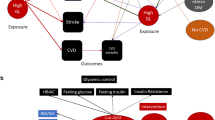
Abstract
Purpose of Review
Despite improvements in treatment, people with type 1 diabetes continue to have increased cardiovascular disease (CVD) risk. Glycemic control does not fully explain this excess CVD risk, so a greater understanding of other risk factors is needed.
Recent Findings
The authors review the relationship between glycemia and CVD risk in adults with type 1 diabetes and summarize evidence regarding other factors that may explain risk beyond glycemia. Insulin resistance, weight gain, sex differences, genetics, inflammation, emerging markers of risk, including lipid subclasses and epigenetic modifications, and future directions are discussed.
Summary
As glycemic control improves, an increased focus on other CVD risk factors is warranted in type 1 diabetes. Novel markers and precision medicine approaches may improve CVD prediction, but a lack of type 1 diabetes-specific guidelines for lipids, blood pressure, and physical activity are likely impediments to optimal CVD prevention in this high-risk population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Landmark findings from the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study that adoption of intensive type 1 diabetes management leads to large reductions in the incidence of complications [1] had practice-changing implications. However, as of 2018 only 21% of US adults with type 1 diabetes achieve the clinical goal of HbA1c < 7% [2] and both micro- and macrovascular complications continue to exert a significant burden in terms of death and disability, quality of life, and health care costs [3]. As a result, people with type 1 diabetes continue to have a dramatically increased risk of developing premature cardiovascular disease (CVD) [4,5,6]. While type 1 diabetes has been shown to be associated with adverse cardiovascular risk factors starting as early as childhood [7], this review will focus on recent data on CVD risk and risk factors in adults with type 1 diabetes.
The Burden of CVD in Type 1 Diabetes
CVD is the major cause of morbidity and mortality in type 1 diabetes [8, 9], affecting an estimated 27% of adults with this chronic disease in the USA [10]. CVD, the majority of which is coronary artery disease, is therefore one of the largest contributors to the overall health care costs associated with diabetes [11]. Indeed, people with type 1 diabetes who also have CVD are estimated to have annual health care expenses averaging > $30,000 per year compared to ~ $12,000 per year for adults with type 1 diabetes but no CVD [10].
Clinical trial evidence for a reduction in CVD complications are scarce in type 1 diabetes. However, the DCCT/EDIC study, in which microvascular complications were the primary outcomes, demonstrated a 42% decrease in CVD risk with intensive, compared to conventional, diabetes therapy [12]. Nonetheless, evidence to date suggests that the excess risk of CVD in type 1 diabetes compared to the general population remains high [4,5,6]. Thus, a large Norwegian registry estimated that adults with childhood-onset type 1 diabetes have ninefold excess risk of acute myocardial infarction (AMI) compared to age- and sex-matched adults without diabetes [5]. Similarly, recent data from the Finnish childhood-onset type 1 diabetes registry pointed to a nearly tenfold higher CVD risk in type 1 diabetes compared to the background population, despite incidence decreasing by 4% per year since 1965 [13••]. In addition to greater risk of incident CVD, type 1 diabetes is also associated with worse prognosis after an event, with 1-year case fatality rate after AMI estimated to be 1.5 times that of patients without diabetes [14•]. As demonstrated by the Pittsburgh Epidemiology of Diabetes Complications (EDC) study of childhood-onset type 1 diabetes, this excess CVD risk is particularly pronounced in younger (< 45 years) adults among whom there was over a 19-fold increased relative risk of CVD mortality, given the low CVD mortality risk in the age-matched background population [4]. Unlike the Finnish data showing a decline in CVD incidence over time [13••], CVD morbidity showed no improvement across three calendar-year diabetes diagnosis subcohorts in EDC (1965–1969, 1970–1974, and 1975–1980), although CVD mortality declined in those with more recent onset [15••]. Yet, CVD affects the vast majority of the type 1 diabetes population at long durations. In the FinnDiane cohort, cumulative incidence of CVD was 64% in those with type 1 diabetes duration of > 50 years [16]. Data from Scotland and Sweden showed slightly lower estimated CVD burden than the other studies, with 80% remaining free of CVD by age 50 and 50% by age 65 [17]. However, it is important to note the Scottish and Swedish cohorts include adult-onset type 1 diabetes cases; thus, average diabetes duration at any given age is significantly shorter than the cohorts of childhood-onset type 1 diabetes and may explain differences in observed CVD rates.
Glycemia and CVD Risk
Despite the strong association between hyperglycemia and microvascular complications, glycemia has historically been an inconsistent predictor of CVD incidence within type 1 diabetes. In DCCT/EDIC, a reduction in HbA1c from 8 to 7.2% was associated with a significant 28% reduction in CVD risk over 20 years [12]. Additionally, HbA1c was the strongest independent risk factor for both the first [18, 19•] and subsequent [19•] CVD events in DCCT/EDIC. In contrast, HbA1c was not a strong risk factor for CVD in the observational type 1 diabetes cohorts [20]. However, more recent analyses suggest that the lack of HbA1c association in those earlier observational studies may have been due to insufficient variability in HbA1c at baseline, preventing the detection of a statistical association [21]. Indeed, in an analysis incorporating longitudinal HbA1c in the EDC study, each 1% increase in HbA1c was significantly associated with a 26% increased risk of CVD over 25 years after adjusting for traditional CVD risk factors [21].
Nevertheless, it is important to recognize that glycemia does not affect CVD risk in isolation of other pathogenic mechanisms. There is evidence that the effect of glycemia on CVD is mediated by other CVD risk factors including lipids and blood pressure, such that hyperglycemia contributes to the derangement of those factors which in turn affect CVD risk over time [22]. There is also evidence that long-term HbA1c may modify effects of other risk factors on CVD risk in type 1 diabetes, such that their effects may be increased or decreased depending on the cumulative exposure to hyperglycemia [23••]. The EDC study has recently shown that over 30 years of follow-up, kidney disease markers were stronger risk factors for CVD, especially major adverse cardiovascular events (MACE), in those with worse long-term glycemic control, but traditional risk factors including lipids, blood pressure, and smoking were stronger risk factors in those with better glycemic control [23••]. These findings support the need for a greater clinical focus on traditional CVD risk factors as glycemic control improves, which may help to reduce the remaining excess CVD risk in type 1 diabetes. Finally, from a clinical perspective, it is important to appreciate that, while HbA1c has been a valuable measure for studying epidemiologic associations between glycemic exposure and complication risk, HbA1c may not be the optimal measure of an individual patient’s glycemic control because as an average measure of glucose exposure it does not capture glucose variability [24]. This point was demonstrated in a recent commentary by Beck et al., which clearly showed that the same HbA1c could result from very different patterns of glycemic control across individuals [24]. As continuous glucose monitoring becomes more widely adopted, utilizing more precise, individualized metrics of glycemic control, such as time in range, will be needed to optimize complication prevention.
Insulin Resistance and Weight Gain Are Associated with CVD Risk
Insulin resistance is associated with poor glycemic control, greater insulin dose requirements, and increased risk of complications [25] and may be a stronger predictor of CVD than glycemic control itself [26]. Concern regarding insulin resistance in type 1 diabetes has increased as intensive insulin therapy has led to concomitant weight gain. In DCCT/EDIC, incidence of becoming overweight was 1.7-fold greater in intensive therapy arm participants compared to the conventional therapy arm [27]. In the EDC study, the prevalence of overweight increased by 47% and obesity sevenfold over 18 years and such weight gain was associated with initiation of intensive therapy during follow-up [28]. Excess body weight is now commonplace in type 1 diabetes and mirrors the general population, with 29% of US adults with type 1 diabetes meeting BMI criteria for overweight and 19% for obesity [29]. Furthermore, weight gain may contribute to worsening of other CVD risk factors. For example, DCCT/EDIC participants with “excessive” weight gain (defined as BMI increase ≥ 4.39 kg/m2 during the trial period) had worse CVD risk factor profiles [30], though more prevalent treatment of lipids and blood pressure in those participants seems to have protected against a concomitant increase in clinical CVD events early on [31].
Weight gain can be difficult to control in type 1 diabetes, especially via exercise, due to challenges associated with managing blood glucose during physical activity. There is also a lack of guidelines and limited data on strategies for weight management specific to type 1 diabetes [32•]. However, research is currently underway to address those gaps. One such initiative is the ongoing Advancing Care for Type 1 Diabetes and Obesity Network (ACT1ON) Consortium, which has examined mechanistic aspects of metabolism and energy requirements of people with type 1 diabetes with the goal of developing and evaluating an adaptive behavioral intervention for weight management with optimization of glycemic control [32•].
Insulin resistance has been proposed as a potential target for adjunctive therapies to insulin in type 1 diabetes [33]. The use of existing pharmacologic agents for insulin resistance and weight, such as metformin, sodium-glucose cotransporter (SGLT) inhibitors, and GLP-1 agonists, may hold promise to reduce CVD risk in type 1 diabetes. In the limited data in adults with type 1 diabetes thus far, metformin does not seem to reduce HbA1c over the long term, but does decrease body weight and insulin dose and has been shown to reduce subclinical markers of CVD risk (e.g., intima-media thickness) in high-risk individuals [33]. SGLT inhibitors have been shown to not only improve glycemic control and reduce body weight in type 1 diabetes but also increase the risk of diabetic ketoacidosis, necessitating caution [33]. Data on GLP-1 agonists in type 1 diabetes is mixed and more research is needed to identify subgroups of patients who may derive the most benefit, while reducing the risk of hypoglycemia [34].
CVD Risk Begins to Increase Below Clinical Targets for Cholesterol and Blood Pressure
Aside from hyperglycemia, on average, people with type 1 diabetes have only minor clinical CVD risk factor differences [35], and oftentimes better risk factor profiles [36], compared to the general population. However, it has long been recognized in type 1 diabetes that CVD and other complication risk may increase at cholesterol and blood pressure levels below established clinical targets [37, 38]. In a recent analysis of long-term cumulative blood pressure levels over the 25-year follow-up of the EDC study, the risk of coronary artery disease began to increase at systolic blood pressure of 120 mmHg and diastolic blood pressure of 80 mmHg [39••]. Similar lower blood pressure targets have also been suggested by prior studies [40, 41]. Earlier data from the EDC study also suggested that CVD risk increases at LDL cholesterol > 100 mg/dl [37]; the same cut-point, which was lower than contemporary general recommendations, was also found to be optimal for CVD risk reduction in a recent report from the Swedish National Diabetes Registry [42••]. Despite such evidence, there remains a lack of type 1 diabetes-specific recommendations due to few trials focused on CVD risk reduction in this population [43]. Current guidelines are based on evidence from type 2 diabetes, despite a lack of knowledge on the appropriateness of such extrapolation.
Loss of Female Protection Against CVD in Type 1 Diabetes
It is now well recognized that the protection against CVD observed in women compared to men in the general population is diminished in diabetes and among individuals with type 1 diabetes women have nearly equivalent absolute CVD risk as men [44, 45]. The reasons underlying this excess relative risk in women are unclear but may relate to differences in the risk factor profile [46] or disparities in risk factor treatment by sex [47]. Thus, in DCCT/EDIC, women were less likely than men to achieve HbA1c < 7% or < 8%, despite being more likely to be using insulin pumps (58% of women versus 38% of men) and as likely to monitor blood glucose ≥ 4 times per day (61% of women versus 58% of men) [47]. However, differences in glucose management and HbA1c do not seem to directly account for the greater relative risk for CVD in women with type 1 diabetes [48] and other cardiovascular risk factors may be more likely to explain their excess CVD risk. Indeed, female participants of DCCT/EDIC were less likely to be prescribed statins, even if they had elevated LDL cholesterol levels [47]. Likewise, in the EDC study, in young adults < 45 years old, women were about half as likely as men to report statin use, despite similar levels of LDL cholesterol on average [4]. This lower rate of statins, particularly in younger women, may have been due to contraindication of statin use in women who could become pregnant. As the US Food and Drug Administration has recently removed the “Pregnancy Category X” label from statins, it remains to be seen whether the disparity in statin use by sex in type 1 diabetes may be reduced in the future [49].
Differences in CVD risk factors by sex appear as early as adolescence in type 1 diabetes [46]. In the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study, women with type 1 diabetes exhibited a more adverse adiposity profile than women without diabetes while there was no difference in men by diabetes status [50]. Furthermore, in the same study, lipids and adiposity measures explained much of the excess coronary artery calcification in women with type 1 diabetes. The CACTI study also showed that type 1 diabetes is associated with greater insulin resistance in women compared to men [51], potentially explaining why women with type 1 diabetes have similar CVD rates as men, despite a greater proportion of women using intensive insulin therapy [52]. Sex hormone disturbances may contribute to that increased insulin resistance, as women with type 1 diabetes have been shown to have lower levels of estradiol and estrogen activity compared to women without diabetes [53]. The hormone disturbances may be related to the role of insulin in maintaining balance of the hypothalamus-pituitary-ovary axis; thus, both endogenous insulin deficiency [54] and exogenous hyperinsulinemia likely contribute [55].
Another reason for this excess CVD risk among women compared with men in type 1 diabetes may relate to sex differences in the association between HDL cholesterol (HDL-C) and CVD. While higher HDL-C is generally protective against CVD, HDL-C > 60 mg/dl offered no additional protection against CAD compared to 50–60 mg/dl among women in the EDC study [56]. Additionally, very high HDL-C > 80 mg/dl was associated with an increased risk of CAD in women only. That U-shaped relationship between HDL-C concentrations and CAD risk suggests that there could be differences in the composition of HDL-C in women with type 1 diabetes. In support of that hypothesis, in the Joslin Medalists study of long-duration type 1 diabetes, the HDL-C subfractions containing apolipoprotein A1 and A2 were lower in women with CVD compared to those without CVD, a difference not observed in men [57].
Lipoprotein Subclasses
Plasma lipid levels are not necessarily elevated in type 1 diabetes, especially among individuals with good glycemic control [36]. However, poor glycemic control, diabetic kidney disease, and insulin resistance are associated with more atherogenic lipid subfractions, which may not be reflected in conventional lipid profiles [36, 58]. Therefore, lipoprotein subclasses may more accurately assess CVD risk associated with lipids in type 1 diabetes. CVD risk has been associated with lipid subclasses including greater VLDL particle number [59], greater large and medium HDL subfractions [59], and total serum ApoC3 and ApoC3 in HDL [60]. Perhaps related to the loss of female protection discussed above, type 1 diabetes may also differentially affect LDL size and particle numbers in women compared to men [61]. In a recent report from Spain, in patients with new onset type 1 diabetes, lipoprotein subclasses substantially improved after achievement of optimal glycemic control [62•]. Improvements were especially marked in the atherogenic ApoB-containing lipoproteins, including intermediate density lipoprotein (IDL), which is not detected in conventional lipid panels.
Genetics of CVD Risk in Type 1 Diabetes
There are very limited genome-wide association studies (GWAS) for CVD in type 1 diabetes, with only two reports to date [63, 64]. The first [63] detected novel associations between three single nucleotide polymorphisms (SNP) at the CDK18, FAM189A2, and PKD1 loci and CAD. The authors also reported that three previously identified SNPs in ANKS1A, COL4A2, and APOE had stronger associations with CAD in type 1 diabetes than in the general population. Apart from CDK18 (odds ratio [OR] = 2.6), the associations were relatively weak (ORs between 1.3 and 1.9). The second, more recent GWAS [64] detected an association between a SNP near CDKN2B-AS1 and CAD that was replicated in independent cohorts, but again the effect was relatively weak (OR = 1.3). Another variant, near DEFB127, had a stronger effect size (OR = 4.2) which, however, could not be replicated.
As CVD is a complex phenotype with multifaceted etiology, polygenic risk scores (PRS) may better reflect genetic risk than single variants detected in GWAS. DCCT/EDIC investigators recently applied a CAD PRS identified in individuals without diabetes based on over 6 million SNPs to type 1 diabetes and found 38% of CVD and 40% of MACE risk per PRS standard deviation [65•]. Importantly, while those associations were statistically independent of established risk factors, including age, HbA1c, lipids, and blood pressure, the PRS only modestly improved prediction of CVD over risk factor levels. Thus, PRS may help improve understanding of pathophysiologic pathways, but clinical utility remains unknown.
Haptoglobin (HP), a copy-number variant, is a major candidate gene for CVD risk in diabetes [66]. Haptoglobin is an acute phase plasma glycoprotein whose function is to bind free hemoglobin, inhibiting release of heme iron, resulting in reduced oxidative potential of free hemoglobin [67]. The HP 2 allele has been associated with an increased risk of CAD, likely via reduced anti-oxidant capacity, first in type 2 diabetes [68] and more recently in type 1 diabetes [69, 70]. HP may also affect CVD risk through anti-inflammatory properties associated with the HP 1 allele (discussed below). In the CACTI study, HP 2–2 genotype predicted progression of coronary artery calcification [71]. While the association between the HP 2 allele and increased CVD risk seems to be specific to diabetes/presence of hyperglycemia, within type 1 diabetes the risk conferred by the HP 2 allele is stronger in individuals with lower glycemic exposure [72], suggesting that as glycemic control improves, the genetic susceptibility conferred by HP becomes more evident. Such an apparently non-glycemic pathway may point to new therapeutic strategies to reduce CVD risk in T1D [73]. Another promising candidate gene is fatty acid binding protein 4 (FABP4), a low-expression variant of which (G allele of rs77878271) was recently associated with 17% increased risk of CVD and 26% increased risk of CAD in a meta-analysis of studies focusing on type 1 diabetes [74].
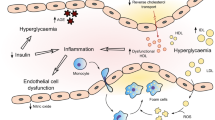
Inflammation and CVD Risk in Type 1 Diabetes
It has long been known that chronic inflammation increases the risk of vascular disease [75]. Both innate and adaptive immune responses are thought to be involved in the vascular damage underlying the development of CVD [76,77,78], related to endothelial injury and increased plaque instability [79]. Compared to people without diabetes, people with type 1 diabetes have increased levels of pro-inflammatory and immune response biomarkers, including cytokines, adhesion molecules, and chemokines, starting as early as childhood [80]. In type 1 diabetes, increased inflammation is correlated with worse glycemic control [81], diabetic kidney disease [82], insulin resistance [83], and hypoglycemia [79]. Thus, hyperglycemia may directly lead to increased levels of circulating inflammatory biomarkers, which may be at least partially responsible for the excess CVD risk associated with type 1 diabetes. Indeed, higher levels of candidate markers of systemic inflammation, including leukocyte count [84, 85], galectin-3 [86], high sensitivity C-reactive protein [87], Lp-PLA2 [87], and kallikrein [88], have been epidemiologically associated with increased CVD risk in type 1 diabetes. Heart failure biomarkers, high sensitivity cardiac troponin-t (hs-cTnT), and N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) have recently been demonstrated to be associated with future total CVD and MACE in type 1 diabetes [89, 90]; however, they do not consistently improve prediction over traditional risk factors [90]. The candidate gene, HP (discussed above), is also associated with increased inflammation [91]. It has been observed that increases in isoprostanes (a measure of oxidative stress) and white blood cell count over time directly correlated with the number of HP 2 alleles relative to HP 1 alleles [92]. These findings suggest that, in addition to deficiencies in protection from oxidative stress, the HP 2 allele may confer inferior anti-inflammatory capacity compared to the HP 1 allele, a possible mechanism through which the HP gene influences CVD risk.
Epigenetics
Though there is currently a lack of data on the association between epigenetic modifications and CVD outcomes in type 1 diabetes to date, epigenetics is an emerging area of research in diabetes complications. Epigenetic associations with CVD in the general population suggest that this research may help improve understanding of how environment/exposures affect gene expression to influence CVD risk [93]. There are recent epigenome-wide association studies (EWAS) examining associations between differential DNA methylation and microvascular complications in type 1 diabetes [94,95,96]. Of note, it has been demonstrated in DCCT/EDIC that DNA methylation of the TXNIP locus mediates the association between HbA1c and microvascular complication development [96]. Its relative affordability and ease of measurement, along with the potential to be pharmacologically modified [97], makes DNA methylation a particularly promising marker to elucidate pathophysiologic pathways and discover new intervention targets.
Future Directions
As research to improve CVD risk prediction moves forward, studies in exclusively type 1 diabetes cohorts are needed to address the unique challenges of developing early interventions for this high-risk population. In particular, it is unclear whether it is appropriate to extrapolate associations detected in type 2 diabetes to type 1 diabetes. In addition to important differences in pathophysiology between type 1 and type 2 diabetes, type 1 diabetes onset occurs most commonly in children and young adults, while type 2 diabetes occurs most commonly in middle to older adulthood. Thus, on average, the length of exposure to diabetes/hyperglycemia is greater in type 1 diabetes than in type 2 diabetes at any given age [98]. Another major limitation of type 1 diabetes complication research in general is the limited data in minority groups, especially in genetic studies. The New Jersey 725 cohort has provided valuable information on black patients with type 1 diabetes but was drawn from a limited geographic region and primarily focused on eye complications [99]. Thus, there is a need for a large multi-ethnic type 1 diabetes cohort.
It is critical to understand that not only CVD risk but also its risk factors may differ by characteristics including but not limited to cumulative glycemic exposure [23••], sex and age at diabetes onset [100], and genetic factors [72]. Risk factors may also differ by the specific manifestation of CVD [101]. Thus, the CVD phenotype definition must be carefully considered to ensure the correct interpretation of the results. The decision to include coronary revascularization in the CVD definition requires careful consideration, as it indicates CVD morbidity but is also a preventative procedure reflecting both access to care and a medical decision; thus, its inclusion or exclusion can affect detection of risk factor associations [101]. Furthermore, strength of risk factor associations for coronary artery disease and cerebrovascular disease may differ [102], so the inclusion or exclusion of stroke may also affect interpretation of results. Finally, precision medicine approaches are needed to identify subgroups of people with type 1 diabetes who may benefit from specific interventions. To that end, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) have instituted a joint Precision Medicine in Diabetes Initiative to promote research and ultimately clinical implementation of precision medicine [103].
Conclusions
This review summarized recent research on the epidemiology of CVD in adults with type 1 diabetes.
While glycemic control is the cornerstone of type 1 diabetes management, an increased focus on other CVD risk factors is needed, particularly as glycemic control improves. Genetic variants, emerging risk markers, and precision medicine approaches may improve CVD risk prediction, but a lack of type 1 diabetes-specific guidelines and clinical target levels for lipids, blood pressure, and physical activity is likely limiting progress on CVD prevention in this high-risk population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nathan DM, Zinman B, Cleary PA, Backlund JYC, Genuth S, Miller R, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: The diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience. Arch Intern Med. 2009;169(14):1307–16.
Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, Dimeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66.
Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Diabetes Care. 2008;31(8):1686–96.
Miller RG, Mahajan HD, Costacou T, Sekikawa A, Anderson SJ, Orchard TJ. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2016;39(12):2296–303.
Saeed M, Stene LC, Ariansen I, Tell GS, Tapia G, Joner G, et al. Nine-fold higher risk of acute myocardial infarction in subjects with type 1 diabetes compared to controls in Norway 1973–2017. Cardiovasc Diabetol. 2022;21(1):59.
Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392(10146):477–86.
Jones S, Khanolkar AR, Gevers E, Stephenson T, Amin R. Cardiovascular risk factors from diagnosis in children with type 1 diabetes mellitus: a longitudinal cohort study. BMJ Open Diabetes Res Care. 2019;7(1):e000625.
Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59(12):3216–22.
Gagnum V, Stene LC, Jenssen TG, Berteussen LM, Sandvik L, Joner G, et al. Causes of death in childhood-onset Type 1 diabetes: long-term follow-up. Diabet Med. 2017;34(1):56–63.
Edelman S, Zhou FL, Preblick R, Verma S, Paranjape S, Davies MJ, et al. Burden of cardiovascular disease in adult patients with type 1 diabetes in the US. PharmacoEconomics - Open. 2020;4(3):519–28.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–28.
Nathan DM, Cleary PA, Backlund JYC, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53.
•• Harjutsalo V, Pongrac Barlovic D, Groop PH. Long-term population-based trends in the incidence of cardiovascular disease in individuals with type 1 diabetes from Finland: a retrospective, nationwide, cohort study. Lancet Diabetes Endocrinol. 2021;9(9):575–85. Recent, large population-based estimates of CVD incidence in type 1 diabetes, showed that while absolute incidence has declined by 4% per year since 1965, CVD risk continues to be approximately 10-fold higher in type 1 diabetes compared to the background population.
• Kerola AM, Juonala M, Palomäki A, Semb AG, Rautava P, Kytö V. Case fatality of patients with type 1 diabetes after myocardial infarction. Diabetes Care. 2022;epub ahead of print. A large, population-based study which observed significantly higher case fatality rates 30 days and 1 year after myocardial infarction in type 1 diabetes compared to nondiabetes.
•• Costacou T, Orchard TJ. Recent trends over time in vascular disease in type 1 diabetes: insights from the Pittsburgh Epidemiology of Diabetes Complications study. Cardiovascular Endocrinology and Metabolism. 2019;8(1):3–13. A cohort study with long-term follow-up of three type 1 diabetes diagnosis calendar year subcohorts which observed a significant decline in CVD mortality with more recent diagnosis, but no change in CVD morbidity.
Harjutsalo V, Barlovic DP, Gordin D, Forsblom C, King G, Groop PH. Presence and determinants of cardiovascular disease and mortality in individuals with type 1 diabetes of long duration: the FinnDiane 50 years of diabetes study. Diabetes Care. 2021;44(8):1885–93.
McGurnaghan SJ, McKeigue PM, Read SH, Franzen S, Svensson AM, Colombo M, et al. Development and validation of a cardiovascular risk prediction model in type 1 diabetes. Diabetologia. 2021;64(9):2001–11.
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Risk factors for cardiovascular disease in type 1 diabetes. Diabetes. 2016;65:1370–9.
• Bebu I, Schade D, Braffett B, Kosiborod M, Lopes-Virella M, Soliman EZ, et al. Risk factors for first and subsequent CVD events in type 1 diabetes: the DCCT/EDIC study. Diabetes Care. 2020;43(4):867–74. A comprehensive examination of risk factors for both first and subsequent CVD events in type 1 diabetes.
Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6).
Miller RG, Anderson SJ, Costacou T, Sekikawa A, Orchard TJ. Hemoglobin A1c and cardiovascular disease incidence in type 1 diabetes: an application of joint modeling of longitudinal and time-to-event data in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study. Am J Epidemiol. 2018;187(7):1520–9.
Bebu I, Braffett BH, Pop-Busui R, Orchard TJ, Nathan DM, Lachin JM, et al. The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia. 2017;60(10):2084–91.
•• Miller RG, Orchard TJ, Costacou T. 30-year cardiovascular disease in type 1 diabetes: risk and risk factors differ by long-term patterns of glycemic control. Diabetes Care. 2022;45(1):142–50. Identified trajectories of glycemic control associated with differential CVD risk over 30 years, as well as imp differences in CVD risk factors by glycemic trajectory.
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–9.
Helliwell R, Warnes H, Kietsiriroje N, Campbell M, Birch R, Pearson SM, et al. Body mass index, estimated glucose disposal rate and vascular complications in type 1 diabetes: beyond glycated haemoglobin. Diabet Med. 2021;38(5).
Orchard TJ, Olsen JC, Erbey JR, Williams K, Forrest KYZ, Smithline Kinder L, et al. Insulin resistance – related factors, but not glycemia, predict coronary artery disease in type 1 diabetes. Diabetes Care. 2003;26(5):1374–9.
Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care. 1995;18(11):1415–27. https://doi.org/10.2337/diacare.18.11.1415. PMID: 8722064.
Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, et al. Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med. 2010;27(4):398–404.
Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, Dimeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange Clinic Registry. Diabetes Care. 2015;38(6):971–8.
Purnell JQ, John EH, Cleary PA, Nathan DM, Lachin JM, Zinman B, et al. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127(2):180–7.
Purnell JQ, Braffett BH, Zinman B, Gubitosi-Klug RA, Sivitz W, Bantle JP, et al. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care. 2017;40(12):1756–62.
• Corbin KD, Igudesman D, Addala A, Casu A, Crandell J, Kosorok MR, et al. Design of the Advancing Care for Type 1 Diabetes and Obesity Network energy metabolism and sequential multiple assignment randomized trial nutrition pilot studies: An integrated approach to develop weight management solutions for individuals with type 1 diabetes. Contemp Clin Trials. 2022;117:106765. Describes a major ongoing effort to develop solutions for weight management that also simultaneously optimize glycemic control in type 1 diabetes.
Warnes H, Helliwell R, Pearson SM, Ajjan RA. Metabolic control in type 1 diabetes: is adjunctive therapy the way forward? Diabetes Therapy. 2018;9(5):1831–51.
von Scholten BJ, Kreiner FF, Gough SCL, von Herrath M. Current and future therapies for type 1 diabetes. Diabetologia. 2021;64(5):1037–48.
Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, et al. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes Care. 2009;32(3):416–20.
Amor AJ, Castelblanco E, Hernández M, Gimenez M, Granado-Casas M, Blanco J, et al. Advanced lipoprotein profile disturbances in type 1 diabetes mellitus: a focus on LDL particles. Cardiovasc Diabetol. 2020;19(1):1–16.
Orchard TJ, Forrest KY, Kuller LH, Becker DJ, Pittsburgh Epidemiology of Diabetes Complications Study. Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2001;24(6):1053–9.
Miller RG, Secrest AM, Ellis D, Becker DJ, Orchard TJ. Changing impact of modifiable risk factors on the incidence of major outcomes of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2013;36(12):3999–4006.
•• Guo J, Brooks MM, Muldoon MF, Naimi AI, Orchard TJ, Costacou T. Optimal blood pressure thresholds for minimal coronary artery disease risk in type 1 diabetes. Diabetes Care. 2019;42(9):1692–9. Showed that 25-year risk of CAD markedly increases at blood pressures below clinical target levels in type 1 diabetes.
Sibal L, Law H, Gebbie J, Dashora U, Agarwal S, Home P. Predicting the development of macrovascular disease in people with type 1 diabetes. Ann N Y Acad Sci. 2006;1084(1):191–207.
Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: The WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(S2):S54-64.
•• Rawshani A, Rawshani A, Sattar N, Franzén S, Mcguire DK, Eliasson B, et al. Relative prognostic importance and optimal levels of risk factors for mortality and cardiovascular outcomes in type 1 diabetes mellitus. Circulation. 2019;139(16):1900–12. Observed that mortality and CVD risk increased at levels of HbA1c, systolic blood pressure, and LDL-c below clinical targets in type 1 diabetes.
American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S144–74.
Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease is associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8.
Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, et al. sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care. 2014;37(3):830–8.
Brown TL, Maahs DM, Bishop FK, Snell-Bergeon JK, Wadwa RP. Influences of gender on cardiovascular disease risk factors in adolescents with and without type 1 diabetes. Int J Pediatr Endocrinol. 2016;2016(1):8.
Larkin ME, Backlund JY, Cleary P, Bayless M, Schaefer B, Canady J, et al. Disparity in management of diabetes and coronary heart disease risk factors by sex in DCCT/EDIC. Diabet Med. 2010;27(4):451–8.
Miller RG, Costacou T. Glucose Management and the sex difference in excess cardiovascular disease risk in long-duration type 1 diabetes. Curr DiabRep. 2019;19(12):1–9.
Mauricio R, Khera A. Statin use in pregnancy: is it time for a paradigm shift? Circulation. 2022;145(7):496–8.
Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52(11):2833–9.
Millstein RJ, Pyle LL, Bergman BC, Eckel RH, Maahs DM, Rewers MJ, et al. Sex-specific differences in insulin resistance in type 1 diabetes: the CACTI cohort. J Diabetes Complications. 2018;32(4):418–23.
Swasey KK, Orchard TJ, Costacou T. Trends in cardiovascular risk factor management in type 1 diabetes by sex. J Diabetes Complications. 2018;32(4):411–7.
Martínez D, Castro A, Merino PM, López P, Lardone MC, Iñiguez G, et al. Oestrogen activity of the serum in adolescents with type 1 diabetes. Diabet Med. 2016;33(10):1366–73.
Thong EP, Codner E, Laven JSE, Teede H. Diabetes: a metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 2020;8(2):134–49.
Kolb H, Kempf K, Röhling M, Martin S. Insulin: too much of a good thing is bad. BMC Med. 2020;18(1):1–12.
Costacou T, Evans R, Orchard T. High density lipoprotein cholesterol in diabetes: is higher always better? J Clin Lipidol. 2011;5(5):387–94.
He ZH, D’Eon SA, Tinsley LJ, Fitzgerald S, Hastings SM, Khamaisi M, et al. Cardiovascular disease protection in long-duration type 1 diabetes and sex differences. Diabetes Care. 2015;38(5):e73–4.
Cree-Green M, Maahs DM, Ferland A, Hokanson JE, Wang H, Pyle L, et al. Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes. 2016;17(4):257–65.
Soedamah-Muthu SS, Chang YF, Otvos J, Evans RW, Orchard TJ. Lipoprotein subclass measurements by nuclear magnetic resonance spectroscopy improve the prediction of coronary artery disease in type 1 diabetes. A prospective report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2003;46(5):674–82.
Basu A, Bebu I, Jenkins AJ, Stoner JA, Zhang Y, Klein RL, et al. Serum apolipoproteins and apolipoprotein-defined lipoprotein subclasses: a hypothesis-generating prospective study of cardiovascular events in T1D. J Lipid Res. 2019;60(8):1432.
Colhoun HM, Otvos JD, Rubens MB, Taskinen MR, Richard Underwood S, Fuller JH. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diabetes. 2002;51(6):1949–56.
• Castelblanco E, Hernández M, Ortega E, Amigó N, Real J, Granado-Casas M, et al. Outstanding improvement of the advanced lipoprotein profile in subjects with new-onset type 1 diabetes mellitus after achieving optimal glycemic control. Diabetes Res Clin Pract. 2021;182:109145. Demonstrated improvements in lipoproteins and lipoprotein subclasses after glycemic control was optimized, particularly for intermediate density lipoprotein which is not reflected in conventional lipid profiles.
Charmet R, Duffy S, Keshavarzi S, Gyorgy B, Marre M, Rossing P, et al. Novel risk genes identified in a genome-wide association study for coronary artery disease in patients with type 1 diabetes. Cardiovasc Diabetol. 2018;17(1):61.
Antikainen AAV, Sandholm N, Trégouët DA, Charmet R, McKnight AJ, Ahluwalia TS, et al. Genome-wide association study on coronary artery disease in type 1 diabetes suggests beta-defensin 127 as a risk locus. Cardiovasc Res. 2021;117(2):600–12.
• Bebu I, Keshavarzi S, Gao X, Braffett BH, Canty AJ, Herman WH, et al. Genetic risk factors for CVD in type 1 diabetes: the DCCT/EDIC Study. Diabetes Care. 2021;44(6):1309–16. Observed that a polygenic risk score was significantly associated with CVD incidence in DCCT/EDIC, however CVD prediction was not improved compared to standard risk factor levels.
Costacou T, Levy AP. Haptoglobin genotype and its role in diabetic cardiovascular disease. J Cardiovasc Transl Res. 2012;5(4):423–35.
Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42(10):1589–600.
Asleh R, Levy AP. In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag. 2005;1(1):19–28.
Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes. 2008;57(6):1702–6.
Orchard TJ, Backlund JYC, Costacou T, Cleary P, Lopes-Virella M, Levy AP, et al. Haptoglobin 2–2 genotype and the risk of coronary artery disease in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). J Diabetes Complications. 2016;30(8):1577–84.
Simpson M, Snell-Bergeon JK, Kinney GL, Lache O, Miller-Lotan R, Anbinder Y, et al. Haptoglobin genotype predicts development of coronary artery calcification in a prospective cohort of patients with type 1 diabetes. Cardiovasc Diabetol. 2011;10:99.
Costacou T, Evans RW, Orchard TJ. Glycaemic control modifies the haptoglobin 2 allele-conferred susceptibility to coronary artery disease in type 1 diabetes. Diabet Med. 2016;33(11):1524–7.
Costacou T, Levy AP, Miller RG, Snell-Bergeon J, Asleh R, Farbstein D, et al. Effect of vitamin E supplementation on HDL function by haptoglobin genotype in type 1 diabetes: results from the HapE randomized crossover pilot trial. Acta Diabetol. 2016;53(2):243–50.
Dahlström EH, Saksi J, Forsblom C, Uglebjerg N, Mars N, Thorn LM, et al. The low-expression variant of fabp4 is associated with cardiovascular disease in type 1 diabetes. Diabetes. 2021;70(10):2391–401.
Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43.
Fukami A, Yamagishi SI, Adachi H, Matusi T, Yoshikawa K, Ogata K, et al. High white blood cell count and low estimated glomerular filtration rate are independently associated with serum level of monocyte chemoattractant protein-1 in a general population. Clin Cardiol. 2011;34(3):189–94.
Gerszten RE, Mach F, Sauty A, Rosenzweig A, Luster AD. Chemokines, leukocytes, and atherosclerosis. J Lab Clin Med. 2000;136:87–92.
Jones DP, True HD, Patel J. Leukocyte trafficking in cardiovascular disease: insights from experimental models. Mediators Inflamm. 2017;2017:1–9.
Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33(6):1389–94.
Snell-Bergeon JK, West NA, Mayer-Davis EJ, Liese AD, Marcovina SM, D’Agostino RB, et al. Inflammatory markers are increased in youth with type 1 diabetes: The SEARCH case-control study. J Clin Endocrinol Metab. 2010;95(6):2868–76.
King DE, Mainous AG, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26(5):1535–9.
Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25(5):805–13.
Piemonti L, Calori G, Lattuada G, Mercalli A, Ragogna F, Garancini MP, et al. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32:2105–10.
Miller RG, Anderson SJ, Costacou T, Sekikawa A, Orchard TJ. Risk stratification for 25-year cardiovascular disease incidence in type 1 diabetes: tree-structured survival analysis of the Pittsburgh Epidemiology of Diabetes Complications study. Diab Vasc Dis Res. 2016;13(4):250–9.
Miller RG, Costacou T, Orchard TJ. Risk factor modeling for cardiovascular disease in type 1 diabetes in the pittsburgh epidemiology of diabetes complications (EDC) study: a comparison with the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabetes. 2019;68(2):409–19.
Saeed M, Tapia G, Ariansen I, Stene LC, Seljeflot I, Tell GS, et al. Serum galectin-3 and subsequent risk of coronary heart disease in subjects with childhood-onset type 1 diabetes: a cohort study. Diabetes Care. 2021;44(3):810–6.
Miller RG, Costacou T, Orchard TJ. Lipoprotein-associated phospholipase A2, C-reactive protein, and coronary artery disease in individuals with type 1 diabetes and macroalbuminuria. Diab Vasc Dis Res. 2010;7(1):47–55.
Jaffa MA, Bebu I, Luttrell D, Braffett BH, Lachin JM, Hunt K, et al. Longitudinal plasma kallikrein levels and their association with the risk of cardiovascular disease outcomes in type 1 diabetes in DCCT/EDIC. Diabetes. 2020;69(11):2440–5.
Galsgaard J, Persson F, Hansen TW, Jorsal A, Tarnow L, Parving HH, et al. Plasma high-sensitivity troponin T predicts end-stage renal disease and cardiovascular and all-cause mortality in patients with type 1 diabetes and diabetic nephropathy. Kidney Int. 2017;92(5):1242–8.
Costacou T, Saenger AK, Orchard TJ. High-sensitivity cardiac troponin-t and n-terminal prohormone of b-type natriuretic peptide in relation to cardiovascular outcomes in type 1 diabetes. Diabetes Care. 2020;43(9):2199–207.
Dalan R, Liew H, Goh LL, Gao X, Chew DEK, Boehm BO, et al. The haptoglobin 2–2 genotype is associated with inflammation and carotid artery intima-media thickness. Diab Vasc Dis Res. 2016;13(5):373–6.
Costacou T, Evans RW, Orchard TJ. Does the concentration of oxidative and inflammatory biomarkers differ by haptoglobin genotype in type 1 diabetes? Antioxid Redox Signal. 2015;23(18):1439–44.
Agha G, Mendelson MM, Ward-Caviness CK, Joehanes R, Huan TX, Gondalia R, et al. Blood leukocyte DNA methylation predicts risk of future myocardial infarction and coronary heart disease. Circulation. 2019;140(8):645–57.
Smyth LJ, Patterson CC, Swan EJ, Maxwell AP, McKnight AJ. DNA methylation associated with diabetic kidney disease in blood-derived DNA. Front Cell Dev Biol. 2020;8:561907.
Smyth LJ, Kilner J, Nair V, Liu H, Brennan E, Kerr K, et al. Assessment of differentially methylated loci in individuals with end-stage kidney disease attributed to diabetic kidney disease: an exploratory study. Clin Epigenetics. 2021;13(1).
Chen Z, Miao F, Braffett BH, Lachin JM, Zhang L, Wu X, et al. DNA methylation mediates development of HbA1c-associated complications in type 1 diabetes. Nat Metab. 2020;2(8):744–62.
Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, et al. Editing DNA methylation in the mammalian genome. Cell. 2016;167(1):233-247.e17.
de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843–63.
Roy MS, Affouf M. Six-year progression of retinopathy and associated risk factors in African American patients with type 1 diabetes mellitus: the New Jersey 725. Arch Ophthalmol. 2006;124(9):1297–306.
Harjutsalo V, Maric-Bilkan C, Forsblom C, Groop PH. Impact of sex and age at onset of diabetes on mortality from ischemic heart disease in patients with type 1 diabetes. Diabetes Care. 2014;37(1):144–8.
Miller RG, Orchard TJ, Costacou T. Risk factors differ by first manifestation of cardiovascular disease in type 1 diabetes. Diabetes Res Clin Pract. 2020;163:108141.
Secrest AM, Prince CT, Costacou T, Miller RG, Orchard TJ. Predictors of and survival after incident stroke in type 1 diabetes. Diab Vasc Dis Res. 2013;10(1):3–10.
Nolan JJ, Kahkoska AR, Semnani-Azad Z, Hivert MF, Ji L, Mohan V, et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care. 2022;45(2):261–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Miller reports grants from American Diabetes Association, outside the submitted work. Dr. Costacou has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Cardiovascular Disease
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miller, R.G., Costacou, T. Cardiovascular Disease in Adults with Type 1 Diabetes: Looking Beyond Glycemic Control. Curr Cardiol Rep 24, 1467–1475 (2022). https://doi.org/10.1007/s11886-022-01763-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01763-9