Docherty NG, Coffey JC, et al. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br J Surg. 2009;96:151–8.
Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med. 2004;229:586–97.
Deplancke B, Gaskins HR. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J. 2003;17:1310–2.
Attene-Ramos MS, Wagner ED, Gaskins HR, et al. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res. 2007;5:455–9.
Ramasamy S, Singh S, Taniere P, et al. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G288–96.
Cai WJ, Wang MJ, Ju LH, et al. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol Int. 2010;34:565–72.
Carbonero F, Benefiel AC, Gaskins HR.Contributions of the microbial hydrogen economy to colonic homeostasis Nature reviews. Gastroenterol Hepatol 2012;9:504–518.
Wu YC, Wang XJ, Yu L, et al. Hydrogen sulfide lowers proliferation and induces protective autophagy in colon epithelial cells. PLoS One. 2012;7:e37572.
Roediger WE, Duncan A, Kapaniris O, et al. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology. 1993;104:802–9.
Pitcher MC, Beatty ER, Cummings JH. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut. 2000;46:64–72.
Vinolo MA, Rodrigues HG, Hatanaka E, et al. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci. 2009;117:331–8.
Zeng H, Combs GF Jr. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem. 2008;19:1–7.
Miller TW, Wang EA, Gould S, et al. Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem. 2012;287:4211–21.
O'Keefe SJ, Ou J, Aufreiter S, et al. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139:2044–8.
Bianchini F, Vainio H. Isothiocyanates in cancer prevention. Drug Metab Rev. 2004;36:655–67.
Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, et al. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol. 2012;3:448.
Larsson SC, Kumlin M, Ingelman-Sundberg M, et al. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–45.
Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–49.
Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61.
•• Cockbain AJ, Volpato M, Race AD, et al. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014;63:1760–8. This randomized controlled study supports the chemopreventive effect of omega-3 fatty acid supplementation on colorectal cancer.
Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl. Health Stat. Rep. 2015:1–16.
West NJ, Clark SK, Phillips RK, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–25.
Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 1851;2015:469–84.
Piazzi G, D'Argenio G, Prossomariti A, et al. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. J Int Cancer. 2014;135:2004–13.
Jiang Y, Djuric Z, Sen A, et al. Biomarkers for personalizing omega-3 fatty acid dosing. Cancer Prev Res (Phila). 2014;7:1011–22.
Calviello G, Di Nicuolo F, Gragnoli S, et al. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis. 2004;25:2303–10.
Bartram HP, Gostner A, Scheppach W, et al. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology. 1993;105:1317–22.
Nowak J, Weylandt KH, Habbel P, et al. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007;28:1991–5.
• Song M, Nishihara R, Cao Y, et al. Marine omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer characterized by tumor-infiltrating T cells. JAMA Oncol. 2016;2:1197–206. This study suggests that the beneficial effect of high omega-3 fatty acid intake may be partly mediated by modulation of regulatory T cells in the tumor microenvironment.
Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22:658–68.
Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10 −/− mice. Nature. 2012;487:104–8.
Ghosh S, DeCoffe D, Brown K, et al. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS One. 2013;8:e55468.
Patterson E, RM OD, Murphy EF, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br J Nutr. 2014;1–13.
Mujico JR, Baccan GC, Gheorghe A, et al. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110:711–20.
Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem. 2014;25:270–80.
Ghosh S, Molcan E, DeCoffe D, et al. Diets rich in n-6 PUFA induce intestinal microbial dysbiosis in aged mice. Br J Nutr. 2013;110:515–23.
Kaliannan K, Wang B, Li XY, et al. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int J Obes (Lond). 2016.
Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–11.
Peran L, Sierra S, Comalada M, et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97:96–103.
Khazaie K, Zadeh M, Khan MW, et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A. 2012;109:10462–7.
• Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9. The study indicates that the efficacy of cancer immunotherapy may depend on the abundance of Bifidobacterium in the gut.
Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70.
Kishino S, Takeuchi M, Park SB, et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc Natl Acad Sci U S A. 2013;110:17808–13.
Kishino S, Ogawa J, Yokozeki K, et al. Metabolic diversity in biohydrogenation of polyunsaturated fatty acids by lactic acid bacteria involving conjugated fatty acid production. Appl Microbiol Biotechnol. 2009;84:87–97.
Hirata A, Kishino S, Park SB, et al. A novel unsaturated fatty acid hydratase toward C16 to C22 fatty acids from Lactobacillus acidophilus. J Lipid Res. 2015;56:1340–50.
Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–42.
Sakurama H, Kishino S, Mihara K, et al. Biohydrogenation of C20 polyunsaturated fatty acids by anaerobic bacteria. J Lipid Res. 2014;55:1855–63.
Druart C, Bindels LB, Schmaltz R, et al. Ability of the gut microbiota to produce PUFA-derived bacterial metabolites: proof of concept in germ-free versus conventionalized mice. Mol Nutr Food Res. 2015;59:1603–13.
Druart C, Neyrinck AM, Vlaeminck B, et al. Role of the lower and upper intestine in the production and absorption of gut microbiota-derived PUFA metabolites. PLoS One. 2014;9:e87560.
Furumoto H, Nanthirudjanar T, Kume T, et al. 10-Oxo-trans-11-octadecenoic acid generated from linoleic acid by a gut lactic acid bacterium Lactobacillus plantarum is cytoprotective against oxidative stress. Toxicol Appl Pharmacol. 2016;296:1–9.
Miyamoto J, Mizukure T, Park SB, et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem. 2015;290:2902–18.
Flint HJ, Duncan SH, Scott KP, et al. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74:13–22.
Belenguer A, Duncan SH, Calder AG, et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72:3593–9.
Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–7.
Arpaia N, Rudensky AY. Microbial metabolites control gut inflammatory responses. Proc Natl Acad Sci U S A. 2014;111:2058–9.
O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691–706.
Kong SY, Tran HQ, Gewirtz AT, et al. Serum endotoxins and flagellin and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Cancer Epidemiol Biomark Prev. 2016;25:291–301.
•• Kaliannan K, Wang B, Li XY, et al. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep. 2015;5:11276. The study proposes a model of mechanisms by which omega-3 fatty acid may influence the gut microbial composition through the influence on epithelial production of intestinal alkaline phosphatase.
Campbell EL, MacManus CF, Kominsky DJ, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107:14298–303.
Polan CE, McNeill JJ, Tove SB. Biohydrogenation of unsaturated fatty acids by rumen bacteria. J Bacteriol. 1964;88:1056–64.
Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology. 2015;148:1107–19.
Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67.
Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–14.
Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013;9:13–27.
Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8.
Doyle SL, Donohoe CL, Lysaght J, et al. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc. 2012;71:181–9.
Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98.
Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57.
Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5.
Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–4.
Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23.
Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Turnbaugh PJ, Backhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23.
Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat. Rev. Endocrinol. 2015;11:182–90.
• Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21. The study suggests that disturbances of the gut microbiome in early life may contribute to subsequent development of obesity in later life.
Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2017.
Kilkkinen A, Rissanen H, Klaukka T, et al. Antibiotic use predicts an increased risk of cancer. Int J Cancer. 2008;123:2152–5.
Boursi B, Haynes K, Mamtani R, et al. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2015;24:534–42.
Dik VK, van Oijen MG, Smeets HM, et al. Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case-control study. Dig Dis Sci. 2016;61:255–64.
Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–84.
Duca FA, Lam TK. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes Metab. 2014;16(Suppl 1):68–76.
Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell Metab. 2013;17:883–94.
Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138:2383–95.
Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101.
Ohtani N, Yoshimoto S, Hara E. Obesity and cancer: a gut microbial connection. Cancer Res. 2014;74:1885–9.
•• Loo TM, Kamachi F, Watanabe Y, et al. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017. The study provides new evidence about how obesity may promote liver cancer through an influence on the functionality of the gut microbiota.
Wang D, DuBois RN. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 2013;19:502–10.
Wang D, DuBois RN. PPARdelta and PGE2 signaling pathways communicate and connect inflammation to colorectal cancer. Inflamm Cell Signal. 2014;1.
Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J. Surg. Oncol. 2014;12:164.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nutrition and Nutritional Interventions in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Song, M., Chan, A.T. Diet, Gut Microbiota, and Colorectal Cancer Prevention: a Review of Potential Mechanisms and Promising Targets for Future Research. Curr Colorectal Cancer Rep 13, 429–439 (2017). https://doi.org/10.1007/s11888-017-0389-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-017-0389-y