Abstract
Introduction/objectives
Cardiovascular risk management of patients with systemic lupus erythematosus (SLE) is medically relevant. The objectives were to estimate the cardiovascular risk by different strategies in patients with SLE, analyzing which proportion of patients would be candidates to receive statin therapy, and identify how many patients with statin indication received such drugs.
Method
A cross-sectional study was performed from a secondary database. Following the recommendations of National Institute for Health and Care Excellence (NICE) guidelines and the Argentine Consensus, the QRISK-3 and the adjusted Framingham (multiplying factor × 2) scores were calculated in primary prevention subjects. The indications for statin therapy according to these recommendations were analyzed.
Results
In total, 110 patients were included. Regarding patients without previous cardiovascular history, the median adjusted Framingham score was 12.8% (4.1–21.9), and 45.2%, 22.6%, and 32.2% of them were classified at low, moderate, or high risk. The median QRISK-3 score was 6.0% (2.1–14.1) and 42.1% of subjects were classified “at risk”. Only 60% of subjects in secondary prevention received statins, although no patient received the recommended doses. Analyzing patients in primary prevention who did not receive statins (87%), 43.4% and 45.2% of the patients were eligible for statin therapy according to NICE guidelines and Argentine Consensus, respectively.
Conclusions
Our findings showed that a large proportion of patients with SLE have a considerable cardiovascular risk and many of them would be eligible for statin therapy. However, the statin use observed was low.
Key Points • A large proportion of patients with lupus have a considerable cardiovascular risk, explained in part by dyslipidemia. • Many patients with SLE would be eligible for statin therapy according to risk stratification based on conventional risk factors. • The use of statins in this population is inadequate. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is associated with accelerated atherosclerosis and increased risk of cardiovascular complications [1]. The prevalence of ischemic heart disease in SLE patients is estimated between 3.8 and 16%, conferring a 10-fold risk compared with the general population and a 50-fold relative risk in young women at reproductive age [2,3,4,5,6]. This association is not fully explained by traditional cardiovascular risk factors such as smoking, hypertension, and elevated cholesterol, and it is believed that immune dysfunction also contributes to cardiovascular risk in SLE [7].
The traditional risk scales used to estimate cardiovascular risk have great limitations, due to the fact that they were not developed specifically for SLE and they have a tendency to underestimate the risk [2, 8]. The low frequency of interventions in cardiovascular prevention, such as statins, may be the result of this deficient evaluation. This point is relevant since the evidence demonstrates that the lipid-lowering treatment with statins decreases between 20 and 25% of the cardiovascular risk, regardless of baseline risk. However, the same relative decrease in the rate of cardiovascular complications will result in a greater absolute reduction when the baseline risk is higher.
Therefore, particular attention should be paid to the conventional cardiovascular risk factor treatment, including dyslipidemia, in these patients. Statins are effective in reducing disease activity (reduced C-reactive protein levels), and in a retrospective cohort study, statins improved lipid levels and cardiovascular outcomes in patients with SLE, supporting statin use in these patients [9, 10]. However, there is no certain indication to use lipid-lowering therapy only on the basis of the presence of the disease. In patients with history of cardiovascular disease, the indication of statins is clearer. However, in primary prevention, the use of such drugs is more controversial.
Several strategies have been proposed for the cardiovascular risk management of these patients. One strategy is to use the British score called QRISK, which in its third version (QRISK-3), includes in addition to the variables commonly used in most risk scores, the history of SLE [11]. Another strategy, recommended by the Consensus of Argentine Society of Cardiology, is to adjust the risk calculated (usually Framingham risk score) by a multiplying factor (× 2) and follow the recommendations for statin therapy of the general population [12].
Taking into account the previously mentioned considerations, the objectives of our work were: (1) to estimate the cardiovascular risk by different strategies in patients with SLE, analyzing which proportion of patients would be candidates to receive statin therapy and (2) to identify how many patients with statin indication received such drugs.
Material and methods
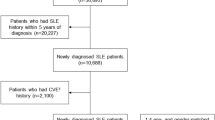
A cross-sectional study was performed from a secondary database (electronic medical records). The sample was obtained from a private health system constituted by two university hospitals and a network of 21 associated peripheral centers distributed in Buenos Aires City and in the province of Buenos Aires, Argentina. All patients older than 18 years with a diagnosis of SLE (fulfilling ACR 1997 and/or SLICC 2012 criteria) [13, 14] from January 1, 2001 to April 30, 2019 were included. The electronic clinical records of the patients included were revised, obtaining information about their history, cardiovascular risk factors, and medication received at the time of the last registered rheumatologic visit.
Two risk scores were calculated in subjects without cardiovascular disease:
- 1.
The QRISK-3 score estimates the 10-year risk of atherosclerotic cardiovascular events used by The National Institute for Health and Care Excellence (NICE) guidelines, defining the population “at risk” when the calculated risk is ≥ 10% [11, 15].
- 2.
The Framingham score for atherosclerotic cardiovascular disease events based on lipids (if the patient had a complete lipid profile) or based on body mass index, defining low, moderate, and high risk as values < 10%, between 10, 19, and ≥ 20%, respectively [16]. The possibility of using the Framingham model in any of its versions is recommended by Argentine Consensuses [12, 17] adjusting the score obtained by a correction factor (× 2).
Following the recommendations of the mentioned guidelines, indications for statins with a level of recommendation I or IIa for patients in primary prevention were selected for our analysis.
Applying recommendations of the NICE guidelines, patients with a QRISK-3 ≥ 10% were considered to receive moderate intensity statins. In cases of chronic renal failure (estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2), familial hypercholesterolemia, or type I diabetes, it is recommended to give statins without considering risk assessment tool to assess cardiovascular risk.
Following the Argentine Consensuses, the following recommendations were taken into account: (a) In patients with diabetes, start moderate/high-intensity statin therapy without estimating cardiovascular risk score; (b) In patients with an low-density lipoprotein cholesterol (LDL-C) level of 190 mg/dL or higher (with or without diagnosis of familial hypercholesterolemia), high-intensity statin therapy is recommended; (c) In subject without evidence of diabetes or C-LDL > 190 mg/dL with a calculated Framingham score ≥ 20%, start moderate/high-intensity statin therapy; (d) In patients without evidence of diabetes or C-LDL > 190 mg/dL with a calculated Framingham score ≥ 10% and < 20 with one or more risk factors, start moderate/high-intensity statin therapy; (e) In subjects with moderate to severe chronic renal insufficiency (eGFR between 30 and 59 ml/min/1.73 m2 or less than 30 ml/min/1.73 m2, respectively) without hemodialysis, start moderate intensity statin therapy.
Following the recommendations of the two guidelines, it was considered that patients in secondary prevention should receive high intensity statins. It was defined as high intensity statins if they were able to reduce ≥ 50% the LDL-C level (atorvastatin 40–80 mg or rosuvastatin 20–40 mg per day).
Finally, those patients already receiving statin therapy were considered as subjects with appropriate prescription according to both guidelines.
Statistical analysis
Continuous data between two groups were analyzed using a Student’s t test if the variables were normally distributed or with a Wilcoxon–Mann–Whitney test otherwise. Categorical data analysis was performed using a chi-squared test. Continuous variables are given as mean ± standard deviation or median (25–75 interquartile range) according to the distribution of the variables, while categorical variables are given as percentages. The agreement between both strategies in selecting patients with statin indication was analyzed, using the Fleiss kappa index. Mild or poor, acceptable or discrete, moderate, significant, or almost perfect agreement was defined if the kappa value was < 0.20, between 0.21 and 0.40, 0.41 and 0.60, 0.61 and 0.80, and 0.81 and 1, respectively. A chi square test for homogeneity was performed to compare between kappa values. Spearman’s test was used to obtain correlation between scores. A value of p < 0.05 was considered statistically significant. STATA 13.0 software packages were used for statistical analysis.
Ethics considerations
The study was conducted in compliance with the recommendations for medical research contained in the Declaration of Helsinki, Good Clinical Practice standards, and the applicable ethical regulations. The protocol was reviewed and approved by the Ethical Board of the Institution.
Results
A total of 110 patients (mean age 55.6 ± 17.9 years, 83.6% women) were included in the study. Globally, the prevalence of type 2 diabetes mellitus in the population was 13.6% and 37.3% of patients were hypertensive. Importantly, 9.1% showed history of cardiovascular disease. The mean time since diagnosis of SLE was 12.9 ± 10.3 years and the median systemic lupus erythematosus disease activity index (SLEDAI) was 4 (interquartile range 2–6). Also, 76.6% were medicated with hydroxychloroquine and 41.1% with corticosteroids (average time of corticosteroid use was 5.3 ± 4.2 years). The baseline characteristics of the population are described in Table 1.
Analyzing the population without cardiovascular history, the median adjusted Framingham score was 12.8% (interquartile range 4.1–21.9). According to this cardiovascular score, 45.2%, 22.6%, and 32.2% of the population was classified at low, moderate, or high risk. Likewise, the median QRISK-3 score was 6.0% (interquartile range 2.1–14.1) and 42.1% of subjects were classified “at risk”.
The patients “at risk” according to the NICE recommendations showed a non-significant tendency to have more years of SLE (14.3 ± 11.9 vs. 11.2 ± 9.1 years, p = 0.21), although the median SLEDAI value (3.5 (interquartile range 2.0–6.0) vs. 2.5 (interquartile range 2.0–6.0), p = 0.87) and the mean time of corticosteroid use (4.8 ± 3.9 vs. 4.2 ± 3.6 years, p = 0.55) were similar to the subjects with lower risk. Moreover, the use of hydroxychloroquine was similar in both groups (65.6% vs. 63.4%, p = 0.86).
Similarly, moderate–high cardiovascular risk patients estimated by the adjusted Framingham score, compared with low-risk subjects, showed the same findings: years of SLE (13.9 ± 9.7 vs. 11.2 ± 7.1 years, p = 0.40), median SLEDAI value (5.0 (interquartile range 0.0–6.0) vs. 3.0 (interquartile range 2.0–8.0), p = 0.73), time of corticosteroid use (6.4 ± 4.5 vs. 5.5 ± 4.8 years, p = 0.62), and use of hydroxychloroquine (70.6% vs. 71.4%, p = 0.95).
The correlation between both cardiovascular risk functions (adjusted Framingham score and QRISK-3 score) was excellent (r = 0.90) Fig. 1.
According to the recommendations of the NICE guidelines and Argentine Consensuses, all patients with cardiovascular disease history should receive high intensity statins. When analyzing the subjects in secondary prevention in our population, 60% received statins, although no patient received these drugs at the recommended doses (high intensity).
Of the total number of patients without a cardiovascular history, 13% were receiving statins. Two strategies in cardiovascular prevention were analyzed from the rest of the population without lipid lowering therapy. Applying the NICE guidelines (based on QRISK-3 score) and the Argentine Consensuses (based on adjusted Framingham score), 43.4% and 45.2% of the population were eligible for statin therapy, respectively. The concordance between both guidelines was discrete to select the population eligible for statin therapy (kappa coefficient 0.31). The reasons why statins were indicated according to both strategies can be seen in Figs. 2 and 3.
The difference between the statin indication recommended by both strategies and the reality observed in our population is shown in Fig. 4.
Discussion
The main finding of our study was that a large proportion of patients with SLE showed an increased cardiovascular risk and many of them were considered candidates for statin therapy.
SLE is a chronic autoimmune inflammatory disease associated with increased cardiovascular morbidity and mortality [4, 6, 18]. Several mechanisms have been proposed to explain the relationship between SLE and cardiovascular risk. Patients with SLE have an increased prevalence of classic cardiovascular risk factors, including obesity, hypertension, diabetes, dyslipidemia, and metabolic syndrome [19,20,21]. Indeed, several lines of evidence support that additional factors, such as autoantibodies, systemic inflammation, endothelial injury due to the autoimmune disease itself, and impaired renal function or end stage renal disease, have been suggested to play an important role in the pathogenesis of premature atherosclerosis in SLE [22]. Likewise, the risk of cardiovascular diseases is increased in SLE with evidence that risk is associated with severity of inflammation or activity [23].
The cardiovascular risk scores are designed to stratify risk in populations in order to define groups of people in whom intervention (e.g., statin therapy) should be recommended. Most traditional cardiovascular risk prediction models developed for the general population do not include non-traditional cardiovascular risk factors. Nevertheless, when applied to patients with SLE, these classic scores have been found to significantly underestimate the true risk of cardiovascular disease [2, 24].
Taking into account the limitations for cardiovascular risk stratification in patients with SLE, two strategies for the management of cardiovascular risk were evaluated in our work. First, we adapt the Framingham score by a correction factor and then follow the recommendations for the general population. Second, we use a score that includes SLE as a predictor variable. This is the case of the QRISK-3 score.
A study conducted in Canada adjusted the Framingham score for several correction factors (1.5, 2, 3, and 4) in a SLE population [25]. The authors determined that the multiplier factor “× 2” showed the best sensitivity and specificity profile to predict coronary events. Only 2.4% of the population was classified as high risk when using the traditional Framingham score, but this proportion increased to 17.3% when applying the score corrected by the multiplier factor. Based on this study, a recent Argentine Consensus recommends adjusting the traditional Framingham score for this correction factor [12]. Consequently, we use this adjustment factor in our work.
Edwards et al. showed that QRISK-3 score capture significantly more patients with SLE with an elevated 10-year risk of developing cardiovascular disease compared with the traditional Framingham score [26]. Unlike this study, our findings showed that a lower proportion of subjects were classified as “at risk” (> 10%) by the QRISK-3 score compared with the Framingham score (42.1% vs. 54.8%), although we used the adjusted version.
Statin utilization is the cornerstone of high cardiovascular disease risk management. The robust evidence showing a reduction of cardiovascular events has made statins essential in a variety of clinical conditions with elevated cardiovascular risk. However, the evidence in patients with SLE is more limited. In a small randomized study, sixty SLE patients in stable clinical conditions were randomized to receive either atorvastatin (40 mg/day) or placebo [27]. After 1 year, statin therapy was associated with a marked decrease in serum lipids and CRP levels. Additionally, coronary calcium deposits increased in the placebo group, but not in the atorvastatin group. In another study, SLE patients with inactive disease were randomized to receive either rosuvastatin (10 mg/day) or placebo [28]. Low-dose rosuvastatin leads to a significant reduction in CRP levels and carotid intima-media thickness in these patients. Statins have also been demonstrated to reduce SLE-related disease activity (as measured by SLEDAI scores) [29]. Finally, a cohort study that included 4095 patients with SLE and hyperlipidemia showed that statin therapy reduce the risk of mortality, cardiovascular disease, and end-stage renal disease [10].
Pleiotropic immunomodulatory effects have recently been demonstrated in the use of statins, rendering them potentially useful in the pharmacological management of SLE [30]. Statins have been found to reduce the production of proinflammatory cytokines in SLE patients by inhibiting the Rho-associated, coiled-coil-containing protein kinase (ROCK) pathway [31]. Also, statins were found to be able to reverse the lipid raft-associated signaling abnormalities in autoreactive T cells from SLE patients [32]. Additionally, statins also reduced the production of IL-6 and IL-10 in vitro in T cells from SLE patients [33]. Another study on SLE patients found a decrease in plasma chemokine ligand 9 (CXCL9) levels upon treatment with atorvastatin [34]. Increased CXCL9 expression resulted in additional recruitment and retention of T lymphocytes in the atherosclerotic lesions [35, 36]. Furthermore, statins were noted to inhibit the production of IFN-α and TNF-α in SLE patients, via its effect on several pathways [37]. Interestingly, statins were also found to decrease the soluble TNF-α receptor type 1 (sTNFR1) in SLE patients as compared with controls [38]. Since sTNFR1 is involved in anti-apoptotic/inflammatory signaling, a reduction in the receptor level potentially ameliorates endothelial dysfunction in SLE patients [39].
In the general population, despite the substantial evidence, a large proportion of patients do not achieve guideline standards [40]. Our study showed that this situation could be worse in patients with SLE. This was observed even in subjects with very high risk (patients with previous cardiovascular history). Application of two strategies showed that nearly half of the primary prevention population had indication for statins. However, very few patients were properly treated. Similarly, not all patients in secondary prevention with statin indication received the treatment and, in those who received it, the doses were inadequate.
The association of cardiovascular risk and cumulative exposure to plasma cholesterol over time has been reported [41], emphasizing the importance of these potentially treatable traditional risk factor in patients with SLE. Consequently, the application of one or another strategy could change the way we treat our patients with SLE.
Finally, our population showed an average age of 55.6 years. In younger populations, the findings of our investigation could be different. In relative terms, young people with SLE have a markedly higher cardiovascular risk compared with subjects without the disease. However, the “weight” of age is substantial in the calculation of scores, underestimating the risk in the young population. Consequently, the indication of statins could be lower.
This study is associated to some limitations. It was a secondary database study (electronic medical record), consequently, there could be information bias. Furthermore, our findings emerge from a small number of patients analyzed from a single health center. Despite its limitations, our study represents a valuable contribution, as we examined different cardiovascular management strategies in this particular group of patients.
In conclusion, our findings showed that a large proportion of patients with SLE have a considerable cardiovascular risk. Importantly, many of them would be eligible for statin therapy according to the two strategies evaluated. However, the statin use observed was extremely low. Understanding the relationship between cardiovascular risk stratification and the statin indication could improve the cardiovascular risk management in patients with SLE.
Taking into account our results, we consider that regular cardiovascular risk estimates with scores on patients with SLE are very important, in addition to offering the usual smoking cessation, reducing steroid doses, and encouraging exercises.
References
Andrades C, Fuego C, Manrique-Arija S, Fernández-Nebro A (2017) Management of cardiovascular risk in systemic lupus erythematosus: a systematic review. Lupus 26:1407–1419
Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Côte R, Grover SA, Fortin PR, Clarke AE, Senécal JL (2001) Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 44:2331–2337
Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, McWilliams LJ et al (1997) Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol 145:408–415
Fernández-Nebro A, Rúa-Figueroa Í, López-Longo FJ, Galindo-Izquierdo M, Calvo-Alén J, Olivé-Marqués A et al (2015) Cardiovascular events in systemic lupus erythematosus: a nationwide study in Spain from the RELESSER registry. Medicine (Baltimore) 94:e1183
Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME (2004) Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun Rev 3:423–453
Gu MM, Wang XP, Cheng QY, Zhao YL, Zhang TP, Li BZ, Ye DQ (2019) A meta-analysis of cardiovascular events in systemic lupus erythematosus. Immunol Investig 48:505–520
Croca S, Rahman A (2017) Atherosclerosis in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31:364–372
Boulos D, Koelmeyer RL, Morand EF, Hoi Y (2017) Cardiovascular risk profiles in a lupus cohort: what do different calculators tell us? Lupus Sci Med 4(1):e000212
Artola RT, Mihos CG, Santana O (2016) Effects of statin therapy in patients with systemic lupus erythematosus. South Med J 109:705–711
Yu HH, Chen PC, Yang YH, Wang LC, Lee JH, Lin YT, Chiang BL (2015) Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: a nationwide population-based cohort study. Atherosclerosis 243:11–18
Hippisley-Cox J, Coupland C, Brindle P (2017) Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 357:j2099
Sociedad Argentina de Cardiología. Área de normas y consensos (2019) Riesgo cardiovascular en las enfermedades inflamatorias crónicas. Rev Arg Cardiol 87(Supl. 2)
Hochberg M (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR et al (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686
D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM et al (2008) General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 117:743–753
Sociedad Argentina de Cardiología. Área de normas y consensos (2018) Uso apropiado de las estatinas en la Argentina. Documento de posición. Rev Arg Cardiol 86(Supl. 1):1–13
Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S (2017) The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford) 56:709–715
Bessant R, Duncan R, Ambler G, Swanton J, Isenberg DA, Gordon C, Rahman A (2006) Prevalence of conventional and lupus-specific risk factors for cardiovascular disease in patients with systemic lupus erythematosus: a case-control study. Arthritis Rheum 55:892–899
Medeiros MM, Xavier de Oliveira ÍM, Ribeiro ÁT (2016) Prevalence of metabolic syndrome in a cohort of systemic lupus erythematosus patients from Northeastern Brazil: association with disease activity, nephritis, smoking, and age. Rheumatol Int 36:117–124
Yang L, Tao J, Tang X, Wang Y, He X, Xu G et al (2012) Prevalence and correlation of conventional and lupus-specific risk factors for cardiovascular disease in Chinese systemic lupus erythematosus patients. J Eur Acad Dermatol Venereol 26:95–101
Westerweel PE, Luyten RK, Koomans HA, Derksen RH, Verhaar MC (2007) Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum 56:1384–1396
Fasano S, Margiotta DPE, Pierro L, Navarini L, Riccardi A, Afeltra A, Valentini G (2019) Prolonged remission is associated with a reduced risk of cardiovascular disease in patients with systemic lupus erythematosus: a GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Clin Rheumatol 38:457–463
Goldberg RJ, Urowitz MB, Ibanez D, Nikpour M, Gladman DD (2009) Risk factors for development of coronary artery disease in women with systemic lupus erythematosus. J Rheumatol 36:2454–2461
Urowitz MB, Ibáñez D, Su J, Gladman DD (2016) Modified Framingham risk factor score for systemic lupus erythematosus. J Rheumatol 43:875–879
Edwards N, Langford-Smith AWW, Parker BJ, Bruce IN, Reynolds JA, Alexander MY et al (2018) QRISK3 improves detection of cardiovascular disease risk in patients with systemic lupus erythematosus. Lupus Sci Med 5(1):e000272
Plazak W, Gryga K, Dziedzic H, Tomkiewicz-Pajak L, Konieczynska M, Podolec P et al (2011) Influence of atorvastatin on coronary calcifications and myocardial perfusion defects in systemic lupus erythematosus patients: a prospective, randomized, double-masked, placebo-controlled study. Arthritis Res Ther 13:R117
Mok CC, Wong CK, To CH, Lai JP, Lam CS (2011) Effects of rosuvastatin on vascular biomarkers and carotid atherosclerosis in lupus: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken) 63:875–883
Ruiz-Limon P, Barbarroja N, Perez-Sanchez C, Aguirre MA, Bertolaccini ML, Khamashta MA, Rodriguez-Ariza A, Almadén Y, Segui P, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Collantes-Estevez E, Cuadrado MJ, Lopez-Pedrera C (2015) Atherosclerosis and cardiovascular disease in systemic lupus erythematosus: effects of in vivo statin treatment. Ann Rheum Dis 74:1450–1458
Tan MKX, Heng TYJ, Mak A (2019) The potential use of metformin, dipyridamole, n-acetylcysteine and statins as adjunctive therapy for systemic lupus erythematosus. Cells Apr 6;8(4). Pii: E323
Rozo C, Chinenov Y, Maharaj RK, Gupta S, Leuenberger L, Kirou KA, Bykerk VP, Goodman SM, Salmon JE, Pernis AB (2017) Targeting the RHOA-ROCK pathway to reverse t-cell dysfunction in SLE. Ann Rheum Dis 76:740–747
Jury EC, Isenberg DA, Mauri C, Ehrenstein MR (2006) Atorvastatin restores LCK expression and lipid raft-associated signaling in t cells from patients with systemic lupus erythematosus. J Immunol 177:7416–7422
Janes PW, Ley SC, Magee AI, Kabouridis PS (2000) The role of lipid rafts in t cell antigen receptor (TCR) signalling. Semin Immunol 12:23–34
Ferreira GA, Teixeira AL, Sato EI (2010) Atorvastatin therapy reduces interferon-regulated chemokine CXCL9 plasma levels in patients with systemic lupus erythematosus. Lupus 19:927–934
Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD (1999) Differential expression of three t lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Investig 104:1041–1050
Sheikh AM, Ochi H, Manabe A, Masuda J (2005) Lysophosphatidylcholine posttranscriptionally inhibits interferon-gamma-induced IP-10, Mig and I-Tac expression in endothelial cells. Cardiovasc Res 65:263–271
Amuro H, Ito T, Miyamoto R, Sugimoto H, Torii Y, Son Y, Nakamichi N, Yamazaki C, Hoshino K, Kaisho T, Ozaki Y, Inaba M, Amakawa R, Fukuhara S (2010) Statins, inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, function as inhibitors of cellular and molecular components involved in type I interferon production. Arthritis Rheum 62:2073–2085
Ferreira GA, Teixeira AL, Calderaro DC, Sato EI (2016) Atorvastatin reduced soluble receptors of TNF-α in systemic lupus erythematosus. Clin Exp Rheumatol 34:42–48
Parameswaran N, Patial S (2010) Tumor necrosis factor-α signaling in macrophages. Crit rev Eukaryot. Gene Expr 20:87–103
De Backer G, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Ž, Rydén L et al (2019) Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 285:135–146
Nikpour M, Urowitz MB, Ibanez D, Harvey PJ, Gladman DD (2011) Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective proof-of-concept cohort study. Arthritis Res Ther 13(5):R156
Ethical approval
The study was conducted in compliance with the recommendations for medical research contained in the Declaration of Helsinki, Good Clinical Practice standards, and the applicable ethical regulations. The protocol was reviewed and approved by the Ethical Board of the Institution.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lorena M Mora-Crespo, Guillermo Cornejo-Peña, Carla Pessio, Mariela Gago, and Rodolfo N Alvarado. The first draft of the manuscript was written by Walter Masson and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masson, W., Rossi, E., Mora-Crespo, L.M. et al. Cardiovascular risk stratification and appropriate use of statins in patients with systemic lupus erythematosus according to different strategies. Clin Rheumatol 39, 455–462 (2020). https://doi.org/10.1007/s10067-019-04856-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04856-z