Abstract
Purpose of the Review
The purpose of the review is to analyze the pathogenetic mechanisms that underlie acute pericarditis, with attention to autoimmune and autoinflammatory pericarditis, and, in addition, to review the available therapeutic armamentarium.
Recent Findings
Several studies have been published on the use of anti-IL-1 drugs in recurrent pericarditis, including anakinra and rilonacept. The latest, the RHAPSODY study, based on the use of rilonacept in recurrent pericarditis, has recently reached phase 3 with promising results in terms of efficacy and safety.
Summary
Alterations in the function of the inflammasome and the consequent overproduction of IL-1 play a pivotal role in the genesis of autoinflammatory pericarditis. Recent studies added evidence to the importance of anti-IL-1 drugs in the treatment of recurrent pericarditis with raised C-reactive protein. In the era of tailored medicine, anti-IL-1 agents may be very useful in the subset of patients with recurrent pericarditis and a clear inflammatory phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pericarditis (AP) represents a frequent and often benign disease, clinically characterized by chest pain and pericardial friction rub. Diagnosis of AP is usually made clinically, although ECG can show a concave or saddle-shaped ST segment elevation or PR-segment depressions, and by imaging, showing pericardial effusion at echocardiography and/or calcifications or cardiomegaly at chest X-ray. Biomarkers also may help in diagnosis of AP, as the majority of patients will show increase in serum CRP [1,2,3,4,5,6].
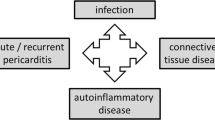
Etiology of AP is heterogeneous and its incidence varies in different regions of the world. In developed countries, idiopathic AP represents the major cause, while the etiological spectrum is more related to infectious diseases in developing regions or in immunosuppressed patients, where tuberculosis plays a relevant role [3, 4] (Table 1). Also, AP may be related to systemic disorders such as connective tissue diseases and other autoimmune disorders, autoinflammatory diseases, malignancies, radiations, and cardiac injuries, both ischemic and surgical as well as traumatic, although some of them may be traced back to autoinflammatory etiology, as we will see below [7, 8].
AP may be self-limiting, with complete resolution in a variable number of weeks, but a subset of patients will develop one or more recurrences, interspersed by asymptomatic periods, or even a chronic symptomatology that lasts without relapses [7].
Rate of recurrences may reach 50% in patients who received steroid-based treatment for the first episode of AP. Recurrence is characterized by pericardial pain and one or more of the following criteria: fever, ECG with the abovementioned changes, pericardial effusion detected by echocardiography, pericardial friction rub at auscultation, and increase in erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP), or white blood cell count.
The pathogenesis of recurrent pericarditis (RP) has been poorly understood for a long time. RP may be a result of the interaction between infectious or environmental triggers and both adaptive and innate immune reactions in specifically susceptible patients, whose immune response may be genetically altered [9•].
Relapses may occur after an asymptomatic period of time and may be caused by a pathological autoinflammatory response [7, 9]. On the contrary, where symptoms are continuous over a long time, chronic symptomatology may be related to an underlying autoimmune disorder [7]. Distinguishing between autoimmune and autoinflammatory AP is important from a pathophysiological point of view, but also for selection of pharmacotherapy, which may vary according to etiology. In this sense, the relevance of detection of pathological processes underlying AP and RP is high, as it may drive therapy properly and obtain better outcomes.
Autoimmune Pericarditis
Pericarditis is a frequent complication of other autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, Behçet’s disease, inflammatory bowel diseases, and vasculitic processes, including granulomatosis with polyangiitis, previously named Wegener’s disease, giant cell arteritis, or Horton’s arteritis [10]. About 30% of patients affected by RA show asymptomatic pericardial effusion at echocardiography, but only 10% or less of patients will be symptomatic, showing typical signs and symptoms of pericarditis. Incidence of pericarditis in RA patients is higher in those having a severe, deforming, and nodular form of disease, characterized also by high levels of seric rheumatoid factor and cyclic citrullinated peptide [11]. In systemic lupus erythematosus (SLE), pericarditis is more frequent, and it occurs in about 50% of patients and is more frequent during disease flares. Other features of SLE often accompany pericarditis, such as malar rash, arthritis, leukopenia, and pleuritis. Its severity is usually related to disease activity and serositic involvement and responds to common therapeutic regimens for lupus [12,13,14]. In systemic sclerosis, a pericardial effusion may be also related to pulmonary hypertension, and not necessarily to the presence of an acute pericarditis [15, 16].
Pericardial involvement may herald the beginning of new autoimmune disorders, and for this reason when a first attack of pericarditis occurs, a detailed anamnestic evaluation is recommended, including the search for signs and symptoms of autoimmune or autoinflammatory disease, such as rash, photosensitivity, aphthosis, Raynaud’s phenomenon, weight loss, fever, arthralgias, dryness of the eyes and mouth, periodic fever, and peritonitis. Further testing is considered based on the pre-test probability that a systemic disorder may be present, but universal testing of all the subjects with pericardial disease for the presence of any underlying systemic disorder is discouraged [17].
Viral infections may also play a pivotal role in the pathogenesis of autoimmune pericarditis, as they can stimulate autoimmune responses by molecular mimicry, when viral antigens share structural similarities or even sequences with pericardial antigens, thus generating an autoimmune response against pericardium. Also, the production of super antigens by microbes or viral-infected cells can activate T cells and generate an immune response against pericardium that is irrespective of an eventual antigen specificity [8].
Autoinflammatory Pericarditis
Recent studies suggest that innate immunity may also play a relevant role in RP [8, 18,19,20]. Such studies initially emphasized the similarities between RP and other forms of autoinflammatory syndromes, such as familial Mediterranean fever (FMF) [21,22,23,24,25,26,27], tumor necrosis factor receptor–associated periodic syndrome (TRAPS) [28,29,30,31,32], and cryopyrin-associated periodic syndromes [33,34,35]. Moreover, a deeper comprehension of mechanisms underlying autoinflammatory syndromes such as FMF and TRAPS helped understanding processes causing RP.
FMF is an autosomal recessive disease, mostly restricted to Mediterranean ethnical populations, that presents with self-limiting and recurrent fevers associated to serositis of pleura, peritoneum, and synovium [21]. Although symptomatically rare, pericardial effusions were detected by echocardiography in about 27% of patients affected by FMF, and in a previous study on pediatric patients affected by FMF, chest pain was present in 56% of patients [23, 25, 26]. FMF is caused by various missense mutations of MEFV gene, encoding pyrin protein, that is a component of NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome [36, 37]. Inflammasomes play a relevant role in innate immunity and can respond to various stimuli such as damage-associated molecular patterns and pathogen-associated molecular patterns (DAMPs and PAMPs respectively) [38]. DAMPs, also known as alarmins, can be released from damaged or dying cells: they are nuclear or cytosolic proteins that, once passing from intra-cellular to extracellular environment, that is from a reducing to an oxidizing milieu, undergo functional denaturation and then exert their inflammasome activation properties via the interaction with pattern recognition receptors (PRRs) [39]. In this way, they can activate the inflammasome and initiate and eventually perpetuate a non-infectious inflammatory response. PAMPs can be identified as small molecular patterns conserved within a class of microbes that are recognized by toll-like receptors (TLRs) and PRRs that can activate the inflammasome in response to infection [40, 41]. Previously, viruses such as Coxsackie, Epstein-Barr, cytomegalovirus, echovirus, adenovirus, parvovirus B19, and human herpesvirus 6 were all acknowledged viruses implicated in the pathogenesis of pericarditis, but the pathogenetic mechanism was unclear.
Inflammasomes are cytosolic macromolecules composed of procaspase 1, ASC adaptor protein, and a sensor molecule that contains a nucleotide-binding oligomerization domain-like receptor (NLR) that is triggered by several stimuli [42]. NLR inflammasome has a pyrin domain-containing sensor molecule, NLRP3, that is genetically altered in patients with FMF. Such alterations lead to an increase of function in NLRP3, with altered pyrin making NLRP3 constitutively activated or characterized by a lower threshold of activation [27]. DAMPs or PAMPs are recognized by the innate immune receptors, toll-like receptors (TLRs) on the cell surface or NLRs inside the cell [40,41,42]. After stimulation, NLRs are integrated into the inflammasome, and, at this point, various mutations can make the process of inflammasome activation pathologically prolonged. This activation leads to an increase in caspase 1 cleaving pro-IL-1beta to IL-1beta and then to a pathological production of IL-1beta, leading to the characteristic symptomatology of FMF and of other syndromes characterized by mutations of pyrins [43,44,45,46]. IL-1 is a master cytokine playing a pivotal role in generating the inflammatory state. There are two types of IL-1, named IL-1alpha and IL-1beta, encoded by two different genes. While IL-1alpha is normally expressed in its precursor form by several cell types, including keratinocytes, mucosal epithelial cells, probably mesothelial cells, and especially endothelial cells, IL-1beta is expressed by macrophages and monocytes after stimulation by mediators including chemokines and bacterial products [35]. Its overproduction leads to prolonged and hyperactive inflammatory states, such as those observed in FMF.
TRAPS is an autosomal dominant syndrome characterized by periodic fever, occurring every 5–6 weeks and lasting for 1–3 weeks, associated to serositis, rash, and migratory myalgias [28, 31, 32]. Symptoms may be unprovoked or triggered by infections, stress, physical exercise, injuries, or hormonal changes. It is caused by missense mutations of the gene for TNF-alfa receptor that lead to activation of TNF-alfa-dependent pathways that in turn upregulate the inflammasome activity, thus producing results, in terms of inflammation and IL-1 production, similar to those observed in FMF [31, 32]. Previous studies reported a prevalence of pericarditis in 7% of patients affected by TRAPS, while 25% of patients reported chest pain. There are also oligo-symptomatic forms of TRAPS, characterized by TNFRSF1A mutations, that lead to late onset of disease and a lower intensity of symptoms. Such patients may experience pericarditis as the only clinical manifestation [32].
All those findings underline the relevance of the inflammasome and of the hyperproduction of IL-1 in the development of autoinflammatory diseases and in RP. PAMPs may be the cause of forms of AP secondary to infections involving the pericardium where the inflammasome function and response are altered [9•] (Fig. 1).
Forms of Acute Pericarditis of Uncertain Classification
Various injuries of the pericardium, such as pericardial surgery and myocardial infarction, or even minor procedures such as percutaneous coronary interventions, pacemaker lead positioning, and radiofrequency ablation may lead to development of pericarditis. Self-autoantigens may be released after oxidative stress, cell death, or tissue injury and may be recognized as non-self consequently to altered expression or post-translational modifications, thus generating tolerance break after epitope spreading. Antinuclear antibodies are a frequent finding in patients having RP, with a prevalence of 43.3% in such patients with respect to 9.8% of healthy patients [9•]. Anti-heart autoantibodies and anti-intercalated disk autoantibodies are present in about 67.5% of patients with RP. The production of such autoantibodies may be due to the abovementioned processes, where the exposition of autoantigens could stimulate an immune response and the activation of T and B lymphocytes. Nevertheless, these autoantibodies may simply be biomarkers without a pathogenic role [9•]. At the same time, the damage produced in the pericardium may lead to the exposure of DAMPs, with the activation of the inflammatory processes typical of inflammatory pericarditis. The hypothesis of an autoinflammatory etiology is corroborated by the brilliant response, in terms of efficacy on symptoms, produced by the anti-IL-1 drugs in these forms [19].
Acute Pericarditis or a Systemic Disease with Pleuropulmonary Involvement?
In some patients, there may be a typical clinical phenotype characterized by the sudden onset of the typical pleuro-pericardial pain, with a form of “antalgic” dyspnea, associated with high fever, high levels of serum CRP, and neutrophil leukocytosis [47, 48]. In these forms, there is an almost ubiquitous pleuropulmonary involvement. Patients present with chest pain associated with fever, prompting doctors to perform a CT scan of the chest, done to rule out aortic problems or pulmonary embolism. This examination allows exclusion of other pathologies, but may incidentally demonstrate pleural effusions, which are often bilateral and associated with pulmonary atelectasis. This may result in the patient being hospitalized with an incorrect diagnosis of pneumonia (in complete absence of cough). In the early stages of this disease, pericardial involvement may be minimal and only clearly develop later [47]. Patients invariably begin intravenous antibiotic therapy, while analgesics are given in dosages that are often insufficient to control pain. Sore throats and diarrhea are often associated, the latter often caused by the therapies administered, including antibiotics and proton pump inhibitors. This condition is not caused by bacteria, so antibiotic therapy is ineffective in controlling symptoms, high fever persists, and consequently second- or even third-line antibiotics are used. Severe, persistent chest pain may prompt further investigations for infectious, hematological, and oncological etiologies. However, when these are inconclusive, corticosteroid therapy is often started empirically. The response to corticosteroids is excellent, but when they are escalated too quickly or stopped, the symptoms return, creating a vicious cycle and a true dependence on corticosteroids. Sometimes NSAIDs are also initiated, but often in ineffective doses in controlling this real “explosion” of the inflammasome, and this makes the use of corticosteroids even easier. This condition is very similar to the aforementioned autoinflammatory diseases, sustained by the activation of the innate immune system, with the release of large amounts of IL-1, as occurs in Still’s disease. The pathogenetic role of IL-1 in these conditions is also demonstrated by the dramatic response to the administration of anti-IL-1 drugs, including anakinra, which exerts beneficial effects on both symptoms and serum CRP levels [48].
Treatment of Acute Pericarditis and Its Relapses
Conventional Therapies
NSAIDs
Non-steroid anti-inflammatory drugs (NSAIDs) represent the cornerstone of therapies in this pathology [17]. The recent discoveries regarding the pathogenesis of acute pericarditis and the participation of the inflammasome in chronic or relapsing inflammatory processes only underline the importance of this therapeutic aid in the management of those patients. Several NSAIDs are used for AP therapy. Aspirin, indomethacin, and ibuprofen are recommended at high dosages by the guidelines of the European Society of Cardiology for the treatment of AP [17], with prolonged duration of therapy. The goal of any therapy is both to reduce the symptoms associated with the acute event and to reduce relapses. However, use of NSAIDs doesn’t seem sufficient to prevent relapses.
Colchicine
Another well-proven treatment is colchicine, which, combined with NSAIDs, is able to improve the response to NSAIDs but also reduce the likelihood of recurrence of AP [17]. The use of colchicine in RP was introduced many years ago after being found to have good results in the treatment and prevention of serositis, along with resolution of pericarditis, in FMF [49,50,51,52]. Colchicine exerts its action in several ways. It inhibits the activation of pore formation carried out by P2X2 and P2X7 receptors, necessary for the activation of the NLRP3 inflammasome. Furthermore, colchicine is also able to inhibit the NACHT-LRRPYD-containing protein 3 inflammasome [53]. Colchicine administration must be started early, does not need an initial load, and can be adapted to the patient according to their weight. Similar to what has been observed for NSAIDs, the use of colchicine, especially to avoid relapses, must be prolonged, and its tapering very slow [17]. The main side effects are gastrointestinal and can be overcome by reducing the dosage [54,55,56,57].
Corticosteroids
The use of corticosteroids in AP remains a moot point. Corticosteroids exert their action by blocking nuclear factor-kappa B (NF-kB) and activator protein-1 (AP-1) that promote the transcription process of other inflammatory mediators [58]. Even though the use of corticosteroids can be indicated in AP secondary to autoimmune diseases, or used as triple therapy with NSAIDs and colchicine in patients with resistant disease, the probability of generating steroid-dependent pericarditis is very high. Many patients with AP on corticosteroid therapy will have a flare-up of RP especially after fast tapering, becoming fully dependent on corticosteroids in order to be asymptomatic [17, 59]. This will produce significant side effects related to the use of corticosteroids, including osteoporosis, with possible vertebral collapse, iatrogenic Cushing, and diabetes mellitus. For such reason, the use of corticosteroids should be strictly limited to patients with underlying autoimmune disease or to patients resistant to conventional therapies, where novel therapeutic regimens are contraindicated.
Azathioprine
In addition to corticosteroids, another immunomodulatory agent is azathioprine, which has previously shown evidence of efficacy in patients with RP resistant to conventional therapies. Azathioprine is a prodrug, which is converted into 6-mercaptopurine and which therefore exerts its function at an intra-cellular level by producing thioinosinic and thioguanilic acids, by interfering with the production of adenine and guanine and consequently generating an inhibition of deoxyribonucleic and ribonucleic acid production [60]. It has long been used in numerous autoimmune pathologies and in chronic intestinal inflammatory pathologies with good efficacy and safety profiles [61]. As observed so far in the RP, azathioprine is a generally well-tolerated drug showing good results, especially in facilitating corticosteroid tapering. At present, however, larger clinical trials regarding its use in RP are needed [62]. So far, there are no available data on the use of other disease-modifying anti-rheumatic drugs, such as methotrexate, leflunomide, or cyclosporine, in RP.
Intravenous Immunoglobulins
Immunoglobulins for intravenous use (IVIG) are normally used in the treatment of autoimmune diseases such as autoimmune thrombocytopenic purpura, Guillain-Barré syndrome, and demyelinating polyneuropathies on an inflammatory basis and in more specific areas such as pregnancies during systemic lupus erythematosus [63,64,65]. Their action is expressed through the blocking of the Fc-gammaRIIB receptors on macrophages and more generally through the blocking of the FC receptors [66]. They are administered with variable dosages between 400 and 500mg per kilogram of body weight with daily infusions, for 5 consecutive days, with a possible booster to be performed 1 month later. Data regarding their use in RP are limited to a few case series [67]. Their use, however, could play an important role in autoimmune-based AP. The high costs related to their use represent another factor that limits their widespread utilization.
Emerging Treatments
Once ascertained the fundamental role played by IL-1 in the genesis of chronic or relapsing inflammatory states mediated by inflammasome and innate immunity, also typical of RP, all drugs that exert their action by blocking the effects of IL-1 could potentially find their rationale of use. This is the case with anakinra, rilonacept, and canakinumab (Table 2).
Anakinra
Anakinra is a short-acting IL-1 receptor antagonist administered by daily subcutaneous injection of 100mg of product, approved in 2001 by the US Food and Drug Administration for the therapy of rheumatoid arthritis and juvenile idiopathic arthritis. There is no need for dosage modifications due to body mass index (BMI), age, or gender. Although its plasma clearance mainly occurs in the kidney, no dosage adjustments are needed for GFR>50ml/min×1.73m2, and dosage or schedule variations are indicated only for patients with more severe renal impairment [73]. The most common adverse reactions to anakinra are local and mild, with injection site swelling, associated to pruritus and pain, that can be reverted with topic therapy with ice or corticosteroids [74]. Such reactions in injection sites seem to present more often in the first 2 months of therapy and can reduce adherence. For such reason, it is recommended to warn patients in advance about this possible occurrence. Latent tuberculosis reactivation (LTR) has been reported, and for such reason, a screening for LTR prior to therapy start is recommended [75]. Anakinra is also contraindicated in patients with known hypersensitivity to Escherichia coli–derived proteins. Transient and mild increases in transaminase serum levels can occur during treatment with anakinra, as well as mild leukopenia. Anakinra was already studied for its use in several cardiovascular pathologies such as myocardial infarction, coronary artery disease, heart failure, and pericardial disease [76]. Evidences on its use in RP that were refractory to NSAIDs and colchicine and corticosteroid dependent are emerging [77, 78•, 79, 80]. The AIRTRIP study [68] tested its efficacy in a double-blind, placebo-controlled trial with 21 patients affected by colchicine-resistant and steroid-dependent RP and elevated CRP. In this trial, anakinra was effective in preventing new flares of RP, with 18% of patients having a new episode of RP in the anakinra group with respect to 90% in the placebo group. Anakinra proved efficacious and safe as well in a subcohort of patients whose etiology for RP was reconducted to post cardiac injury pericarditis [69]. In the AIRTRIP study, no serious adverse events were observed in the anakinra arm, while only mild adverse events, mainly represented by local injection site reactions, were reported, thus showing a good safety profile. Moreover, retrospective analyses showed that anakinra can be rapidly effective also in patients whose pericarditis was resistant to azathioprine and IVIG administration [69]. A very slow tapering of anakinra is suggested, as new flares of disease were observed in patients undergoing anakinra treatment for less than 3 months or those who stopped its administration by tapering in less than 3 months. Data suggest that anakinra is safe and effective in patients with RP refractory to conventional treatments, and a treatment longer than 3-month duration, with slow tapering, may grant better outcomes in the long time [69].
Anakinra also proved efficacious in reducing corticosteroid dependence for symptom control in patients affected by RP. In the IRAP (International Registry of Anakinra for Pericarditis) study [69], performed to evaluate the effectiveness of anakinra in a real-world population of patients affected by RP that was colchicine resistant and corticosteroid dependent, need for corticosteroids to control RP was strongly decreased in patients receiving anakinra, with the percentage of patients needing corticosteroids for symptom control decreasing from 80 to 27% (p<0.001).
Rilonacept
Rilonacept is a dimeric fusion protein consisting of the ligand-binding domains of IL-R and the accessory IL-1 receptor protein, linked in-line to the FC portion of human IgG1 that blocks the activities of both IL-1alfa and IL-1beta [81]. Steady state of this drug is similar between male and female patients and is not affected by body weight. Renal or hepatic impairments do not affect its pharmacokinetics. For such reason, rilonacept is prescribed in adult patients at the fixed dose of 160mg by weekly subcutaneous injections [82]. Rilonacept is generally well tolerated, with mild injection site reactions as the most common adverse event, similar to anakinra. In 2014, a randomized controlled trial proved the efficacy and safety profiles of rilonacept for systemic-onset juvenile idiopathic arthritis [83]. FDA also recognized rilonacept as orphan drug for the treatment of cryopyrin-associated periodic syndromes, as it proved to be efficacious in reducing symptoms with good safety profiles [84, 85]. The lack of long-term efficacy and safety data prevented its approval for administration in gout arthritis. Its relevance in RP is now emerging. The recent RHAPSODY study [70••] focused on the use of rilonacept in RP with elevated CRP and concluded its phase 3 with encouraging results. The RHAPSODY trial was a multicenter, double-blind, event-driven randomized withdrawal trial using rilonacept in 86 patients with RP and elevated serum levels of CRP. Adult and adolescent patients (12 years old or older), presenting with acute signs and symptoms of RP who met 2015 European Society of Cardiology criteria for pericarditis [17] during at least a second recurrence, despite NSAIDs or colchicine or corticosteroid treatment, or any combination of these three therapies, were enrolled. Rilonacept was administered at a dosage of 320mg at first injection and subsequently at 160mg weekly administrations for a 12-week run-in period. All background medications were discontinued. Patients achieving a clinical response were then randomly assigned to continue rilonacept monotherapy or placebo. Primary efficacy endpoint was time to first recurrence of pericarditis. Interestingly, during double-blind phase, only 7% of patients in the rilonacept arm experienced a pericarditis recurrence, while 74% of patients in the placebo group had a recurrence. Patients with RP related to prior cardiac injury were included in the study and demonstrated similar results. Adverse events were reported in 74 of 86 patients and were rated as mild to moderate in the majority of cases: injection site reactions, arthromyalgia, and mild to moderate infections of the upper respiratory ways represented the most common adverse events. Higher rates of infections in this trial account for the inclusion of mild upper respiratory way infections that were not recorded in other studies on anakinra and canakinumab. In four patients, adverse events led to the discontinuation of rilonacept therapy.
So far, rilonacept represents a promising tool for the management of RP, and data regarding its efficacy and safety profiles are emerging.
It is to note that numbers of patients enrolled in the AIRTRIP and RHAPSODY studies (21 and 86, respectively) are small, simply given the large treatment effect. Sample sizes calculated for the two studies are due to the high expected efficacy.
Canakinumab
Canakinumab is a human monoclonal antibody directed against IL-beta. Compared with anakinra, it has a longer half-life that ranges from 22 to 26 days. For such reason, it can be administered every 4 to 8 weeks. The dosage used on adults is 150mg/4 weeks by subcutaneous injection [86, 87]. Canakinumab is the only drug actually approved for the treatment of colchicine-resistant gout arthritis and is also approved for FMF, TRAPS, and cryopyrin-associated periodic syndromes and in systemic-onset idiopathic juvenile arthritis [88,89,90,91]. Data regarding its use in RP are limited. It was used in a case series where local reactions to anakinra prevented its use, and canakinumab seemed efficacious [92]. Canakinumab might be less effective than anakinra and rilonacept in the treatment of RP, thus suggesting that the blockade of both IL1 alfa and beta may exert more beneficial effects in this condition [93].
Anakinra and rilonacept share the same route of injection, but the weekly administration of rilonacept compared to the daily administration of anakinra may represent an advantage for patients. In contrast, in patients with severe infections, the short half-life of anakinra may reduce immunosuppression time. While good efficacy profiles have been seen for rilonacept and anakinra, efficacy of canakinumab in RP requires further evaluations. All anti-IL-1 drugs are burdened with high costs, while conventional therapies for RP are characterized by much lower costs, thus indicating that the use of anti-IL-1 drugs should be reserved for when standard, guideline-driven therapies do not prove to be effective.
Utility of IL-1 Agents in RP
The correct allocation of such therapies is fundamental.
-
Prior to the administration of anti-IL-1 agents, patients affected by RP should receive guideline-driven therapy, incorporating use of high-dosage NSAIDs and low-dose colchicine.
-
Patients with a RP characterized by a severe systemic inflammation, with elevated serum levels of CRP and a history of repeated hospitalizations, fever, neutrophil leukocytosis, and pleural effusions, are the best candidates for anti-IL-1 therapy, since all such clinical features are likely associated with the pivotal role of IL-1.
-
Anti-IL-1 agents may be considered even before corticosteroid use, and this is particularly relevant in pediatric patients.
-
Anakinra and rilonacept may be used in patients having relevant contraindications to the use of NSAIDs or corticosteroids such as in patients who are anticoagulated or have renal failure, heart failure, gastrointestinal hemorrhages, ischemic heart disease, or recent cardiac surgery.
On the other hand, they should be avoided in patients with mild symptoms or with uncertainty of diagnosis, as well as in patients who have low levels of CRP, where IL-1 is not expected to play a relevant role.
Conclusions
Innate immunity may play a relevant role in the pathogenesis of RP, with inflammasome and IL-1 overproduction acting as drivers for systemic inflammatory response. Conventional first-line therapy with NSAIDs and colchicine is always recommended, unless contraindicated by other comorbidities. The use of corticosteroids in RP remains controversial but may be helpful for autoimmune forms of RP. Use of corticosteroids in autoinflammatory RP may result in recurrent disease with steroid dependence, so should be avoided where possible. The use of anti-IL-1 drugs in recurrent inflammatory pericarditis represents a big opportunity for patients. While anti-IL-1 agents represent the latest advance in RP therapy, with promising efficacy and safety profiles for both anakinra and rilonacept, their correct allocation is essential. Recent evidence supports that patients with RP resistant to conventional therapies should be considered for anti-IL-1-based therapy when systemic inflammation signs are present. The use of anti-IL-1 agents reduces recurrences and hospitalizations, thus granting patients with RP a better quality of life. However, there are several questions that need to be addressed in future studies, such as duration of therapy and tapering timeline for anti-IL-1 agents, their use as monotherapy without colchicine, and their eventual routine initiation before corticosteroids.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Snyder MJ, Bepko J, White M. Acute pericarditis: diagnosis and management. Am Fam Physician. 2014;89(7):553–60.
Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: a systematic review. JAMA. 2015;314(14):1498–506.
Imazio M, Battaglia A, Gaido L, Gaita F. Recurrent pericarditis. Rev Med Interne. 2017;38(5):307–11.
Imazio M, Brucato A, Derosa FG, Lestuzzi C, Bombana E, Scipione F, et al. Aetiological diagnosis in acute and recurrent pericarditis: when and how. J Cardiovasc Med (Hagerstown). 2009;10(3):217–30.
Doctor NS, Shah AB, Coplan N, Kronzon I. Acute pericarditis. Prog Cardiovasc Dis. 2017;59(4):349–59.
Imazio M, Gribaudo E, Gaita F. Recurrent pericarditis. Prog Cardiovasc Dis. 2017;59(4):360–8.
Andreis A, Imazio M, de Ferrari GM. Contemporary diagnosis and treatment of recurrent pericarditis. Expert Rev Cardiovasc Ther. 2019;17(11):817–26.
Maestroni S, Di Corato PR, Cumetti D, Chiara DB, Ghidoni S, Prisacaru L, et al. Recurrent pericarditis: autoimmune or autoinflammatory? Autoimmun Rev. 2012;12(1):60–5.
• Lopalco G, Rigante D, Cantarini L, Imazio M, Lopalco A, Emmi G, Venerito V, Fornaro M, Frediani B, Nivuori M, Brucato A, Iannone F. The autoinflammatory side of recurrent pericarditis: Enlightening the pathogenesis for a more rational treatment. Trends Cardiovasc Med. 2021;31(5):265–74 This paper reports on the autoinflammatory processes underlying recurrent pericarditis and its pathogenesis.
Gawrysiak W, Skrypnik K, Suliburska J, Skrypnik D, Bogdański P. Cardiac complications in rheumatoid arthritis, systemic lupus erythematosus and systemic sclerosis. Przegl Lek. 2017;74(4):179–82 Polish.
El Hasbani G, Masri BK, Rebeiz AG, Uthman I. Recurrent pericarditis as an initial presentation of rheumatoid arthritis. Am J Med. 2020;133(2):e50–1.
Ward NKZ, Linares-Koloffon C, Posligua A, Gandrabur L, Kim WY, Sperber K, Wasserman A, Ash J. Cardiac Manifestations of Systemic Lupus Erythematous: An Overview of the Incidence, Risk Factors, Diagnostic Criteria, Pathophysiology and Treatment Options. Cardiol Rev. 2020;24.
Dein E, Douglas H, Petri M, Law G, Timlin H. Pericarditis in lupus. Cureus. 2019;11(3):e4166.
Ryu S, Fu W, Petri MA. Associates and predictors of pleurisy or pericarditis in SLE. Lupus Sci Med. 2017;4(1):e000221.
Fenstad ER, Le RJ, Sinak LJ, et al. Pericardial effusions in pulmonary arterial hypertension: characteristics, prognosis, and role of drainage. Chest. 2013;144(5):1530–8.
Butt SA, Jeppesen JL, Torp-Pedersen C, Sam F, Gislason GH, Jacobsen S, et al. Cardiovascular manifestations of systemic sclerosis: a Danish nationwide cohort study. J Am Heart Assoc. 2019;8(17):e013405.
Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921–64.
Cantarini L, Imazio M, Brizi MG, Lucherini OM, Brucato A, Cimaz R, et al. Role of autoimmunity and autoinflammation in the pathogenesis of idiopathic recurrent pericarditis. Clin Rev Allergy Immunol. 2013;44(1):6–13.
Brucato A, Imazio M, Cremer PC, Adler Y, Maisch B, Lazaros G, et al. Recurrent pericarditis: still idiopathic? The pros and cons of a well-honoured term. Intern Emerg Med. 2018;13(6):839–44.
Bonaventura A, Vecchié A, Mauro AG, Brucato AL, Imazio M, Abbate A. An update on the pathophysiology of acute and recurrent pericarditis. Panminerva Med. 2020.
Salehzadeh F, Enteshari MA. Coexisting diseases in patients with familial Mediterranean fever. Open Access Rheumatol. 2020;12:65–71.
Perricone C, Katz D, Ciccacci C, Ceccarelli F, Valesini G, Shoenfeld Y, et al. The Heart Matters: contribution of genetic factors in recurrent pericarditis. Isr Med Assoc J. 2019;21(7):487–90.
Alsarah A, Alsara O, Laird-Fick HS. Cardiac manifestations of familial Mediterranean fever. Avicenna J Med. 2017;7(4):158–63.
Hintenberger R, Falkinger A, Danninger K, Pieringer H. Cardiovascular disease in patients with autoinflammatory syndromes. Rheumatol Int. 2018;38(1):37–50.
Erken E, Erken E. Cardiac disease in familial Mediterranean fever. Rheumatol Int. 2018;38(1):51–8.
Imazio M, Brucato A, Pluymaekers N, Breda L, Calabri G, Cantarini L, et al. Recurrent pericarditis in children and adolescents: a multicentre cohort study. J Cardiovasc Med (Hagerstown). 2016;17(9):707–12.
Kilic A, Varkal MA, Durmus MS, Yildiz I, Yıldırım ZN, Turunc G, et al. Relationship between clinical findings and genetic mutations in patients with familial Mediterranean fever. Pediatr Rheumatol Online J. 2015;13:59.
Gaggiano C, Vitale A, Obici L, Merlini G, Soriano A, Viapiana O, et al. Clinical features at onset and genetic characterization of pediatric and adult patients with TNF-α receptor-associated periodic syndrome (TRAPS): a series of 80 cases from the AIDA network. Mediat Inflamm. 2020;2020:8562485.
Navallas M, Inarejos Clemente EJ, Iglesias E, Rebollo-Polo M, Zaki FM, Navarro OM. Autoinflammatory diseases in childhood, part 1: monogenic syndromes. Pediatr Radiol. 2020;50(3):415–30.
Camprubí D, Mitjavila F, Arostegui JI, Corbella X. Efficacy of anakinra in an adult patient with recurrent pericarditis and cardiac tamponade as initial manifestations of tumor necrosis factor receptor-associated periodic syndrome due to the R92Q TNFRSF1A variant. Int J Rheum Dis. 2017;20(4):510–4.
Cantarini L, Rigante D, Merlini G, Vitale A, Caso F, Lucherini OM, et al. The expanding spectrum of low-penetrance TNFRSF1A gene variants in adults presenting with recurrent inflammatory attacks: clinical manifestations and long-term follow-up. Semin Arthritis Rheum. 2014;43(6):818–23.
Cantarini L, Lucherini OM, Brucato A, Barone L, Cumetti D, Iacoponi F, et al. Clues to detect tumor necrosis factor receptor-associated periodic syndrome (TRAPS) among patients with idiopathic recurrent acute pericarditis: results of a multicentre study. Clin Res Cardiol. 2012;101(7):525–31.
Welzel T, Kuemmerle-Deschner JB. Diagnosis and management of the cryopyrin-associated periodic syndromes (CAPS): what do we know today? J Clin Med. 2021;10(1):128.
Insalaco A, Prencipe G, Buonuomo PS, Ceccherini I, Bracaglia C, Pardeo M, et al. A novel mutation in the CIAS1/NLRP3 gene associated with an unexpected phenotype of cryopyrin-associated periodic syndromes. Clin Exp Rheumatol. 2014;32(1):123–5.
Malcova H, Strizova Z, Milota T, Striz I, Sediva A, Cebecauerova D, et al. IL-1 inhibitors in the treatment of monogenic periodic fever syndromes: from the past to the future perspectives. Front Immunol. 2021;11:619257.
Bouomrani S, Masmoudi I, Teber SB. Familial Mediterranean fever: what associations to screen for? Reumatologia. 2020;58(3):150–4.
Krainer J, Siebenhandl S, Weinhäusel A. Systemic autoinflammatory diseases. J Autoimmun. 2020;109:102421.
Yang X, Lin G, Han Z, Chai J. Structural Biology of NOD-Like Receptors. Adv Exp Med Biol. 2019;1172:119–41.
Sundaram B, Kanneganti TD. Advances in understanding activation and function of the NLRC4 inflammasome. Int J Mol Sci. 2021;22(3):1048.
Zong Z, Zhang Z, Wu L, Zhang L, Zhou F. The functional deubiquitinating enzymes in control of innate antiviral immunity. Adv Sci (Weinh). 2020;8(2):2002484.
Jiang L, Shao Y, Tian Y, Ouyang C, Wang X. Nuclear alarmin cytokines in inflammation. J Immunol Res. 2020;2020:7206451.
Vong CT, Tseng HHL, Yao P, Yu H, Wang S, Zhong Z, Wang Y. Specific NLRP3 inflammasome inhibitors: promising therapeutic agents for inflammatory diseases. Drug Discov Today. 2021;26(6):1394–408.
Hansmann S, Lainka E, Horneff G, Holzinger D, Rieber N, Jansson AF, et al. Consensus protocols for the diagnosis and management of the hereditary autoinflammatory syndromes CAPS, TRAPS and MKD/HIDS: a German PRO-KIND initiative. Pediatr Rheumatol Online J. 2020;18(1):17.
Hausmann JS. Targeting cytokines to treat autoinflammatory diseases. Clin Immunol. 2019;206:23–32.
Sag E, Bilginer Y, Ozen S. Autoinflammatory diseases with periodic fevers. Curr Rheumatol Rep. 2017;19(7):41.
Ter Haar NM, Frenkel J. Treatment of hereditary autoinflammatory diseases. Curr Opin Rheumatol. 2014;26(3):252–8.
Wu MA, Costedoat-Chalumeau N, Maestroni S, Brucato A. Acute pericarditis or a systemic disease with pleuropulmonary involvement? Intern Emerg Med. 2019;14(5):731–3.
Lazaros G, Antonopoulos AS, Imazio M, Solomou E, Lazarou E, Vassilopoulos D, et al. Clinical significance of pleural effusions and association with outcome in patients hospitalized with a first episode of acute pericarditis. Intern Emerg Med. 2019;14(5):745–51.
Liantinioti G, Argyris AA, Protogerou AD, Vlachoyiannopoulos P. The role of colchicine in the treatment of autoinflammatory diseases. Curr Pharm Des. 2018;24(6):690–4.
Leung YY, Yao Hui LL, Kraus VB. Colchicine--update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–50.
Imazio M, Brucato A, Trinchero R, Spodick D, Adler Y. Colchicine for pericarditis: hype or hope? Eur Heart J. 2009;30(5):532–9.
Markel G, Imazio M, Brucato A, Adler Y. Colchicine for the prevention of recurrent pericarditis. Isr Med Assoc J. 2008;10(1):69–72.
Leung YY, Yao Hui LL, Kraus VB. Colchicine--update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–50.
Lazaros G, Imazio M, Brucato A, Vlachopoulos C, Lazarou E, Vassilopoulos D, et al. The role of colchicine in pericardial syndromes. Curr Pharm Des. 2018;24(6):702–9.
Imazio M, Brucato A, Cemin R, Ferrua S, Maggiolini S, Beqaraj F, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med. 2013;369(16):1522–8. https://doi.org/10.1056/NEJMoa1208536.
Imazio M, Brucato A, Cemin R, Ferrua S, Belli R, Maestroni S, et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med. 2011;155(7):409–14.
Imazio M, Belli R, Brucato A, Cemin R, Ferrua S, Beqaraj F, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet. 2014;383(9936):2232–7.
Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500(1-3):51–62.
Imazio M, Lazaros G, Brucato A, Gaita F. Recurrent pericarditis: new and emerging therapeutic options. Nat Rev Cardiol. 2016;13(2):99–105.
Elion GB. The purine path to chemotherapy. Science. 1989;244(4900):41–7.
Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol. 1996;91(3):423–33.
Vianello F, Cinetto F, Cavraro M, Battisti A, Castelli M, Imbergamo S, et al. Azathioprine in isolated recurrent pericarditis: a single centre experience. Int J Cardiol. 2011;147(3):477–8.
Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013;12:CD001797.
Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2014;2014(9):CD002063.
Salib M, Clayden R, Clare R, Wang G, Warkentin TE, Crowther MA, et al. Difficulties in establishing the diagnosis of immune thrombocytopenia: an agreement study. Am J Hematol. 2016;91(8):E327–9.
Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–3.
Imazio M, Lazaros G, Picardi E, Vasileiou P, Carraro M, Tousoulis D, et al. Intravenous human immunoglobulins for refractory recurrent pericarditis: a systematic review of all published cases. J Cardiovasc Med (Hagerstown). 2016;17(4):263–9.
Brucato A, Imazio M, Gattorno M, Lazaros G, Maestroni S, Carraro M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316(18):1906–12.
Imazio M, Andreis A, De Ferrari GM, Cremer PC, Mardigyan V, Maestroni S, et al. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol. 2020;27(9):956–64.
•• Klein AL, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2021;384(1):31–41 Findings in this study represent a relevant step forward in the therapy of recurrent pericarditis, adding evidence to the importance in IL-1 in its pathogenesis and reporting on the efficacy of rilonacept, a new therapeutic tool for its management.
Klein AL, Lin D, Cremer PC, Nasir S, Luis SA, Abbate A, et al. Efficacy and safety of rilonacept for recurrent pericarditis: results from a phase II clinical trial. Heart. 2020. https://doi.org/10.1136/heartjnl-2020-317928 Epub ahead of print.
De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med. 2018;378(20):1908–19.
Yang BB, Baughman S, Sullivan JT. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin Pharmacol Ther. 2003;74(1):85–94.
Kaiser C, Knight A, Nordström D, Pettersson T, Fransson J, Florin-Robertsson E, et al. Injection-site reactions upon Kineret (anakinra) administration: experiences and explanations. Rheumatol Int. 2012;32(2):295–9.
Goletti D, Petrone L, Ippolito G, Niccoli L, Nannini C, Cantini F. Preventive therapy for tuberculosis in rheumatological patients undergoing therapy with biological drugs. Expert Rev Anti-Infect Ther. 2018;16(6):501–12.
Emmi G, Urban ML, Imazio M, Gattorno M, Maestroni S, Lopalco G, et al. Use of interleukin-1 blockers in pericardial and cardiovascular diseases. Curr Cardiol Rep. 2018;20(8):61.
Lazaros G, Imazio M, Brucato A, Vassilopoulos D, Vasileiou P, Gattorno M, et al. Anakinra: an emerging option for refractory idiopathic recurrent pericarditis: a systematic review of published evidence. J Cardiovasc Med (Hagerstown). 2016;17(4):256–62.
• Chiabrando JG, Bonaventura A, Vecchié A, Wohlford GF, Mauro AG, Jordan JH, et al. Management of acute and recurrent pericarditis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(1):76–92 This paper reports on current available therapies for recurrent pericarditis.
Brucato A, Emmi G, Cantarini L, Di Lenarda A, Gattorno M, Lopalco G, et al. Management of idiopathic recurrent pericarditis in adults and in children: a role for IL-1 receptor antagonism. Intern Emerg Med. 2018;13(4):475–89.
Tombetti E, Mulè A, Tamanini S, Matteucci L, Negro E, Brucato A, et al. Novel pharmacotherapies for recurrent pericarditis: current options in 2020. Curr Cardiol Rep. 2020;22(8):59.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. Rilonacept. 2020.
Radin A, Marbury T, Osgood G, Belomestnov P. Safety and pharmacokinetics of subcutaneously administered rilonacept in patients with well-controlled end- stage renal disease (ESRD). J Clin Pharmacol. 2010;50(7):835–41.
Ilowite NT, Prather K, Lokhnygina Y, Schanberg LE, Elder M, Milojevic D, et al. Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis. Arthritis Rheum. 2014;66(9):2570–9.
Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58(8):2443–52.
Hoffman HM, Throne ML, Amar NJ, Cartwright RC, Kivitz AJ, Soo Y, et al. Long-term efficacy and safety profile of rilonacept in the treatment of cryopryin-associated periodic syndromes: results of a 72-week open-label extension study. Clin Ther. 2012;34(10):2091–103.
Chioato A, Noseda E, Colin L, Matott R, Skerjanec A, Dietz AJ. Bioequivalence of canakinumab liquid pre-filled syringe and reconstituted lyophilized formulations following 150 mg subcutaneous administration: a randomized study in healthy subjects. Clin Drug Investig. 2013;33(11):801–8.
Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti- interleukin-1β monoclonal antibody. Clin Pharmacokinet. 2012;51(6):e1–18.
Feist E, Quartier P, Fautrel B, Schneider R, Sfriso P, Efthimiou P, et al. Efficacy and safety of canakinumab in patients with Still’s disease: exposure-response analysis of pooled systemic juvenile idiopathic arthritis data by age groups. Clin Exp Rheumatol. 2018;36(4):668–75.
Landmann EC, Walker UA. Pharmacological treatment options for cryopyrin-associated periodic syndromes. Expert Rev Clin Pharmacol. 2017;10(8):855–64.
Gattorno M, Obici L, Cattalini M, Tormey V, Abrams K, Davis N, et al. Canakinumab treatment for patients with active recurrent or chronic TNF receptor-associated periodic syndrome (TRAPS): an open-label, phase II study. Ann Rheum Dis. 2017;76(1):173–8.
Giancane G, Minoia F, Davì S, Bracciolini G, Consolaro A, Ravelli A. IL-1 inhibition in systemic juvenile idiopathic arthritis. Front Pharmacol. 2016;7:467.
Lazaros G, Antonatou K, Vassilopoulos D. The therapeutic role of interleukin-1 inhibition in idiopathic recurrent pericarditis: current evidence and future challenges. Front Med (Lausanne). 2017;4:78.
Signa S, D'Alessandro M, Consolini R, Miniaci A, Bustaffa M, Longo C, et al. Failure of anti interleukin-1 β monoclonal antibody in the treatment of recurrent pericarditis in two children. Pediatr Rheumatol Online J. 2020;18(1):51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Trotta and Pancrazi report grants from Kiniksa. Dr. Brucato reports grants from SOBI, grants from ACARPIA, other from SOBI, and other from KINIKSA. The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pericardial Disease
Rights and permissions
About this article
Cite this article
Bizzi, E., Trotta, L., Pancrazi, M. et al. Autoimmune and Autoinflammatory Pericarditis: Definitions and New Treatments. Curr Cardiol Rep 23, 128 (2021). https://doi.org/10.1007/s11886-021-01549-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01549-5