Abstract
Angiogenesis is a complex biologic process critical to growth and proliferation of colorectal cancer. The safety and efficacy of various anti-angiogenic agents have been investigated in many treatment settings. Bevacizumab, an anti-vascular endothelial growth factor agent, has efficacy in both the first-line setting and beyond progression in metastatic colorectal cancer. The decoy vascular endothelial growth factor receptor aflibercept has been approved in combination with 5-fluorouracil-, leucovorin-, and irinotecan-based chemotherapy in metastatic colorectal cancer patients whose disease has progressed on a prior oxaliplatin-based chemotherapy regimen. The multikinase inhibitor regorafenib is modestly effective in the refractory colorectal cancer setting but confers significant toxicity. Ramucirumab, an anti-vascular endothelial growth factor receptor 2 molecule, has efficacy in combination with 5-fluorouracil, leucovorin, and irinotecan after disease progression on a first-line bevacizumab-, oxaliplatin-, and fluoropyrimidine-containing regimen. Questions regarding optimal treatment setting, predictive biomarkers of response, and cost-effectiveness of these anti-angiogenic agents and others are as yet unanswered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
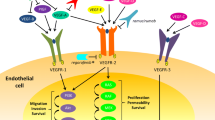
Angiogenesis, or the formation of new blood vessels, is a critically important process in tumor growth and proliferation [1]. For decades, the process by which cancers cause new blood vessel formation has been studied in the hope that angiogenesis could be therapeutically inhibited, thereby halting tumor growth. Angiogenesis, however, is an extremely complex and likely biologically variable process that is a result of the interplay of multiple pro- and anti-angiogenic factors and several intricate and overlapping signaling pathways. Key players in angiogenesis include the vascular endothelial growth factor (VEGF) family, which consists of growth factors such as VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF), and receptors such as VEGF receptor (VEGFR) 1, VEGFR-2, and VEGFR-3 [2]. Other important players in tumor angiogenesis include platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and c-kit, among others. Within the last decade, many studies have demonstrated the survival benefit for patients with metastatic colorectal cancer (mCRC) treated with anti-angiogenic therapies that target these important growth factors and receptors. This review will discuss recent evidence regarding the varying mechanisms and efficacy of anti-angiogenic agents in colorectal cancer.
Bevacizumab (Avastin®, Genentech)
The first anti-angiogenic agent to be approved for use in metastatic colorectal cancer was bevacizumab (Table 1). This agent is a humanized monoclonal antibody that binds to VEGF-A and prevents its binding to the VEGF receptor [3]. A randomized phase III trial of first-line irinotecan, bolus fluorouracil, and leucovorin (IFL) plus either bevacizumab 5 mg/kg intravenously (IV) or placebo every 2 weeks resulted in the demonstration of an overall survival (OS) benefit (median OS, 20.3 vs. 15.6 months, hazard ratio (HR) 0.66, p < 0.001) and progression-free survival (PFS) benefit (median PFS, 10.6 vs. 6.2 months, HR 0.54, p < 0.001) for the bevacizumab arm [4]. Several other studies then confirmed the efficacy of bevacizumab in combination with standard first- and second-line chemotherapy regimens in the treatment of bevacizumab-naïve patients with metastatic colorectal cancer [5, 6].
It was unknown, however, whether continuation of an anti-angiogenic therapy such as bevacizumab beyond disease progression on a first-line bevacizumab-containing regimen conferred any advantage. It was hypothesized that bevacizumab resistance was unlikely to occur at the same time or via the same mechanism as resistance to cytotoxic chemotherapy. Data from observational registry studies such as BRiTE and ARIES appeared promising in this regard [7, 8], but prospective data for bevacizumab beyond progression was needed. As a result, the phase III TML (ML18147) trial was undertaken to answer this question [9••]. This trial randomized 820 mCRC patients to second-line chemotherapy with or without bevacizumab 2.5 mg/kg per week equivalent (either 5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks intravenously). Median overall survival was 11.2 months for the bevacizumab arm and 9.8 months for chemotherapy alone (HR 0.81, 95 % confidence interval (CI) 0.69–0.94; unstratified log-rank test, p = 0.0062). As expected from an anti-angiogenic agent such as bevacizumab, grade 3 or higher bleeding, gastrointestinal perforation, and venous thromboembolism were more common in the bevacizumab arm. This study confirmed the survival benefit to the continuation of bevacizumab beyond disease progression with first-line therapy, although debates have ensued regarding whether the statistically significant survival advantage translates into a meaningful or cost-effective prolongation of life.
Unlike the findings in multiple studies of patients with metastatic colorectal cancer, bevacizumab has not been found to improve outcomes in patients with early stage disease. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) trial C-08, 2673 patients with stage II or III colon cancer were randomized to modified 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) for 6 months versus mFOLFOX6 for 6 months plus bevacizumab 5 mg/kg for 12 months beginning concurrently with chemotherapy [10, 11]. The addition of bevacizumab to mFOLFOX6 did not result in an overall significant increase in disease-free survival (DFS; 78 vs. 75 %, HR 0.93, 95 % CI 0.81–1.08, p = 0.35) or 5-year overall survival (83 vs. 81 %, HR 0.95, 95 % CI 0.79–1.13, p = 0.56). Overall survival in the subset of stage III patients was also not improved with the addition of bevacizumab (HR 1.0, 95 % CI 0.83–1.21, p = 0.99). This was despite an early separation of the DFS curves favoring the bevacizumab-containing arm of the study; however, the delay in progression disappeared soon after the bevacizumab was discontinued, suggesting that it was exerting a cytostatic effect but that disease progression resumed once the agent was stopped.
The European AVANT trial also failed to show a benefit to the addition of bevacizumab in early stage colon cancer [12]. In this study, 2867 patients with stage III disease were randomized to FOLFOX4 (oxaliplatin 85 mg/m2, leucovorin 200 mg/m2, and fluorouracil 400 mg/m2 bolus plus 500 mg/m2 22-h continuous infusion on day 1; leucovorin 200 mg/m2 plus fluorouracil 400 mg/m2 bolus plus 600 mg/m2 22-h continuous infusion on day 2) every 2 weeks for 12 cycles, bevacizumab 5 mg/kg plus FOLFOX4 every 2 weeks for 12 cycles plus bevacizumab monotherapy 7.5 mg/kg every 3 weeks (8 cycles over 24 weeks), or bevacizumab 7.5 mg/kg plus XELOX (oxaliplatin 130 mg/m2 on day 1 plus oral capecitabine 1000 mg/m2 two times daily on days 1–15) every 3 weeks for 8 cycles followed by bevacizumab monotherapy 7.5 mg/kg every 3 weeks (8 cycles over 24 weeks). The DFS hazard ratio for bevacizumab-FOLFOX4 versus FOLFOX4 was 1.17 (95 % CI 0.98–1.39, p = 0.07), and for bevacizumab-XELOX versus FOLFOX4 was 1.07 (95 % CI 0.90–1.28, p = 0.44). The OS hazard ratio for bevacizumab-FOLFOX4 versus FOLFOX4 was 1.27 (95 % CI 1.03–1.57, p = 0.02), and for bevacizumab-XELOX versus FOLFOX4 was 1.15 (95 % CI 0.93–1.42, p = 0.21), indicating a potential detrimental effect with bevacizumab plus oxaliplatin-based adjuvant therapy in stage III colon cancer patients. As a result of these studies, bevacizumab is not indicated in the adjuvant treatment of patients with colon cancer.
Despite the clear survival benefit of bevacizumab in metastatic colorectal cancer, biomarkers to predict response to therapy or to select appropriate patients for its use have not yet been discovered. As reviewed by Lambrechts et al., challenges to the development of validated biomarkers predictive of bevacizumab treatment outcome include the sheer number of molecules and pathways involved in tumor angiogenesis, the heterogeneous effect of bevacizumab on tumors and the development of resistance, and the limitations in obtaining serial biopsies or samples from patients receiving therapy in a multitude of clinical settings [13]. Potential biomarkers of interest at various stages of development include that of circulating levels of VEGF-A, expression of neuropilin-1 and VEGFR1 in tumors or plasma, genetic variants in VEGFA or its receptors, and imaging biomarkers such as dynamic-contrast-enhanced magnetic resonance imaging (DCE-MRI) [13]. Development of validated biomarkers predictive of response to bevacizumab therapy or to that of other anti-angiogenics is greatly needed.
Aflibercept (Zaltrap®, Sanofi)
Additional efforts to target the VEGF pathway by employing other agents have been explored for the treatment of colorectal cancer. For example, aflibercept is a fully humanized recombinant fusion protein composed of portions of the extracellular domains of VEGF receptors 1 and 2 fused to the Fc portion of human immunoglobulin G1 (Table 1) [14]. As a decoy VEGF receptor, it prevents ligands such as VEGF-A, VEGF-B, placental growth factor (PlGF)-1, and PlGF-2 from binding and activating their respective receptors. Aflibercept binds with a higher affinity to VEGF-A than bevacizumab, and since PlGF levels can increase with exposure to other anti-VEGF agents, aflibercept was hypothesized to potentially overcome resistance to other anti-VEGF therapies.
Early clinical investigation of aflibercept included a phase I study of 47 patients with advanced solid tumors who received aflibercept in doses ranging from 0.3 to 7.0 mg/kg intravenously every 2 weeks [15]. From the results of this study, the chosen recommended phase II dose of aflibercept was 4.0 mg/kg IV every 2 weeks. A phase I trial of aflibercept in combination with irinotecan, 5-fluorouracil, and leucovorin was undertaken in patients with advanced solid tumors [16]. Though not the primary endpoint, 9 of 34 evaluable patients in this study had a partial response (PR), and 22 patients had stable disease; 7 of the PRs were seen in patients who had received irinotecan previously. Subsequent double-blind expansion cohort of this phase I study confirmed efficacy of this regimen [17]. Including crossover, 4 of 26 patients (anal, colon, ovarian, and liver cancers) had PRs, and 17 patients had stable disease.
As a result of these early studies, more trials with aflibercept as a single agent and in combination with cytotoxic chemotherapy were performed in patients with colorectal cancer. A phase II study of single agent aflibercept in patients with refractory colorectal cancer demonstrated little efficacy but showed significant toxicity in this population [18]. At the same time, an international, randomized double-blind phase III trial named VELOUR investigated the efficacy of aflibercept versus placebo in combination with 5-fluorouracil (5-FU), leucovorin, and irinotecan (FOLFIRI) in patients with metastatic colorectal cancer (mCRC) who had progressed on an oxaliplatin-based regimen [19••]. A total of 1226 patients were included in the efficacy analysis, of which nearly one third had previously received bevacizumab. Patients in the aflibercept plus FOLFIRI arm had longer overall survival than patients receiving placebo plus FOLFIRI (median OS, 13.50 vs. 12.06 months, p = 0.0032; HR = 0.817, 95.34 % CI, 0.713–0.937). PFS was also increased with aflibercept (median PFS, 6.90 vs. 4.67 months, p < 0.001; HR = 0.758; 95 % CI, 0.661–0.869), and response rate was better with aflibercept as well (19.8 vs. 11.1 %, p = 0.001). Grade 3 and 4 toxicities were reported at a higher incidence in the aflibercept arm, including diarrhea, stomatitis, infections, neutropenia, and thrombocytopenia, as well as anti-VEGF-treatment-related toxicities such as hypertension, bleeding, arterial thromboembolism, and proteinuria. Due to these side effects, 26.8 % of patients in the aflibercept arm permanently discontinued study treatment compared with 12.1 % of patients in the placebo arm. As a result of aflibercept’s efficacy in VELOUR, this agent was approved in combination with FOLFIRI for patients with mCRC who had progressed on a prior oxaliplatin-based chemotherapy regimen by the US Food and Drug Administration (FDA) in August 2012 and by the European Medicines Agency in February 2013.
Many questions regarding the optimal role of aflibercept in mCRC remain. The VELOUR trial did not contain a FOLFIRI plus bevacizumab arm, a current standard, because the data supporting the use of bevacizumab beyond first-line disease progression was not yet available at the time of trial accrual. Prespecified subgroup analyses from VELOUR did not reveal particular patient groups that benefited more from the addition of aflibercept to chemotherapy [20]. Furthermore, many subsequent studies have been unable to identify particular biomarkers of response with which to select patients for aflibercept therapy. Clinical trials investigating the potential role of aflibercept in adjuvant CRC therapy, in combination with chemotherapy for maintenance treatment of mCRC, in combination with oxaliplatin-based chemotherapy in front-line treatment of mCRC, and others (including biomarker studies), are ongoing.
Regorafenib (Stivarga®, Bayer)
Regorafenib is an oral multikinase inhibitor that targets VEGFR1-3, TIE2, KIT, RET, RAF, PDGFR, and FGFR, among other kinases involved in tumor angiogenesis, oncogenesis, and the tumor microenvironment (Table 1) [21]. This multi-pronged approach was hypothesized to benefit patients with mCRC once preclinical colorectal cancer models showed anti-tumor activity [21]. A first-in-man, phase I dose-escalation study of regorafenib in patients with advanced solid tumors was performed, with 53 patients receiving doses between 10 and 200 mg daily on a 21 days on, 7 days off schedule every 28 days [22]. The most common regorafenib-related grade 3 or 4 adverse events included hand-foot skin reaction, desquamation/rash, hypertension, and diarrhea, and the frequency of these toxicities increased with increasing dose level. Under the dose-escalation schema, the maximum tolerated dose level for single agent regorafenib was found to be 160 mg once daily in a 21 days on, 7 days off schedule. In terms of efficacy, 35 of 47 (66 %) evaluable patients had disease control, defined as partial response or stable disease, with one mCRC patient having a PR at the 220-mg dose. An expansion cohort was then formed, consisting of an additional 23 patients with mCRC treated at the 160-mg dose; [23] a total of 38 mCRC patients were treated with regorafenib in this study. A total of 22/38 (58 %) of patients developed grade 3 or higher regorafenib-related adverse events, with 6/25 patients treated at the 160 mg dose level permanently discontinuing regorafenib due to treatment-related adverse events. Best responses of the 27 evaluable patients included one partial response (4 %) and 19 patients (70 %) with stable disease.
Despite the significant toxicity of regorafenib in the phase I study, its development moved into phase III testing in an international setting. In the CORRECT trial, 753 patients with refractory metastatic colorectal cancer were randomized to either regorafenib 160 mg daily for the first 3 weeks of each 4-week cycle or placebo [24••]. The primary endpoint of overall survival was met at a preplanned interim analysis, with median OS of 6.4 months in the regorafenib group and 5.0 months in the placebo group (HR 0.77, 95 % CI 0.64–0.94, one-sided p = 0.0052). Median progression-free survival was 1.9 months in the regorafenib group and 1.7 months in the placebo group (HR 0.49, 95 % CI 0.42–0.58, p < 0.0001). Significantly, 93 % of patients receiving regorafenib had treatment-related adverse events compared to 61 % of those receiving placebo, with hand-foot skin reaction, fatigue, diarrhea, hypertension, and rash being the most common toxicities seen. On the basis of these data, the US FDA approved regorafenib for the treatment of patients with metastatic colorectal cancer previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, anti-VEGF therapy, and anti-EGFR therapy if KRAS wild type on September 27, 2012.
Another international phase III trial of regorafenib versus placebo for patients with treatment-refractory metastatic colorectal cancer, CONCUR, was undertaken to determine whether a broader Asian patient population might benefit differently from regorafenib [25••]. A total of 204 patients were treated on this study. Overall survival in this trial was significantly better with regorafenib than with placebo to a greater extent than CORRECT, with median OS of 8.8 months in the regorafenib arm and 6.3 months in the placebo arm (HR 0.55, 95 % CI 0.40–0.77, one-sided p = 0.00016). Median PFS with regorafenib in CONCUR was also better than with placebo (3.2 vs. 1.7 months, respectively; HR 0.31, 95 % CI 0.22–0.44, p < 0.0001). Adverse events and frequencies seen were similar to the prior study, with hand-foot skin reactions (74 % in CONCUR vs. 47 % in CORRECT) and hyperbilirubinemia (37 % in CONCUR vs 20 % in CORRECT) both more prevalent in CONCUR. It has been hypothesized that the larger benefit of regorafenib seen in CONCUR could be due to differences in receipt of prior VEGF-directed drugs. In CORRECT, all patients had received prior bevacizumab and EGFR antibodies (for KRAS wild-type cancers), whereas only 41 % of patients in CONCUR had received biologics [24••, 25••]. An exploratory analysis of CONCUR showed that the HR for overall survival favoring regorafenib was 0.31 (95 % CI 0.19–0.53) in the portion of patients who had not received prior targeted therapy, compared to a HR of 0.78 (95 % CI 0.51–1.19) for the population more similar to that of CORRECT. This raises the hypothesis that perhaps regorafenib could have more benefit in the less heavily pretreated mCRC patient population.
Accordingly, clinical trials investigating the safety and efficacy of regorafenib in combination with chemotherapy in earlier stage settings have been undertaken. A phase Ib study of regorafenib in combination with chemotherapy in first- or second-line treatment of mCRC was performed in which 45 patients received standard 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or FOLFIRI plus regorafenib 160 mg on days 4–10 of every 2-week cycle [26]. Grade 3 or higher drug-related adverse events occurred in 32/45 patients (71 %), including neutropenia and leukopenia, hand-foot skin reaction, and hypophosphatemia. Seven out of 38 evaluable patients achieved a partial response (FOLFOX, n = 4; FOLFIRI, n = 3), and 26 patients had stable disease as their best response (FOLFOX, n = 14; FOLFIRI, n = 12). Given the historical data for disease control utilizing FOLFOX and FOLFIRI, the addition of regorafenib to cytotoxic chemotherapy in his trial was of limited efficacy.
More recently, regorafenib was combined with modified FOLFOX (mFOLFOX6) in first-line treatment of mCRC patients in a phase II study [27]. In the CORDIAL trial, 54 patients with mCRC were treated with mFOLFOX6 every 2 weeks at standard doses and regorafenib 160 mg on days 4–10 and 18–24 of each 28-day cycle. The primary endpoint of objective response rate (ORR) was 43.9 %, with no complete responses seen; disease control rate (DCR) was 85.4 %. Median PFS was 8.5 months, and median OS was not reached. Although this was a single-arm study, the mFOLFOX6 plus regorafenib regimen did not appear to improve ORR over historical controls. Importantly, treatment-emergent adverse events led to dose modifications in 51 patients (96.2 %), with discontinuation of a component of study treatment required by 19 patients (35.8 %) and discontinuation of full study treatment in 4 patients (8 %).
Like with aflibercept, studies are also currently underway to investigate the potential efficacy of regorafenib in the adjuvant colorectal cancer setting. Other settings of ongoing investigation include regorafenib in combination with radioembolization in patients with liver metastases, regorafenib in combination with other targeted therapies such as the next-generation activin-receptor like kinase-1 (ALK) inhibitor PF-03446962, and regorafenib in combination with FOLFIRI in the second-line setting, among others. Furthermore, the phase II ReDOS study is currently evaluating differing doses of regorafenib and strategies to optimally manage resultant hand-foot syndrome symptoms.
Finally, much remains to be discovered regarding potential biomarkers predictive for response to regorafenib. Thus far, no single biomarker has been identified to predict efficacy of regorafenib, and current investigations have shifted to develop molecular signatures to predict response. For example, Tabernero et al. recently reported an analysis of circulating DNA utilizing beads, emulsions, amplification, and magnetic (BEAMing) technology that showed a trend toward clinical benefit with regorafenib defined by KRAS and PIK3CA mutational status [28]. Much additional work is needed to prospectively clarify the patients who have the greatest opportunity to benefit from regorafenib, particularly given its known toxicities.
Ramucirumab (Cyramza®, Eli Lilly)
Ramucirumab is a fully human immunoglobulin G1 monoclonal antibody that binds with high affinity to the extracellular VEGF-binding domain of VEGFR2, thereby blocking VEGF ligands from binding and activating the receptor (Table 1) [29]. During development, it was thought that this approach would lead to more complete target inhibition, given that all VEGF ligands would be blocked from binding to VEGFR2. A phase I study was therefore performed in which 37 patients with advanced solid tumors were treated with weekly doses of intravenous ramucirumab [30]. The maximum tolerated dose (MTD) of ramucirumab was determined to be 13 mg/kg after dose-limiting toxicities of hypertension and deep venous thrombosis at 16 mg/kg. A total of 22 patients (60 %) experienced grade 3 or higher adverse events, including hypertension, abdominal pain, anorexia, vomiting, headache, and deep venous thrombosis (DVT), among others. In terms of efficacy, 4 of 27 patients (15 %) with measurable disease had a PR, and 11 out of 37 patients (30 %) had PR or stable disease (SD) lasting at least 6 months. Interestingly, serum VEGF-A levels at 7 days after treatment were 1.5- to 3.5-fold higher than pretreatment concentrations, validating the hypothesis that ramucirumab could effectively prevent binding of VEGF ligands to VEGFR2. Furthermore, dynamic-contrast-enhanced magnetic resonance imaging (DCE-MRI) demonstrated decreased tumor perfusion and vascularity in 9 of 13 patients (69 %): 5 of 7 receiving weekly treatment in this study and 4 of 6 in another trial receiving treatment every 2 to 3 weeks [31]. This latter phase I trial treated 25 advanced solid tumor patients with ramucirumab at escalating doses from 6 to 20 mg/kg every 2 or 3 weeks [31]. Treatment-related adverse events were as expected for an anti-angiogenic agent, and no dose-limiting toxicities (DLTs) were seen. Ramucirumab has a relatively long half-life of 110–160 h, and trends were seen toward elevated VEGF-A and a transient decline in soluble VEGFR-2 with ramucirumab treatment. A total of 15 out of 25 patients (60 %) had stable disease, including 3 patients with colorectal cancer. This study led to a recommended phase II ramucirumab dose of 8 mg/kg every 2 weeks and 10 mg/kg every 3 weeks.
After the initial phase I trials of ramucirumab in all tumor types, a small phase Ib study evaluated the safety of ramucirumab in combination with second-line FOLFIRI in six Japanese patients with mCRC [32]. This trial also aimed to confirm the recommended dose of 8 mg/kg ramucirumab every 2 weeks in combination with standard FOLFIRI. One patient had a PR and four had SD; importantly, only one patient had a DLT with grade 2 proteinuria and grade 4 neutropenia. These results, in combination with the phase II trial results showing that the pharmacokinetics of irinotecan and its metabolite were not affected when administered with ramucirumab, [33] led to phase III testing of ramucirumab in mCRC.
In the randomized, placebo-controlled phase III RAISE trial, ramucirumab was evaluated in combination with second-line FOLFIRI in 1072 metastatic colorectal cancer patients [34••]. Patients enrolled in this study all had disease progression on first-line bevacizumab, oxaliplatin, and fluoropyrimidine therapy and were treated with either 8 mg/kg IV ramucirumab or placebo every 2 weeks. Median OS was 13.3 months for the ramucirumab arm compared to 11.7 months for the placebo arm (HR 0.844, 95 % CI 0.730–0.976, log-rank p = 0.0219), with similar off-study systemic therapy post-protocol specified therapy being fairly similar among the two groups. PFS was also longer in the ramucirumab group (5.7 vs. 4.5 months, HR 0.793, 95 % CI 0.697–0.903, log-rank p = 0.0005). Patients achieving objective responses to therapy were similar in the groups receiving ramucirumab and placebo (13.4 vs. 12.5 %, p = 0.63). Frequency of grade 3 or worse adverse events was higher in the ramucirumab arm (79 vs. 62 %), including neutropenia, febrile neutropenia, hypertension, diarrhea, and fatigue, among others; no new safety signals were identified.
As a result of the RAISE trial data, on April 24, 2015, the FDA approved ramucirumab in combination with FOLFIRI for metastatic colorectal cancer patients whose disease had progressed on a first-line bevacizumab-, oxaliplatin-, and fluoropyrimidine-containing regimen. These results confirmed the efficacy of continuing anti-angiogenic therapy after disease progression. Despite the hazards inherent in cross-trial comparisons, the hazard ratios for OS and improvement in OS were similar in the RAISE, TML, and VELOUR trials [9••, 19••, 34••]. Thus far, biomarkers predictive for response to ramucirumab in mCRC have not been identified, although this work is underway. Additional questions regarding the optimal role of ramucirumab in other treatment lines or in combination with oxaliplatin-containing regimens have not yet been definitively answered [35].
Other Anti-angiogenics in Development for Colorectal Cancer
Other anti-angiogenic agents are also being developed and tested for the treatment of metastatic colorectal cancer beyond those already discussed. For example, nintedanib (Ofev®, Boehringer Ingelheim), a potent, oral multikinase agent that inhibits VEGFR1-3, FGFR1-3, PDGFR-α/β, RET, and Flt3 [36], currently seems promising given early phase trial results and the biologic hypothesis that it could potential overcome resistance to previous anti-VEGF treatments given its multiple targets (Table 1). A phase I accelerated dose-escalation trial of nintedanib in 61 patients with advanced solid tumors showed that nintedanib-related adverse events were frequent but generally manageable, with a maximum tolerated dose of 250 mg for one time and two times daily dosing [37•]. One patient with colorectal cancer had a partial response, and 24 out of 30 advanced CRC patients (80 %) on this trial achieved stable disease for at least 8 weeks. Interestingly, clinically relevant effects of nintedanib on tumor blood flow and permeability in colorectal cancer patients evaluable for DCE-MRI analysis were seen [38]. Further studies of nintedanib with chemotherapy or versus placebo for refractory CRC are ongoing. Clinical data on other anti-angiogenic agents in development for the treatment of mCRC are awaited.
Cost-Effectiveness of Anti-angiogenics for mCRC
In addition to understanding the optimal biologic role of anti-angiogenics in mCRC, navigating the cost effectiveness of these expensive agents is imperative. As the incidence of CRC increases and patients are living longer with mCRC, the cost of anti-angiogenics in relation to their efficacy must be addressed. One systematic review by Lange et al. evaluated published analyses of cost-effectiveness and cost-utility studies involving monoclonal antibody use for colorectal cancer [39•]. The incremental cost-effectiveness ratios (ICERs) for first-line treatment with bevacizumab plus chemotherapy are very high. In some countries with socialized medicine systems, regulatory bodies charged with balancing cost and efficacy have made the judgment that bevacizumab does not have sufficient benefit to be judged as cost-effective in the treatment of mCRC [39•]. Few cost-effectiveness analyses have been performed in the USA, however [40, 41].
Another study by Wade et al. published the clinical and cost-effectiveness evidence for aflibercept in combination with FOLFIRI for mCRC, as evaluated by the Evidence Review Group and the National Institute for Health and Care Evidence (NICE) in the UK [42]. Ultimately, the NICE Appraisal Committee concluded that aflibercept in combination with FOLFIRI could not be considered a cost-effective use of National Health Service resources for treating mCRC resistant to or that had progressed after an oxaliplatin-containing regimen.
In the USA, the pricing of aflibercept even when compared to bevacizumab has been controversial as well. Despite a similar benefit, aflibercept was initially priced approximately two times that of its competitor bevacizumab, leading to a New York Times editorial in which some oncologists refused to prescribe aflibercept based on its inferior cost-effectiveness [43]. Ultimately, this public outcry led to 50 % discounts on aflibercept offered to doctors and hospitals from Sanofi. As newer anti-angiogenics are approved and more widely used, attention should be paid by physicians and the public alike on cost-effectiveness of therapy. Development of biomarkers to prospectively predict effectiveness of therapy in particular patient populations would be very valuable in this regard.
Conclusions
Given the importance of angiogenesis in solid tumors such as colorectal cancer, attempts to inhibit this process are critical to the successful treatment of such cancers. Development of the anti-angiogenic agents discussed in this article represents significant advances in the understanding of the biology of angiogenesis and its intricate pathways. The relatively modest improvements in survival for patients with metastatic colorectal cancer treated with these agents suggest that their use has not yet been optimized. A better understanding of primary and acquired resistance to these agents, perhaps through as yet unknown mechanisms of action or compensatory pathways, is needed. Prospective selection of patients most likely to benefit from these agents, perhaps from development of validated predictive biomarkers, is also imperative. Finally, cost-effectiveness of anti-angiogenic agents must continue to be evaluated in light of their efficacy, particularly in the era of rapidly rising cancer treatment costs.
Abbreviations
- 5-FU :
-
5-Fluorouracil
- ALK :
-
Activin-receptor like kinase-1
- BEAMing :
-
Beads, emulsions, amplification and magnetic
- CI :
-
Confidence interval
- CRC :
-
Colorectal cancer
- DCE-MRI :
-
Dynamic-contrast-enhanced magnetic resonance imaging
- DCR :
-
Disease control rate
- DFS :
-
Disease-free survival
- DLT :
-
Dose-limiting toxicity
- DVT :
-
Deep venous thrombosis
- EGFR :
-
Epidermal growth factor receptor
- FGFR :
-
Fibroblast growth factor receptor
- FDA :
-
Food and Drug Administration
- FOLFOX :
-
5-Fluorouracil, leucovorin, and oxaliplatin
- FOLFOX4 :
-
Oxaliplatin 85 mg/m2, leucovorin 200 mg/m2, and fluorouracil 400 mg/m2 bolus plus 500 mg/m2 22-h continuous infusion on day1; leucovorin 200 mg/m2 plus fluorouracil 400 mg/m2 bolus plus 600 mg/m2 22-h continuous infusion on day 2
- FOLFIRI :
-
5-Fluorouracil, leucovorin and irinotecan
- HR :
-
Hazard ratio
- ICER :
-
Incremental cost-effectiveness ratio
- IFL :
-
Irinotecan, bolus fluorouracil, and leucovorin
- IV :
-
Intravenous
- KRAS :
-
Kirsten ras oncogene
- mCRC :
-
Metastatic colorectal cancer
- mFOLFOX6 :
-
Modified FOLFOX (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, 5-FU 400 mg/m2 bolus, and 5-FU2400 mg/m2 over 46 h)
- MTD :
-
Maximum tolerated dose
- NICE :
-
National Institute for Health and Care Evidence
- NSABP :
-
National Surgical Adjuvant Breast and Bowel Project
- ORR :
-
Overall response rate
- OS :
-
Overall survival
- PDGFR :
-
Platelet-derived growth factor receptor
- PR :
-
Partial response
- PlGF :
-
Placental growth factor
- PFS :
-
Progression-free survival
- SD :
-
Stable disease
- TBD :
-
To be determined
- VEGF :
-
Vascular endothelial growth factor
- XELOX :
-
Capecitabine and oxaliplatin
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76.
Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593–9.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern cooperative oncology group study E3200. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(12):1539–44.
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26(12):2013–9.
Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26(33):5326–34.
Grothey A, Flick ED, Cohn AL, et al. Bevacizumab exposure beyond first disease progression in patients with metastatic colorectal cancer: analyses of the ARIES observational cohort study. Pharmacoepidemiol Drug Saf. 2014;23(7):726–34.
Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. This phase III trial demonstrated the survival benefit to the continuation of bevacizumab beyond disease progression with first-line therapy in mCRC.
Allegra CJ, Yothers G, O'Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the national surgical adjuvant breast and bowel project C-08 trial. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(3):359–64.
Allegra CJ, Yothers G, O'Connell MJ, et al. Initial safety report of NSABP C-08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2009;27(20):3385–90.
de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13(12):1225–33.
Lambrechts D, Lenz HJ, de Haas S, et al. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(9):1219–30.
Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–8.
Lockhart AC, Rothenberg ML, Dupont J, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(2):207–14.
Van Cutsem E, Khayat D, Verslype C, et al. Phase I dose-escalation study of intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours. Eur J Cancer. 2013;49(1):17–24.
Khayat D, Tejpar S, Spano JP, et al. Intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours: results from the expansion cohort of a phase I study. Eur J Cancer. 2013;49(4):790–7.
Tang PA, Cohen SJ, Kollmannsberger C, et al. Phase II clinical and pharmacokinetic study of aflibercept in patients with previously treated metastatic colorectal cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18(21):6023–31.
Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30(28):3499–506. This phase III trial demonstrated the superiority of aflibercept versus placebo in combination with FOLFIRI for second-line mCRC therapy.
Tabernero J, Van Cutsem E, Lakomy R, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. 2014;50(2):320–31.
Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer J Int du Cancer. 2011;129(1):245–55.
Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73–4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18(9):2658–67.
Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73–4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106(11):1722–7.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12. This phase III trial showed the survival benefit of regorafenib in comparison to placebo in the refractory mCRC setting.
Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–29. This phase III trial in an Asian patient population demonstrated increased efficacy of regorafenib in refractory mCRC patients when compared with the CORRECT trial.
Schultheis B, Folprecht G, Kuhlmann J, et al. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study. Annals Oncol: Off J Eur Soc Med Oncol / ESMO. 2013;24(6):1560–7.
Argiles G, Saunders MP, Rivera F, et al. Regorafenib plus modified FOLFOX6 as first-line treatment of metastatic colorectal cancer: a phase II trial. Eur J Cancer. 2015;51(8):942–9.
Tabernero J, Lenz HJ, Siena S, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015.
Lu D, Jimenez X, Zhang H, et al. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer J Int du Cancer. 2002;97(3):393–9.
Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28(5):780–7.
Chiorean EG, Hurwitz HI, Cohen RB, et al. Phase I study of every 2- or 3-week dosing of ramucirumab, a human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2 in patients with advanced solid tumors. Annals Oncol: Off J Eur Soc Med Oncol / ESMO. 2015;26(6):1230–7.
Yoshino T, Yamazaki K, Gotoh M, et al. Safety and pharmacokinetics of second-line Ramucirumab plus FOLFIRI in Japanese patients with metastatic colorectal carcinoma. Anticancer Res. 2015;35(7):4003–7.
Wang DBF, Lee JJ, Denlinger CS, Shepard DR, Chaudhary A, Lin Y, et al. Phase II study evaluating the effect of concomitant ramucirumab on the pharmacokinetics of irinotecan and its metabolite SN-38 when coadministered with folinic acid and 5-fluorouracil in patients with advanced malignant solid tumors. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33 suppl 3:691.
Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. This phase III trial proved the efficacy of ramucirumab in combination with FOLFIRI in the second-line mCRC setting.
Garcia-Carbonero R, Rivera F, Maurel J, et al. An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer. Oncologist. 2014;19(4):350–1.
Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68(12):4774–82.
Mross K, Stefanic M, Gmehling D, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res: Off J Am Assoc Cancer Res. 2010;16(1):311–9. This phase I study demonstrated the safety and potential efficacy of nintedanib in patients with advanced solid tumors.
Mross K, Buchert M, Frost A, et al. Vascular effects, efficacy and safety of nintedanib in patients with advanced, refractory colorectal cancer: a prospective phase I subanalysis. BMC Cancer. 2014;14:510.
Lange A, Prenzler A, Frank M, et al. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50(1):40–9. This systematic review evaluated published analyses of cost effectiveness studies involving monoclonal antibody use for mCRC.
Shankaran V, Mummy D, Koepl L, et al. Survival and lifetime costs associated with first-line bevacizumab use in older patients with metastatic colorectal cancer. Oncologist. 2014;19(8):892–9.
Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33(10):1112–8.
Wade R, Duarte A, Simmonds M, et al. The clinical and cost effectiveness of aflibercept in combination with irinotecan and fluorouracil-based therapy (FOLFIRI) for the treatment of metastatic colorectal cancer which has progressed following prior oxaliplatin-based chemotherapy: a critique of the evidence. Pharmaco Econ. 2015;33(5):457–66.
Bach PB SL, Wittes RE. In cancer care, cost matters. New York Times. 2012:A25.
Compliance with Ethics Guidelines
Conflict of Interest
Kristen K. Ciombor declares that she has no conflict of interest.
Richard M. Goldberg has received research support through grants for a clinical trial from Sanofi and Bayer to The Ohio State University and has received compensation from Eli Lilly & Co. for service on a data safety monitoring committee for a clinical trial and from Sanofi for conducting a lecture at a symposium.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Topical Collection on Translational Colorectal Oncology
Rights and permissions
About this article
Cite this article
Ciombor, K.K., Goldberg, R.M. Update on Anti-angiogenesis Therapy in Colorectal Cancer. Curr Colorectal Cancer Rep 11, 378–387 (2015). https://doi.org/10.1007/s11888-015-0292-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-015-0292-3