Abstract
Purpose of the Review
To review cardiovascular outcomes (CVE) in systemic lupus erythematosus (SLE) that evolves over time.
Recent Findings
Inception cohorts now report long-term data, and large population registries add to our knowledge. Mortality and cardiovascular morbidity remain high with a risk ratio of 2–3. SLE disease activity–related inflammation accounts for higher CVE incidence ratio in the first year following diagnosis with accelerated atherosclerosis contributing to CVE in about a quarter to a third of the patients later in the disease course. Immunomodulation and disease control are associated with improved cardiovascular outcomes. Validation of modified risk stratification tools and studies evaluating primary prevention with aspirin and hydroxychloroquine are reported.
Summary
Increased awareness of high mortality associated with cardiac inflammation, improved outcomes with early disease control, aggressive management of risk factors, hypertension, obesity, and high cholesterol with modifying risk stratification will result in more favorable outcomes in SLE patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cardiovascular outcomes in SLE
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem inflammatory disease affecting the kidneys, heart, lungs, skin, joints, and nervous system. The disease course is unpredictable with episodic disease flares and periods of remission. The severity of the disease varies from indolent chronic inflammation to death. Incidence of disease ranges between 3.7 and 49 per 100,000 person years in the US Medicare population [1]. Prevalence is estimated to be 48–366.6 per 100,000 individuals [2].
Mortality Trends
Once a fatal disease with 5-year mortality reported to be as high as 40% in the 1950s, current 10-year survival is between 85 and 93% [3, 4•, 5••]. Since the 1990s, the USA has seen the SLE mortality rate as well as the SLE mortality to non-SLE mortality ratio decline, with crude annual mortality reported at 19.1 per 1000 person-years [2, 6, 7•]. The WHO database reported an age standardized mortality rate (ASMR) of 2.68 per million inhabitants in 2014, [95% CI: 2.62–2.75]. However, regional differences are observed with lower ASMR in Europe, 1.06 [95% CI: 0.98–1.13] and higher ASMR in Asia, 2.16 [2.03–2.29], Latin America, 5.53 [5.33–0.73], and North America, 2.69 [2.53–2.84] [7•].
Infections and cardiovascular diseases account for most morbidity and mortality in SLE [1,2,3]. Early in the disease course, complications due to organ specific inflammation, vascular inflammation, and thrombosis are the major contributors to high morbidity and mortality [2, 3]. Development of atherosclerosis over time and accrued comorbidities from inflammation contribute to greater proportion of cardiovascular related deaths observed years after diagnosis. Regional differences in the proportion of deaths from cardiovascular disease (CVD) and infection are observed [2, 3].
Cardiac Inflammation in Active SLE and Its Outcome
Cardiac inflammation results in pericarditis, myositis, and endocarditis. Vascular inflammation and thrombosis result in ischemic events like myocardial infarction (MI), stroke, and pulmonary arterial hypertension (PAH). Heart failure and conduction abnormalities, such as atrial fibrillation, result from multifactorial causes including inflammatory and ischemic causes.
Pericarditis
Pericarditis is the most frequent cardiac manifestation of SLE with asymptomatic pericardial effusion identified by echocardiogram in 50% of the patients over time [8]. Symptomatic pericardial effusion and pericarditis is seen in 16–39% of the patients [8,9,10,11]. Majority of the patients respond to medical management. Cardiac tamponade and constrictive pericarditis occur rarely, in 1–2% of patients. About 5–13% of hospitalized patients with symptomatic pericarditis go on to develop tamponade, some requiring surgical intervention [10, 12]. Recurrence after the first episode is common and seen in 15–30% of patients and is usually associated with lupus serositis flares [11]. Differentiating lupus pericarditis from other causes, especially infectious, remains a challenge. Constrictive pericarditis is rare, mostly reported in the literature as case reports. The presence of anti-smith antibodies and antibodies to double stranded DNA (anti-dsDNA) are associated with lupus pericarditis [11].
Myocarditis
Myocarditis is less frequent with a clinical prevalence of 3–9% [13•]. However, with the use of cardiac magnetic resonance tomography, asymptomatic myocardial abnormalities were noted in 43% of SLE patients [14]. Myocarditis was the first manifestation of SLE in 58% of patients hospitalized with lupus myocarditis, mean left ventricular ejection fraction (LVEF) of 37%. Majority of those patients, 86%, required ICU care [15]. Myocarditis mortality was observed at a rate between 4 and 10% despite treatment [15, 16]. Following hospitalization, recovery of LVEF to 55% or greater was observed over time. After 1 month, 43% of the patients and in 3 years, 81% of the patients had recovered [15]. In long-term prospective studies, overall complete recovery was observed in 60% of the patients, partial recovery in 20% of the patients, and mortality was reported in 20% [13•].
Valvular Disease and Endocarditis
Asymptomatic tricuspid and mitral regurgitation are the most common findings, 45 and 25–40%, respectively, of mild severity noted on the echocardiogram [17]. Valvular thickening is seen in 17–19% with Libman-Sacks endocarditis in 11% [17, 18]. The patients with Libman-Sacks often presented with cerebroembolic stroke. Valvular vegetations appear to decrease in size in majority of the patients who received medical management, with quarter of the patients experiencing disease progression especially those with MR or AS over 46.24 ± 17.13 months follow-up [17]. Some of whom required valvular surgeries. Compared to non-SLE patients with Libman-Sacks endocarditis, SLE patients had better outcomes and 76% report improvement with resolution of regurgitation or reduction in vegetation size [19]. Advancing age increases the risk of valvular disease both in the SLE patients and in the general population. The presence of antiphospholipid antibodies (aPL) increases the risk of valvular disease by threefold when compared to SLE without aPL [18].
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) prevalence was reported to be 0.5–17% in older studies; however, recent studies using right heart catheterization report prevalence to be 2.6–3.8% [20]. In a study of 310 SLE patients with mean pulmonary artery pressure of 46 ± 12 mmHg, survival rates were reported as 92% 1-year, 85% 3-year, and 73% 5-year. The treatment goal was achieved with medical management at 1-, 3-, and 5 years in 31.5%, 54% and 63% of the SLE patients, respectively [21••]. Medical management included glucocorticoids in 99% of the patients, immunosuppressants in 93% of the patients, endothelin receptor antagonists in 57% of the patients, phosphodiesterase inhibitors in 58% of the patients, and prostacyclin analogues in 7% of the patients [21••]. Independent prognostic factors of improvement were baseline serositis, 6-min walk distance of more than 380 m and cardiac index more than 2.5 L/min/m2 [21••].
Cardiovascular Events in SLE
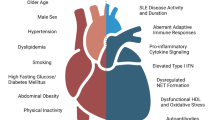
CVD events MI, stroke, heart failure, and atrial fibrillation follow a bimodal pattern similar to the mortality trend. In the initial years following diagnosis, disease activity–related cardiac and vascular inflammation results are more frequent MI, stroke, heart failure, and atrial fibrillation. Higher incidence per 1000 patients is reported in the first year following diagnosis and decreases over the next few years. Subsequent increase in CVE after 10–15 years of disease duration was observed [22]. With better understanding of the disease and available diagnostic and therapeutic options the 10-year survival is close to 86–90%. Accrued damage, development of hypertension, and other cardiovascular risk factors, accelerated atherosclerosis contribute to excess CVE observed late in the disease course. Figure 1 represents a schematic diagram of the natural history of the disease in SLE patients.
Schematic representation of the natural history in SLE and the observed trend in incidence ratio for MI over time. a: Schematic representation of inflammation observed in a patient with SLE and the likely causes for the relative increase in CVE observed in SLE patients. b: Representation of the observed incidence ratio of MI and angina over time
Myocardial Infarction
The cumulative incidence of MI increases steadily with time from diagnosis over the years. The incidence per 1000 patient-year is highest the first year following diagnosis, 12.18/1000 person-years, and decreased to 7.07/1000 years over the next 5 years [23•]. The 10-year absolute prevalence for myocardial infarction in SLE patients is 2.17% (95% CI: 1.66 to 2.80%), while it was 1.49% (95% CI: 1.26 to 1.75%) in control subjects [5••]. SLE was associated with an increased risk of MI, with a pooled RR of 2.99 (95% CI: 2.34 to 3.820 [24]. Age and sex-adjusted risk (HR) for MI in SLE was highest in the first year following diagnosis with a steady decrease over the years.
Recent studies report similar outcomes following hospitalization for MI with no significant difference observed in the frequency of re-ischemia, death, and procedures in SLE patients vs. control [25]. However, when adjusted for age and comorbidity, SLE was independently associated with overall mortality (HR = 2.20) [26]. Worse outcomes with bare metal stents and following cardiac surgery was noted in SLE patients compared to control [27, 28].
Cerebrovascular Events
The prevalence of both ischemic and hemorrhagic stroke in patients with SLE ranges from 2 to 15% [29]. In a multicenter and multiracial cohort of SLE patients followed for the mean of 6.6 ± 4.1 year, cerebrovascular event was reported in 4.5% with ischemic stroke and TIA being the most common (80%). Intracranial hemorrhage and sinus thrombosis were less frequent (8.3 and 2.8% of all cerebrovascular events) [30••]. Increased risk of stroke is observed in SLE patients with higher pooled relative risk (RR) for ischemic stroke (RR 2.18) intracerebral hemorrhagic stroke (RR 1.84), subarachnoid hemorrhage (RR 1.95), and composite stroke (RR 2.13) as identified in a meta-analysis [24]. The increased risk occurs early in the disease course with the highest relative risk being within the first year following SLE diagnosis and in those with active disease (HR 3.7) [30••, 31]. The presence of lupus anticoagulant antibodies at enrollment was a significant risk factor (HR 2.23 [95% CI: 1.11, 4.45], P = 0.024) for stroke [30••]. Age < 40, Black and Hispanic race were other risk factors associated with stroke in SLE patients [22, 32].
Following the initial event, 60% of the stroke patients experienced a clinical resolution [30••]. Among those hospitalized and discharged to rehabilitation centers, functionality was similar to older patients without SLE [33]. Recurrence was common, and about half of the patients had a second cerebrovascular event and a quarter had a third event [30••]. The estimated 10-year cumulative incidence of any CVE is 3.75 –5% (95% CI: 3.9–6.2) increasing to 11.4% at 5 years for those patients with previous CVE [5••, 30••].
Heart Failure
Heart failure in SLE is multifactorial with disease-related cardiac inflammation involving the myocardium, valves, and conduction abnormalities contributing to the observed increase. Absolute 10-year risk of incident HF was 3.71% (95% confidence interval [CI]: 3.02–4.51%) for SLE patients vs. 1.94% (95% CI: 1.68–2.24%) for controls [5••]. Incidence of HF per 1000 person-years was highest in the first year following diagnosis. It is an important cause of heart failure in the young, with relative risk of 20–36 among patients under the age of 35 [5••, 34]. Later in the course of the disease atherosclerosis and CAD accounted for 29% of HF [34]. Renal involvement in SLE correlated with earlier and higher incidence of HF [35]. SLE with subsequent HF was associated with higher mortality compared with HF without SLE (adjusted hazard ratio: 1.50; 95% CI: 1.08 to 2.08) [5••].
Atrial Fibrillation
AFib and atrial flutter reported prevalence in SLE is 2.27%–5.5% [5••, 36]. SLE patients had a higher long-term risk of incident AFib with HR of 2.84 (95% CI: 2.50–3.23) compared with matched control subjects [5••, 36]. In a study of general Medicaid US population with SLE, the risk of incident AFib hospitalization among patients with SLE was 1.79-fold higher (95% CI: 1.43–2.24) than the age- and sex-matched controls. This risk reduced when adjusted for race/ethnicity, CVD, and hypertension (HTN) (HR 1.17, 95% CI: 0.92–1.48) [37]. Inpatient mortality, length of stay, likelihood of pharmacologic, and electrical cardioversion were similar to controls in a study of national inpatient sample. While ablation was more frequent in the SLE group (6.8% vs. 4.2%, AOR: 1.9, 95% CI: 1.3–2.7, P < 0.0001) compared to those without SLE [38].
Cardiovascular Risk Factors Among Lupus Patients: Prevalence and Risk for CVE
Prevalence of traditional cardiovascular risk factors, especially HTN and diabetes, are higher in SLE patients compared to controls. HTN was the most prevalent CV risk factor, 26–53%, and it increases with age and disease duration with a relative risk of 2.59 (95% CI: 1.79–3.75) [22, 39, 40]. Resistant HTN (defined as HTN requiring 4 antihypertensive medications to control HTN or those whose HTN was uncontrolled with 3 antihypertensive medications) was nearly twice as prevalent in patients with SLE compared to control subjects (10.2% and 5.3%, respectively) [39]. Diabetes (DM) prevalence is reported around 5–7% with relative risk of 6 in some cohorts [3, 41].
In a 3-year follow-up of SLE inception cohort, 48.6% increase in hypertension, 65.3% in hypercholesterolemia, and 55.6% in diabetes was observed. Increase in non-traditional risk factors such waist to hip ratio > 0.8 (79.5%) and low physical activity (71.6%) were reported [42]. Use of glucocorticoids significantly increased the development of HTN and DM in SLE patients [42, 43].
CVE were significantly associated with HTN and obesity in SLE patients with consistent findings in most studies [43,44,45]. Not all studies were able to establish DM or dyslipidemia as an independent risk factor for CVE in SLE patients compared to non-SLE patients [43,44,45,46]. In studies with longer term follow-up (> 15 years), the traditional risk factors HTN, cholesterol > 250, and DM were significant predictors of atherosclerotic cardiovascular events in SLE patients with no active disease [44].
SLE as an Independent Risk Factor for Cardiovascular Events and Atherosclerosis
Both large multicenter prospective studies and population registries have established SLE as an independent risk factor in developing CVD with a relative risk greater than 2, after controlling for comorbid factors of HTN, DM, and age [5••, 40, 45]. SLE patients without active disease or inflammation have increased CV events, and SLE is recognized as an independent risk factor for accelerated atherosclerosis.
Atherosclerosis is an inflammatory condition where monocytes adhere to endothelial cells as they migrate to the intima and differentiate into macrophages and foam cells in the presence of inflammatory cytokines. Several abnormalities are observed in the SLE pathologic process which contributes to increased endothelial cell activation, death, accelerated atherosclerosis, and impaired endothelial cell repair [47,48,49,50,51,52]. Table 1 lists some abnormalities observed in SLE that could contribute to accelerated atherosclerosis.
SLE: Clinical Features and Antibodies Associated with CVD
Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) is an instrument score used to measure disease activity in SLE. More frequent CVE are observed in patients with active disease and in those with high SLEDAI scores. SLE patients who present with renal involvement have higher risk for CVE compared to those without renal involvement. More frequent MI, stroke, and CV mortality are observed in lupus nephritis compared to SLE numerically, and statistical significance with HR 8.5 (95% CI: 2.2, 33; P = 0.002) was noted for MI [53•]. Higher prevalence of hypertension, dyslipidemia, and corticosteroid use is recorded in patients with lupus nephritis than those without renal involvement [54].
SLE Patients with aPL and Without Co-existent Antiphospholipid Syndrome
Transient and persistent antiphospholipid antibodies are common and present in 20–38% of patients with SLE [55, 56]. About a third of these patients develop antiphospholipid syndrome (APS) with occurrence of either arterial or venous thrombosis [57]. CVE are more frequent in patients who have double or triple aPL positivity (positive for lupus anticoagulant, anti-β2-glycoprotein-I antibodies, and anticardiolipin antibody) compared to those with no aPL or had single aPL positivity with venous thrombosis being most prevalent [55]. The presence of aPL is a significant predictor of thrombosis with 3–fourfold increased risk of CVE.
Cardiac Risk Stratification in SLE
Risk stratification tools such as the Framingham risk score (FRS) calculates 10-year estimated risk of future CVD events for individuals in the general population but significantly underestimates CVE risk in patients with SLE, a female predominant disease. Among SLE patients with CVE, 78% was stratified as low risk when risk stratified with the Framingham calculator [58]. In patients with SLE, a predicted FRS score was 4.1% at 8 years; observed CVE 11.4%, respectively [44]. Urowitz MB et al. suggested modifying the Framingham score (mFRS) by doubling it to predict CVD risk more accurately in patients with SLE [59]. The Hopkins Lupus Cohort showed that this risk in SLE was increased by a factor of 2.66 [60].
The excess risk of a cardiovascular events occurring among patients with SLE is dependent on disease-related factors such as disease activity and presence of aPL. Petri MA et al. developed a SLE cardiovascular risk equation to estimate cardiovascular risk based on both traditional and SLE-related risk factors by including a global disease activity score (the SELENA-SLEDAI score), low C3, and history of lupus anticoagulant [60].
An updated QRISK3 algorithm was recently created by taking account of additional risk factors such as SLE and steroid use [61]. Recognizing that individuals with chronic inflammatory conditions are at underestimated risk, the AHA considers SLE a “risk-enhancing” condition that can be used to revise the 10-year ASCVD risk estimate [62]. A single center study comparing the different scores reported sensitivities and specificities of 22 and 93% for FRS, 46 and 83% for mFRS, 47 and 78% for QRISK3, and 61 and 64% for SLE cardiovascular risk equation [58].
Drugs in SLE and Cardiovascular Outcomes: Associations, Risk Reduction and Primary Prevention
Treatment of SLE with corticosteroids and immunomodulation improves survival and outcomes in pericarditis, myocarditis, valvular lesions, and PAH, where cardiac inflammation dominates. However, use of corticosteroids results in increasing occurrence of hypertension, diabetes, and dyslipidemia [44].
Interest in use of aspirin and hydroxychloroquine as medications in primary prevention stems from studies that have reported inverse associations and fewer CV events among its users with SLE [63,64,65]. A protective effect for primary prevention with use of statins could not be established in SLE. Meta-analysis of studies with a mean follow-up of 6–11 years reported pooled RR 0.72, 95% CI: 0.56–0.94, for CVE among hydroxychloroquine users [64]. Protective role of hydroxychloroquine in studies with longer term follow–up of 15 years or in short term studies was not observed. A combination of aspirin with hydroxychloroquine use was observed to be more beneficial than either drug alone [65].
Aspirin use in SLE patients during pregnancy is recommended to prevent pregnancy-induced hypertension. Among SLE patients with aPL without arterial or venous thrombosis (aPL carriers) a beneficial effect of aspirin for arterial (HR: 0.43 [95% CI: 0.20–0.93]) but not venous (HR: 0.49 [95% CI: 0.22–1.11]) thrombosis was observed, HR 0.43 (95% CI: 0.25–0.750 [66••]. Anticoagulation is the standard of care for patients with SLE and APS.
Until larger, multicenter prospective studies define the role of aspirin and hydroxychloroquine role in primary prevention, aggressive control of CV risk factors, and aiming for disease remission will benefit patients the most [67].
Conclusions
There are increased CV events in SLE. The risk is about 2–3 times that of the general population with active disease–related inflammation accounting for most of the risk. The incidence ratio for CVE per 1000 SLE patients is the highest in the first year of diagnosis and decreases over the next 5–6 years. Gradual increase is observed after 6–7 years, especially for MI and angina. Increasing occurrence of comorbid conditions and accelerated atherosclerosis accounts for about a quarter of the CVE in SLE. With better awareness, early diagnosis of the disease, and treatment with immunomodulatory therapy, favorable outcomes are observed for most adverse cardiac presentations associated with disease activity. Using the modified risk tools for calculating risk and aggressive control of traditional risk factors are likely to result in better outcomes. While aspirin is associated with fewer events especially in patients with aPL, its role in primary prevention is yet to be established.
Change history
22 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11886-022-01665-w
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Li S, Gong T, Peng Y, Nieman KM, Gilbertson DT. Prevalence and incidence of systemic lupus erythematosus and associated outcomes in the 2009–2016 US Medicare population. Lupus. 2020;29(1):15–26. https://doi.org/10.1177/0961203319888691.
Barber MRW, Drenkard C, Falasinnu T, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17(9):515–32. https://doi.org/10.1038/s41584-021-00668-1.
Yee CS, Su L, Toescu V, et al. Birmingham SLE cohort: outcomes of a large inception cohort followed for up to 21 years. Rheumatology (Oxford). 2015;54(5):836–43. https://doi.org/10.1093/rheumatology/keu412.
• Urowitz MB, Gladman DD, Farewell V, et al. Accrual of atherosclerotic vascular events in a multicenter inception systemic lupus erythematosus cohort. Arthritis Rheumatol. 2020;72(10):1734–40. https://doi.org/10.1002/art.41392. (This study separates the atherosclerotic VE by identifying disease inactive SLE patients)
•• Yafasova A, Fosbøl EL, Schou M, et al. Long-term cardiovascular outcomes in systemic lupus erythematosus. J Am Coll Cardiol. 2021;77(14):1717–27. https://doi.org/10.1016/j.jacc.2021.02.029. (This study calculates the 10 year absolute risk for varies outcome)
Singh RR, Yen EY. SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus. 2018;27(10):1577–81. https://doi.org/10.1177/0961203318786436.
• Scherlinger M, Mertz P, Sagez F, et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun Rev. 2020;19(6): 102531. https://doi.org/10.1016/j.autrev.2020.102531. (Stark differences in different nations are observed)
Dein E, Douglas H, Petri M, Law G, Timlin H. Pericarditis in lupus. Cureus. 2019;11(3):e4166. Published 2019 Mar 1. https://doi.org/10.7759/cureus.4166
Smiti M, Salem TB, Larbi T, et al. Péricardites lupiques : prévalence, caractéristiques cliniques et immunologiques [Pericarditis in systemic lupus erythematosus: prevalence and clinical and immunologic characteristics]. Presse Med. 2009;38(3):362–5. https://doi.org/10.1016/j.lpm.2008.08.010.
Goswami RP, Sircar G, Ghosh A, Ghosh P. Cardiac tamponade in systemic lupus erythematosus. QJM. 2018;111(2):83–7. https://doi.org/10.1093/qjmed/hcx195.
Ryu S, Fu W, Petri MA. Associates and predictors of pleurisy or pericarditis in SLE. Lupus Sci Med. 2017;4(1):e000221. Published 2017 Oct 23. https://doi.org/10.1136/lupus-2017-000221
Buppajamrntham T, Palavutitotai N, Katchamart W. Clinical manifestation, diagnosis, management, and treatment outcome of pericarditis in patients with systemic lupus erythematosus. J Med Assoc Thai. 2014;97(12):1234–40.
• Tanwani J, Tselios K, Gladman DD, Su J, Urowitz MB. Lupus myocarditis: a single center experience and a comparative analysis of observational cohort studies. Lupus. 2018;27(8):1296–302. https://doi.org/10.1177/0961203318770018. (Prospective data and long term outcomes in a lupus myocarditis)
Burkard T, Trendelenburg M, Daikeler T, et al. The heart in systemic lupus erythematosus - a comprehensive approach by cardiovascular magnetic resonance tomography. PLoS One. 2018;13(10):e0202105. Published 2018 Oct 1. https://doi.org/10.1371/journal.pone.0202105
Thomas G, Cohen Aubart F, Chiche L, et al. Lupus myocarditis: initial presentation and longterm outcomes in a multicentric series of 29 patients. J Rheumatol. 2017;44(1):24–32. https://doi.org/10.3899/jrheum.160493.
Zhang L, Zhu YL, Li MT, et al. Lupus myocarditis: a case-control study from China. Chin Med J (Engl). 2015;128(19):2588–94. https://doi.org/10.4103/0366-6999.166029.
Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM. Libman-Sacks endocarditis in systemic lupus erythematosus: prevalence, associations, and evolution. Am J Med. 2007;120(7):636–42. https://doi.org/10.1016/j.amjmed.2007.01.024.
Zuily S, Regnault V, Selton-Suty C, et al. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: meta-analysis of echocardiographic studies. Circulation. 2011;124(2):215–24. https://doi.org/10.1161/CIRCULATIONAHA.111.028522.
Roldan CA, Sibbitt WL Jr, Greene ER, Qualls CR, Jung RE. Libman-Sacks endocarditis and associated cerebrovascular disease: the role of medical therapy. PLoS One. 2021;16(2):e0247052. Published 2021 Feb 16. https://doi.org/10.1371/journal.pone.0247052
Pérez-Peñate GM, Rúa-Figueroa I, Juliá-Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43(2):323–9. https://doi.org/10.3899/jrheum.150451.
•• Qian J, Li M, Zhang X, et al. Long-term prognosis of patients with systemic lupus erythematosus-associated pulmonary arterial hypertension: CSTAR-PAH cohort study. Eur Respir J. 2019;53(2):1800081. Published 2019 Feb 14. https://doi.org/10.1183/13993003.00081-2018. (Valuable data on the outcome in CTD- PAH)
Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176(8):708–19. https://doi.org/10.1093/aje/kws130.
• Aviña-Zubieta JA, To F, Vostretsova K, De Vera M, Sayre EC, Esdaile JM. Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res (Hoboken). 2017;69(6):849–56. https://doi.org/10.1002/acr.23018. (Identifies incidence ratios in different age group as well as based on disease duration)
Yazdany J, Pooley N, Langham J, et al. Systemic lupus erythematosus; stroke and myocardial infarction risk: a systematic review and meta-analysis. RMD Open. 2020;6(2): e001247. https://doi.org/10.1136/rmdopen-2020-001247.
Ando T, Adegbala O, Akintoye E, et al. Acute myocardial infarction outcomes in systemic lupus erythematosus (from the nationwide inpatient sample). Am J Cardiol. 2019;123(2):227–32. https://doi.org/10.1016/j.amjcard.2018.09.043.
Lai CH, Lai WW, Chiou MJ, et al. Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Ann Rheum Dis. 2016;75(7):1350–6. https://doi.org/10.1136/annrheumdis-2015-207719.
Tejeda-Maldonado J, Quintanilla-González L, Galindo-Uribe J, Hinojosa-Azaola A. Cardiac surgery in systemic lupus erythematosus patients: clinical characteristics and outcomes. Cirugía cardiaca en pacientes con lupus eritematoso sistémico: características clínicas y desenlaces. Reumatol Clin (Engl Ed). 2018;14(5):269–277. https://doi.org/10.1016/j.reuma.2017.01.012
Lee CH, Chong E, Low A, Lim J, Lim YT, Tan HC. Long-term follow-up after percutaneous coronary intervention in patients with systemic lupus erythematosus. Int J Cardiol. 2008;126(3):430–2. https://doi.org/10.1016/j.ijcard.2007.01.117.
Timlin H, Petri M. Transient ischemic attack and stroke in systemic lupus erythematosus. Lupus. 2013;22(12):1251–8. https://doi.org/10.1177/0961203313497416.
•• Hanly JG, Li Q, Su L, et al. Cerebrovascular events in systemic lupus erythematosus: results from an international inception cohort study. Arthritis Care Res (Hoboken). 2018;70(10):1478–87. https://doi.org/10.1002/acr.23509. (Large study evaluating outcome of cerebrovascular events)
Arkema EV, Svenungsson E, Von Euler M, Sjöwall C, Simard JF. Stroke in systemic lupus erythematosus: a Swedish population-based cohort study. Ann Rheum Dis. 2017;76(9):1544–9. https://doi.org/10.1136/annrheumdis-2016-210973.
Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30(2):144–50. https://doi.org/10.1097/BOR.0000000000000480.
Nguyen-Oghalai TU, Wu H, McNearney TA, Granger CV, Ottenbacher KJ. Functional outcome after stroke in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2008;59(7):984–8. https://doi.org/10.1002/art.23816.
Urowitz MB, Gladman D, Ibañez D, et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62(6):881–7. https://doi.org/10.1002/acr.20122.
Kim CH, Al-Kindi SG, Jandali B, Askari AD, Zacharias M, Oliveira GH. Incidence and risk of heart failure in systemic lupus erythematosus. Heart. 2017;103(3):227–33. https://doi.org/10.1136/heartjnl-2016-309561.
Lim SY, Bae EH, Han KD, et al. Systemic lupus erythematosus is a risk factor for atrial fibrillation: a nationwide, population-based study. Clin Exp Rheumatol. 2019;37(6):1019–25.
Chen SK, Barbhaiya M, Solomon DH, et al. Atrial fibrillation/flutter hospitalizations among US Medicaid recipients with and without systemic lupus erythematosus. J Rheumatol. 2020;47(9):1359–65. https://doi.org/10.3899/jrheum.190502.
Rivera Pavon MM, Shahi A, Akuna E, et al. Outcomes of atrial fibrillation hospitalizations in patients with systemic lupus erythematosus: a report from the national inpatient sample [published online ahead of print, 2021 Jan 13]. J Investig Med. 2021;jim-2020–001707. https://doi.org/10.1136/jim-2020-001707
Gandelman JS, Khan OA, Shuey MM, et al. Increased incidence of resistant hypertension in patients with systemic lupus erythematosus: a retrospective cohort study. Arthritis Care Res (Hoboken). 2020;72(4):534–43. https://doi.org/10.1002/acr.23880.
Hak AE, Karlson EW, Feskanich D, Stampfer MJ, Costenbader KH. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum. 2009;61(10):1396–402. https://doi.org/10.1002/art.24537.
Urowitz MB, Gladman D, Ibañez D, et al. Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Lupus. 2007;16(9):731–5. https://doi.org/10.1177/0961203307081113.
Urowitz MB, Gladman D, Ibañez D, et al. Accumulation of coronary artery disease risk factors over three years: data from an international inception cohort. Arthritis Rheum. 2008;59(2):176–80. https://doi.org/10.1002/art.23353.
Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum. 2013;43(1):77–95. https://doi.org/10.1016/j.semarthrit.2012.12.002.
Tselios K, Gladman DD, Su J, Ace O, Urowitz MB. Evolution of risk factors for atherosclerotic cardiovascular events in systemic lupus erythematosus: a longterm prospective study. J Rheumatol. 2017;44(12):1841–9. https://doi.org/10.3899/jrheum.161121.
Bengtsson C, Ohman ML, Nived O, Rantapää DS. Cardiovascular event in systemic lupus erythematosus in northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus. 2012;21(4):452–9. https://doi.org/10.1177/0961203311425524.
Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93(5):513–9. https://doi.org/10.1016/0002-9343(92)90578-y.
Atehortúa L, Rojas M, Vásquez GM, Castaño D. Endothelial alterations in systemic lupus erythematosus and rheumatoid arthritis: potential effect of monocyte interaction. Mediators Inflamm. 2017;2017:9680729. https://doi.org/10.1155/2017/9680729.
Atehortúa L, Rojas M, Vásquez G, et al. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):34. Published 2019 Jan 23. https://doi.org/10.1186/s13075-018-1796-4
Cieślik P, Hrycek A. Pentraxin 3 as a biomarker of local inflammatory response to vascular injury in systemic lupus erythematosus. Autoimmunity. 2015;48(4):242–50. https://doi.org/10.3109/08916934.2014.983264.
Haque S, Alexander MY, Bruce IN. Endothelial progenitor cells: a new player in lupus?. Arthritis Res Ther. 2012;14(1):203. Published 2012 Feb 20. https://doi.org/10.1186/ar3700
Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE--mechanisms and management. Nat Rev Rheumatol. 2012;8(4):214–223. Published 2012 Feb 14. https://doi.org/10.1038/nrrheum.2012.14
Tydén H, Lood C, Gullstrand B, et al. Endothelial dysfunction is associated with activation of the type I interferon system and platelets in patients with systemic lupus erythematosus. RMD Open. 2017;3(2):e000508. Published 2017 Oct 25. https://doi.org/10.1136/rmdopen-2017-000508
• Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford). 2017;56(5):709–15. https://doi.org/10.1093/rheumatology/kew475. (Study supports lupus nephritis as a significant risk factor)
Drakoulogkona O, Barbulescu AL, Rica I, Musetescu AE, Ciurea PL. The outcome of patients with lupus nephritis and the impact of cardiovascular risk factors. Curr Health Sci J. 2011;37(2):70–4.
Pericleous C, D’Souza A, McDonnell T, et al. Antiphospholipid antibody levels in early systemic lupus erythematosus: are they associated with subsequent mortality and vascular events? Rheumatology (Oxford). 2020;59(1):146–52. https://doi.org/10.1093/rheumatology/kez239.
Petri M. Epidemiology of antiphospholipid syndrome. In: Khamashta M, editor. Hughes syndrome—antiphospholipid syndrome. 2nd ed. London: Springer-Verlag; 2006. p. 234.
Cervera R, Serrano R, Pons-Estel GJ, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74(6):1011–8. https://doi.org/10.1136/annrheumdis-2013-204838.
Sivakumaran J, Harvey P, Omar A, et al. Assessment of cardiovascular risk tools as predictors of cardiovascular disease events in systemic lupus erythematosus. Lupus Sci Med. 2021;8(1): e000448. https://doi.org/10.1136/lupus-2020-000448.
Urowitz MB, Ibañez D, Su J, Gladman DD. Modified Framingham risk factor score for systemic lupus erythematosus. J Rheumatol. 2016;43(5):875–9. https://doi.org/10.3899/jrheum.150983.
Petri MA, Barr E, Magder LS. Development of a systemic lupus erythematosus cardiovascular risk equation [published correction appears in Lupus Sci Med. 2020 Feb 6;7(1):e000346corr1]. Lupus Sci Med. 2019;6(1):e000346. Published 2019 Oct 10. https://doi.org/10.1136/lupus-2019-000346
Edwards N, Langford-Smith AWW, Parker BJ, et al. QRISK3 improves detection of cardiovascular disease risk in patients with systemic lupus erythematosus. Lupus Sci Med. 2018;5(1):e000272. Published 2018 Aug 13. https://doi.org/10.1136/lupus-2018-000272
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in J Am Coll Cardiol. 2019 Sep 10;74(10):1428–1429] [published correction appears in J Am Coll Cardiol. 2020 Feb 25;75(7):840]. J Am Coll Cardiol. 2019;74(10):1376–1414. https://doi.org/10.1016/j.jacc.2019.03.009
Haugaard JH, Dreyer L, Ottosen MB, Gislason G, Kofoed K, Egeberg A. Use of hydroxychloroquine and risk of major adverse cardiovascular events in patients with lupus erythematosus: a Danish nationwide cohort study. J Am Acad Dermatol. 2021;84(4):930–7. https://doi.org/10.1016/j.jaad.2020.12.013.
Liu D, Li X, Zhang Y, et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:1685–1695. Published 2018 Jun 11. https://doi.org/10.2147/DDDT.S166893
Fasano S, Margiotta DP, Navarini L, et al. Primary prevention of cardiovascular disease in patients with systemic lupus erythematosus: case series and literature review. Lupus. 2017;26(14):1463–72. https://doi.org/10.1177/0961203317722847.
•• Arnaud L, Mathian A, Devilliers H, et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun Rev. 2015;14(3):192–200. https://doi.org/10.1016/j.autrev.2014.10.019. (Addresses the role of aspirin in SLE patients with aPL without APS)
Fasano S, Margiotta DPE, Pierro L, et al. Prolonged remission is associated with a reduced risk of cardiovascular disease in patients with systemic lupus erythematosus: a GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Clin Rheumatol. 2019;38(2):457–63. https://doi.org/10.1007/s10067-018-4286-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Ischemic Heart Disease
The original online version of this article was revised: The copyright holder name was incorrect.
Rights and permissions
About this article
Cite this article
Sairam, S., Sureen, A., Gutierrez, J. et al. Cardiovascular Outcomes in Systemic Lupus Erythematosus. Curr Cardiol Rep 24, 75–83 (2022). https://doi.org/10.1007/s11886-021-01626-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-021-01626-9