Abstract
Purpose of Review
To summarize differences in plaque depositions, coronary artery calcium (CAC) scoring, and the role of CAC in predicting atherosclerotic cardiovascular disease (ASCVD) mortality in men and women.
Recent Findings
Women have coronary plaque that is more lipid-rich, dense, and less calcified than their male counterparts. CAC scoring has emerged as a useful tool to quantify ASCVD burden. However, recent evidence favors the use of sex-adjusted CAC cutoffs for women to account for the relatively lower overall CAC burden and therefore risk stratify women appropriately. Several studies have identified CAC distribution patterns in women associated with increased CV mortality, particularly the number of lesions involved, CAC volume, and size.
Summary
Multiple studies have shown that the pathophysiology and associated risks of ASCVD are different in women when compared with men. CAC scoring is a tool that is widely being used for ASCVD risk stratification. Recent studies have shown that although men have higher CAC burdens, women are more likely to develop plaque erosions with non-calcified plaque that carries a greater risk for cardiovascular events. Providers should be aware of sex-specific CAC patterns carrying increased mortality risk for women, particularly increasing lesion size and number. Given the differences in plaque composition and distribution, revised sex-adjusted CAC scoring is suggested to better risk stratify patients, especially those deemed intermediate risk, and decrease CV mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in women, accounting for approximately 1 out of every 5 deaths in the USA [1]. Numerous studies have revealed a higher absolute number of deaths from CVD as well as higher 30-day mortality rates in women compared with men [2, 3]. Yet there is relatively little known about sex-based differences in the development of CVD. Women present on average 10 years later than men with CVD. The leading explanation for this timing relates to the onset of menopause and the noted increase in the presence of ASCVD risk factors after this point.
Often manifestations of atherosclerotic cardiovascular disease (ASCVD) in women are underrecognized leading to delays in care and treatment [4,5,6,7,8]. This often makes identification, risk stratification, and management challenging in this population. For these reasons, primary prevention and risk stratification of women are of vital importance. Therefore, considering sex-specific data in our interpretation of current diagnostic evaluation and risk stratification tools is necessary in order to optimize our care of women.
Sex differences in the pathogenesis and clinical presentation of atherosclerosis and acute coronary syndrome (ACS) have been well described in literature. Women are less likely to have obstructive coronary artery disease (CAD) with more multi-vessel diffuse disease when presenting with ACS [9•]. This has been attributed to smaller coronary vessel anatomy, an increase in arterial stiffness, and relatively low flow reserves compared with men [10,11,12]. In a study by Schiefer et al., women had smaller mean uncorrected left main and left anterior descending artery sizes than men (21.53 vs. 26.95 mm2, P < 0.001, and 14.68 vs. 19.94 mm2, P = 0.002, respectively), and smaller luminal areas for the same (15.94 vs. 18.79 mm2, P = 0.020, and 10.13 vs. 12.71 mm2, P = 0.036, respectively) [13]. In a study involving 142 patients who underwent intravascular ultrasonography and coronary endothelial function assessment, men were found to have higher atheroma burden in the left main and proximal left anterior descending arteries (median, 23.0% vs. 14.1%, P = 0.002; median, 40.1% vs. 29.3%, P = 0.001, respectively) and more eccentric atheroma (median, 0.89 vs. 0.80, P = 0.04), and women had lower maximal coronary flow reserve (2.80 vs. 3.30, P < 0.001) [14].
Early recognition of atherosclerosis may assist in identifying those who may benefit most from preventive strategies [15, 16]. The detection of plaque has a strong, independent association with ASCVD risk [17]. Non-invasive cardiovascular imaging technology has emerged as a means to quantify and qualitatively analyze plaque composition [18, 19]. Several studies have found lipid-rich, non-calcified plaques to be strongly associated with ACS as opposed to calcified plaques [20,21,22]. Studies with heterogeneous results can also be found in literature. Yet, the differences in clinical presentations, risk factors, and pathological manifestations suggest distinct processes of atherosclerotic plaque development between men and women.
Presence of coronary artery calcium (CAC) is strongly indicative of subclinical coronary atherosclerosis and is also an independent risk predictor for major cardiovascular events [23, 24]. It has also been shown to increase predictive accuracy when combined with other risk stratification tools, such as the Framingham Risk Score.
Is All Plaque Created Equal for Both Sexes?
Atherosclerosis has traditionally been thought of as an insidious process leading to a ruptured thrombus causing ACS. However, women typically have plaque erosions while men present more classically with acute plaque rupture [25]. While plaque rupture is still considered the primary cause of ACS, a study looking at patients with ST segment-elevation myocardial infarctions (STEMI) found up to 47% of women over the age of 50 years had plaque erosions rather than rupture [26]. Erosions are derived from nodular calcifications that protrude into the coronary artery lumen without evidence of plaque rupture [27]. While the exact reason for this is largely unknown, a higher incidence of plaque erosions has been associated with younger age, female sex, lack of multi-vessel disease, and less severe disease compared with plaque rupture [26]. This suggests an alternative pathogenic process that may play a role in sex-specific differences in CVD presentations.
Women also tend to have higher CVD mortality rates despite less obstructive CAD [28]. Yet little pathological evidence has been found to explain this discrepancy. A rising interest in sex-specific disparities in women’s cardiovascular health has led to research efforts investigating coronary artery plaque development in women. A study of 697 patients presenting with ACS looked at plaque characteristics using intravascular ultrasound (IVUS) and invasive coronary angiography. In this study, women had less extensive CAD despite more comorbid conditions compared with men and plaque rupture was less common in women (6.6% vs. 16.3%; P = 0.002). The extent of CAD was characterized by the number of non-culprit lesions, which was significantly less in women (P = 0.002) [29].
Data from prior studies on plaque composition has reported heterogeneous findings for women. In a large study based out of the CONFIRM registry consisting of 5632 patients, more plaques of every type were found in men than in women [30]. A similar study found more non-calcified than calcified plaque in women [31]. A similar distribution of results was found in a study of over 1000 patients with matched cohorts, showing a significantly higher proportion of non-calcified plaques in females [32•]. This pattern of relatively increased non-calcified, lipid-rich plaque development in women is important in identification and risk stratification using sex-specific patterns of atherosclerotic plaque development.
Coronary Artery Calcium
CAC occurs with deposition of calcium on the intimal layers of the coronary arteries. It is a direct indicator of atherosclerotic disease and is incrementally predictive of future cardiovascular events, independent of traditional cardiovascular risk factors [33]. Computed tomography (CT) scanning has emerged as a vital tool that detects and quantifies coronary plaque, given its non-invasiveness, diagnostic accuracy, reproducibility, relatively less radiation hazard, and less cost [34]. In 1990, Agatston et al. first described the use of quantifying coronary calcium burden and its correlation with coronary atherosclerosis [35]. Of the different methods available to quantify CAC, the most commonly used is where calcium is quantified and scored using the measured area times weighted densities (in Hounsfield units) of the lesions [35]. Absolute cut points were Initially defined as 0, 1–100, 101–400, and > 400 for absent, mild, moderate, and high amount of CAC burden respectively [36, 37]. In 2016, this was modified with CAC 0 being defined as very low risk, 1–99 as mildly increased risk, 100–299 as moderately increased risk and > 300 as moderate to severely increased risk [38••]. Higher CAC scores in asymptomatic patients have been shown to strongly correlate with histopathological coronary disease [39, 40]. On the other hand, absence of CAC (score = 0) makes the likelihood of having a cardiovascular event extremely unlikely, with an approximate 97–99% negative predictive value [33, 41].
Sex-Specific CAC Patterns
It is well established that high CAC is associated with atherosclerotic plaque development [42]. CAC detects and quantifies subclinical coronary atherosclerosis in order to risk stratify asymptomatic patients. Application of calcium scoring alone has been shown to drastically underestimate women at risk for cardiovascular events compared with men [20]. In a large study of 10,377 asymptomatic individuals, women had lower CAC scores despite higher all-cause mortality than men. This was true across all groups, most prominently in groups with CAC scores > 400 and > 1000 (HR = 5.5, P < 0.0001) [43]. In a case-control study of 5718 smokers, CAC was more prevalent and more severe in men (prevalence 81% vs. 60%; median volume 104 mm3 vs. 12 mm3) and women had CAC more comparable with men who were in a 10-year younger age group. CAC was associated with an increase in both all-cause mortality and cardiovascular mortality for women and men [44].
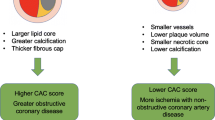
Women have less quantifiable CAC, which is detected on average 10 years after men [9•]. This creates a substantial dilemma in how to risk stratify women. Initially, absolute calcium scoring was used assuming the standard Agatston score thresholds > 0, > 100, and > 400. However, introduction of relative calcium scoring adjusting for sex and age suggested an improved method for risk stratification (Fig. 1). For this reason, sex-specific CAC cutoffs were developed in order to account for discrepancies in calcium distribution [20, 45, 46].
Using coronary artery calcification (CAC) in women. The role of coronary artery calcification (CAC) has evolved with increasing evidence beyond the CAC scoring alone (original model). In 2016, the modified CAC model proposed was adjusted for age and sex, and had better prognostic power. With increasing data, we propose the use of a sex-adjusted model in women but also take into consideration features of the plaque that improve the prognostic power of CAC
In a large, prospective study by the Multi-Ethnic Study of Atherosclerosis (MESA) investigators, men had much higher coronary calcium burdens than women at all ages [47]. Estimated CAC for the 90th percentile of white patients aging 55–64 was 102 for women and 452 for men, representing a large yet consistent difference in CAC measurement between sexes. In this cohort, 62% of women had a CAC score of zero compared with only 40% of men. Measured calcium levels and overall prevalence increased steadily with age [45]. Using sex-adjusted CAC scores to reclassify patients has been validated in several other studies [48,49,50]. A large prospective cohort study of 3238 participants from New England consisting of 48% women found a large difference in risk stratification groups using relative cutoffs. These findings were especially pronounced in women. In this cohort, only 9.1% of women had a severely elevated absolute CAC score > 400; however, 42.7% were reclassified into this category based on the 90th percentile relative cutoff [20].
Evidence comparing CAC distribution in women also varies substantially among racial groups and ethnicities suggesting sex-specific differences may play an even more complex role in identifying at-risk patients. In the MESA study, CAC measurement and prevalence varied significantly between races. A total of 62% women had calcium scores of zero when compared with 40% men. Caucasian women had the highest non-zero calcium scores, while Hispanic women had the lowest [47]. A multicenter, prospective cohort ELSA-Brasil study from Brazil looked at CAC distribution in 3616 individuals, 54.3% of which were female. The reported overall zero CAC prevalence was much lower than in MESA for women (17% vs. 62%) and to a lesser degree in men (36% vs. 40%). When compared with MESA, the CAC 90th percentile values in the ELSA-Brasil study were similar in younger women ages 44–54 (7 vs. 8) but were significantly lower in older women ages 65–74 (157 vs. 391) [51].
Although CAC has been shown to be an independent predictor for cardiovascular events and mortality risk, CAC presence varies tremendously between males and females. In an effort to explain this discrepancy, several groups have compared plaque patterns and distributions in women and men. In a large multicenter study of over 63,000 people, Shaw et al. studied calcified plaque distribution with respect to sex and different CAC subgroups (CAC score 1–100, 101–399, ≥ 400) [9•]. They discovered that across subgroups, women tend to have fewer calcified lesions (2.6 vs. 3.5, 6.9 vs. 9.5, 15.9 vs. 20.2; P < 0.0001), fewer calcified vessels (1.5 vs. 1.6, 2.4 vs. 2.6, 3.2 vs. 3.3; P = 0.017), less volume of calcifications (24 vs. 25, 161 vs. 172, 790 vs. 932 mm3; P < 0.0001), more dense plaques (192 vs. 184, 248 vs. 234, 268 vs. 258 HU; P = 0.013), and greater mean lesion size (12 vs. 10, 36 vs. 28, 65 vs. 57 mm3; P < 0.0001) than their male counterparts respectively. Higher CAC scores were also associated with higher plaque density. Women with larger and higher number of CAC lesions were found to have at least a twofold higher CVD mortality than men (P < 0.0001). Women had a 1.3 times increased risk of cardiovascular death than men if CAC was detected in any amount. Interestingly, the number of lesions was the greatest predictor for CVD mortality with women having a proportionally higher risk per each lesion involved (28% vs. 14%) [9].
Traditional CAC scoring is weighted up with increasing CAC density; however, data from several studies has shown that CAC density is not predictive of CV events in women (9, 10, 52). A prospective, multicenter observational MESA study of 3398 participants in the USA, composed of 42% females, analyzed CVD risk and CAC characteristics seen on CT. This study showed no significant interaction for CVD with respect to density score and sex (P = 0.55) [18]. Another study in the same population by Criqui et al. found that CAC density was inversely related to CV events although this did not differ significantly between men (HR = 0.802; 95% CI 0.665–0.966) and women (HR = 0.661; 95% CI 0.526–0.831, P value for interaction = 0.581) (52). Conversely, CAC volume had a greater correlation with CV events for both men and women [18]. Although women on average have lower CAC volumes, when comparing similar volumes, women have a significantly elevated mortality risk compared with men. This is also most evident at higher plaque volumes (relative hazard 28.81 vs. 9.90, P < 0.0001) [9•]. Literature also exists that incorporating factors like regional calcium depositions and extra coronary calcification in to calcium scoring would help further risk stratify patients more effectively [52•].
Cardiovascular Mortality
Cardiovascular disease remains the leading cause of morbidity and mortality among both men and women in the USA, accounting for approximately 840,768 deaths in 2016 [53]. Historically, women have had consistently higher CVD mortality rates than men [54]. However, over the last several decades, there has been a drastic reduction in these numbers largely due to an emphasis on education and awareness. Recent data has supported unique sex-based differences in underlying plaque pathology, clinical presentation, and cardiovascular outcomes. This has led to further investigations into what features of coronary atherosclerosis are unique to women and portend an increased mortality risk.
Higher CVD mortality rates have been attributed to women’s tendency to present later than men. The AHA 2019 Heart Disease and Stroke Statistics Update noted the average age for women and men presenting with their first myocardial infarction (MI) is 72.0 and 65.6 years respectively [53]. This may be related to differences in underlying pathology as women compared with men more commonly present with non-ST segment-elevation MI (NSTEMI) and stable angina [55].
Younger women represent perhaps the most overlooked but vulnerable population with respect to ASCVD and presenting with ACS. While women in various age groups have shown decreased mortality rates over the past several years, the mortality rate of women < 55 years of age have remained either stagnant or higher than previous years [56]. Mortality in this age group of women continues to be higher than its male counterparts [57]. This is largely attributed to higher cardiovascular risk factor burden, including diabetes and obesity, comorbidities, and delays in presentation [58]. Presentations of ASCVD in young women are often misunderstood and overlooked. In addition, our traditional risk stratification tools frequently underestimate ASCVD risk, leading to missed diagnoses and opportunities for prevention. A broad category of these misunderstood diagnoses have been coined MI with non-obstructive coronary arteries (MINOCA). The prevalence of this group which includes spontaneous coronary artery dissection (SCAD) and other non-obstructive coronary patterns has been reported in 3.5–15% of patients with acute MI [59].
Use of CAC in Risk Reduction Strategies
Risk stratifying ASCVD in asymptomatic women can be challenging. Screening using traditional cardiovascular risk factors often categorizes women as lower risk compared with their male counterparts. In a study by Michos et al., involving 2447 non-diabetic, asymptomatic women subjects in the age range of 55 ± 10 years, 84% women with CAC ≥ 75th percentile were classified as low risk per Framingham Risk Score (FRS) [60]. In another study in the MESA cohort, 90% non-diabetic women aged < 79 years were deemed as low risk per FRS. A total of 32% (n = 870) of these women had CAC > 0. These women were at an increased risk of CVD (hazard ratio, 6.5; 95% confidence interval, 2.6–16.4) and CVE (hazard ratio, 5.2; 95% confidence interval, 2.5–10.8) when compared with women with no CAC [61]. The MESA risk score calculator hence has CAC incorporated for CVD risk assessment.
CAC is highly predictive for major CV events and mortality, as the MESA cohort and others have demonstrated. Budoff et al., compared serial CAC scans in asymptomatic patients and found that regardless of cutoff points, with CAC > 0, progression of CAC translates to at least a threefold increase in mortality [62]. In a study of 2363 asymptomatic patients with a low-intermediate Framingham Risk Score, the 15-year mortality ranged from 5 to 23.5% for CAC scores of 0 and > 400 respectively (P < 0.001) [63].
The 2019 American College of Cardiology and American Heart Association (ACC/AHA) CVD prevention guidelines recommend CAC measurement in intermediate risk patients. Proposed guidelines for ASCVD risk estimates of 5–7.5% and > 7.5–20% use CAC > 1 to consider and recommend statins respectively [64, 65].
Conclusions
Despite growing awareness over the past several decades around ASCVD in women, much is still unknown regarding the pathophysiological sex-based mechanisms of coronary atherosclerosis. Several studies have observed variable profiles of calcification and plaque characteristics suggesting a unique phenotype of plaque development in women. Women have smaller plaques that are less calcified and obstructive yet portend higher acute and long-term CV mortality. This may be explained by both relative extensiveness of plaque burden and volume. Women seem to display a distinct plaque profile that should be strongly considered when assessing for cardiovascular risk. CAC scoring with relative cutoff values is a vital tool in this regard and helps with risk stratification and sex-specific tailored preventive therapy. CAC measurements other than calcium density might be predictive of CVD risk and CVE in women and further larger studies are warranted for the same.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Murphy SL, Xu J, Kochanek KD, Arias E, Mortality in the United States. NCHS. Data Brief. 2017;2018(328):1–8.
Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global use of strategies to open occluded coronary arteries in acute coronary syndromes IIb investigators. N Engl J Med. 1999;341(4):226–32.
Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302(8):874–82.
Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307(8):813–22.
Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–9.
Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the global registry of acute coronary events. Heart. 2009;95(1):20–6.
Johnston N, Schenck-Gustafsson K, Lagerqvist B. Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur Heart J. 2011;32(11):1331–6.
Leifheit-Limson EC, D’Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, et al. Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: the VIRGO study. J Am Coll Cardiol. 2015;66(18):1949–57.
• Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC consortium. Eur Heart J. 2018;39(41):3727–35. This was an important study of sex-specific CAC distribution patterns and long-term CV mortality.
Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54(17):1561–75.
Pepine CJ, Kerensky RA, Lambert CR, Smith KM, von Mering GO, Sopko G, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47(3 Suppl):S30–5.
Jacobs AK. Coronary intervention in 2009: are women no different than men? Circ Cardiovasc Interv. 2009;2(1):69–78.
Sheifer SE, Canos MR, Weinfurt KP, Arora UK, Mendelsohn FO, Gersh BJ, et al. Sex differences in coronary artery size assessed by intravascular ultrasound. Am Heart J. 2000;139(4):649–53.
Han SH, Bae JH, Holmes DR, Lennon RJ, Eeckhout E, Barsness GW, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29(11):1359–69.
Li L, Wang L, Liu SS, Zhao ZY, Li M, Wang TG, et al. Association between coronary atherosclerotic plaque composition and cardiovascular disease risk. Biomed Environ Sci. 2019;32(2):75–86.
Lee H, Yoon YE, Kim YJ, Kim HL, Lee SP, Kim HK, et al. Presence and extent of coronary calcified plaque evaluated by coronary computed tomographic angiography are independent predictors of ischemic stroke in patients with suspected coronary artery disease. Int J Cardiovasc Imaging. 2015;31(7):1469–78.
Pundziute G, Schuijf JD, Jukema JW, Boersma E, de Roos A, van der Wall EE, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49(1):62–70.
Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311(3):271–8.
Bharadwaj AS, Vengrenyuk Y, Yoshimura T, Baber U, Hasan C, Narula J, et al. Multimodality intravascular imaging to evaluate sex differences in plaque morphology in stable CAD. JACC Cardiovasc Imaging. 2016;9(4):400–7.
Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102(9):1136–41 41.e1.
Hoshino M, Yonetsu T, Usui E, Kanaji Y, Ohya H, Sumino Y, et al. Clinical significance of the presence or absence of lipid-rich plaque underneath intact fibrous cap plaque in acute coronary syndrome. J Am Heart Assoc. 2019;8(9):e011820.
ElFaramawy A, Youssef M, Abdel Ghany M, Shokry K. Difference in plaque characteristics of coronary culprit lesions in a cohort of Egyptian patients presented with acute coronary syndrome and stable coronary artery disease: an optical coherence tomography study. Egypt Heart J. 2018;70(2):95–100.
Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158–65.
Becker A, Leber A, Becker C, Knez A. Predictive value of coronary calcifications for future cardiac events in asymptomatic individuals. Am Heart J. 2008;155(1):154–60.
Wang L, Mintz GS, Witzenbichler B, Metzger DC, Rinaldi MJ, Duffy PL, et al. Differences in underlying culprit lesion morphology between men and women: an IVUS analysis from the ADAPT-DES study. JACC Cardiovasc Imaging. 2016;9(4):498–9.
Dai J, Xing L, Jia H, Zhu Y, Zhang S, Hu S, et al. In vivo predictors of plaque erosion in patients with ST-segment elevation myocardial infarction: a clinical, angiographical, and intravascular optical coherence tomography study. Eur Heart J. 2018;39(22):2077–85.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66.
Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of myocardial infarction 2 participants. N Engl J Med. 1999;341(4):217–25.
Lansky AJ, Ng VG, Maehara A, Weisz G, Lerman A, Mintz GS, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S62–72.
Schulman-Marcus J, Hartaigh B, Gransar H, Lin F, Valenti V, Cho I, et al. Sex-specific associations between coronary artery plaque extent and risk of major adverse cardiovascular events: the CONFIRM long-term registry. JACC Cardiovasc Imaging. 2016;9(4):364–72.
Sun Y, Qi G, Yu X, Zhi Y, Geng S, Li H, et al. Prevalence, severity, characteristics and coronary calcified score of coronary artery plaques are different in women than men with suspected coronary artery disease. Zhonghua Yi Xue Za Zhi. 2015;95(41):3337–42.
• Plank F, Beyer C, Friedrich G, Wildauer M, Feuchtner G. Sex differences in coronary artery plaque composition detected by coronary computed tomography: quantitative and qualitative analysis. Neth Heart J. 2019;27(5):272–80. This was a comprehensive study comparing plaque composition and major cardiovascular events between sexes.
Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, Council on Clinical Cardiology. Circulation. 2006;114(16):1761–91.
Shekar C, Budoff M. Calcification of the heart: mechanisms and therapeutic avenues. Expert Rev Cardiovasc Ther. 2018;16(7):527–36.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32.
Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):e584–636.
Neves PO, Andrade J, Monção H. Coronary artery calcium score: current status. Radiol Bras. 2017;50(3):182–9.
•• Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017;11(1):74–84. SCCT guidelines for current CAC and risk model.
Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92(8):2157–62.
Simons DB, Schwartz RS, Edwards WD, Sheedy PF, Breen JF, Rumberger JA. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol. 1992;20(5):1118–26.
Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Hoffman U, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675–88.
Ceponiene I, Nakanishi R, Osawa K, Kanisawa M, Nezarat N, Rahmani S, et al. Coronary artery calcium progression is associated with coronary plaque volume progression: results from a quantitative semiautomated coronary artery plaque analysis. JACC Cardiovasc Imaging. 2018;11(12):1785–94.
Raggi P, Shaw LJ, Berman DS, Callister TQ. Gender-based differences in the prognostic value of coronary calcification. J Women’s Health (Larchmt). 2004;13(3):273–83.
Lessmann N, de Jong PA, Celeng C, Takx RAP, Viergever MA, van Ginneken B, et al. Sex differences in coronary artery and thoracic aorta calcification and their association with cardiovascular mortality in heavy smokers. JACC Cardiovasc Imaging. 2019;12(9):1808–17.
Mitchell TL, Pippin JJ, Devers SM, Kimball TE, Cannaday JJ, Gibbons LW, et al. Age- and sex-based nomograms from coronary artery calcium scores as determined by electron beam computed tomography. Am J Cardiol. 2001;87(4):453–6 A6.
Nasir K, Raggi P, Rumberger JA, Braunstein JB, Post WS, Budoff MJ, et al. Coronary artery calcium volume scores on electron beam tomography in 12,936 asymptomatic adults. Am J Cardiol. 2004;93(9):1146–9.
McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113(1):30–7.
Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001;87(12):1335–9.
Schmermund A, Möhlenkamp S, Stang A, Grönemeyer D, Seibel R, Hirche H, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL study. Risk factors, evaluation of coronary calcium and lifestyle. Am Heart J. 2002;144(2):212–8.
Erbel R, Delaney JA, Lehmann N, McClelland RL, Möhlenkamp S, Kronmal RA, et al. Signs of subclinical coronary atherosclerosis in relation to risk factor distribution in the Multi-Ethnic Study of Atherosclerosis (MESA) and the Heinz Nixdorf Recall Study (HNR). Eur Heart J. 2008;29(22):2782–91.
Pereira AC, Gomez LM, Bittencourt MS, Staniak HL, Sharovsky R, Foppa M, et al. Age, gender, and race-based coronary artery calcium score percentiles in the Brazilian longitudinal study of adult health (ELSA-Brasil). Clin Cardiol. 2016;39(6):352–9.
• Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary artery calcium scoring: is it time for a change in methodology? JACC Cardiovasc Imaging. 2017;10(8):923–37. Paper outlining a proposed CAC model.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133(9):916–47.
Bellasi A, Raggi P, Merz CN, Shaw LJ. New insights into ischemic heart disease in women. Cleve Clin J Med. 2007;74(8):585–94.
Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64(4):337–45.
Bullock-Palmer RP, Shaw LJ, Gulati M. Emerging misunderstood presentations of cardiovascular disease in young women. Clin Cardiol. 2019;42(4):476–83.
Bucholz EM, Strait KM, Dreyer RP, Lindau ST, D’Onofrio G, Geda M, et al. Editor’s choice-sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6(7):610–22.
Tamis-Holland JE, Jneid H. Myocardial infarction with nonobstructive coronary arteries (MINOCA): it’s time to face reality! J Am Heart Assoc. 2018;7(13).
Michos ED, Nasir K, Braunstein JB, Rumberger JA, Budoff MJ, Post WS, et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184(1):201–6.
Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as "low risk" based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167(22):2437–42.
Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3(12):1229–36.
Kelkar AA, Schultz WM, Khosa F, Schulman-Marcus J, O’Hartaigh BW, Gransar H, et al. Long-term prognosis after coronary artery calcium scoring among low-intermediate risk women and men. Circ Cardiovasc Imaging. 2016;9(4):e003742.
Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–47.
Kavousi M, Desai CS, Ayers C, Blumenthal RS, Budoff MJ, Mahabadi AA, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA. 2016;316(20):2126–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Allison Bigeh, Chandana Shekar, and Martha Gulati declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Bigeh, A., Shekar, C. & Gulati, M. Sex Differences in Coronary Artery Calcium and Long-term CV Mortality. Curr Cardiol Rep 22, 21 (2020). https://doi.org/10.1007/s11886-020-1267-9
Published:
DOI: https://doi.org/10.1007/s11886-020-1267-9