Abstract
Purpose of Review
The importance of cardiovascular disease (CVD) in women has long been underestimated. Therefore, we need to understand the impact of sex differences on CVD.
Recent Findings
Traditional risk factors contribute to coronary artery disease (CAD) differently in women and men. There are female-specific risk factors and comorbid conditions that affect the risk of CAD. Plaque erosion is frequently seen in younger women who smoke, while plaque rupture is common in older women and men who have elevated blood cholesterol. Coronary artery calcification is also different in both sexes. Thus, coronary artery calcification score-based risk stratification in women is challenging.
Summary
A deeper understanding of the sex differences in the risk factors and plaque morphology of coronary atherosclerosis may lead to improved outcomes of CVD in women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in both men and women worldwide [1]. Although significant reductions in overall mortality in both men and women from coronary artery disease (CAD) have continued in the USA in recent years, these encouraging trends hide age-specific differences in younger individuals, especially women [2]. Young women in the USA exhibit stagnation or even an increase in CAD mortality, mirroring statistics outside the USA [3, 4].
The importance of CVD in women has long been underestimated because most CVD studies have focused primarily on men and the knowledge of CAD in women has been extrapolated from studies that were focused primarily on men. In 2017, United States CVD deaths were numerically greater in men (440,460 [51.3%]) than women (418,655 [48.7%]), but the mortality rate of CVD was higher in women than in men (2). Although a general understanding that manifestations and outcomes of CVD differ in male and female patients, and the number of sex- and gender-specific research publications has been increasing rapidly, there has been an underestimation of the pathologic differences in cardiovascular disease in women for many years. This review focuses on sex differences plaque morphology as related to coronary atherosclerosis risk factors.
Morphology of Atherosclerosis
Coronary Thrombosis
There are three types of plaque morphology that cause coronary acute thrombosis; the most frequent cause of luminal thrombi is plaque rupture (65%), followed by erosion (30%), and the least frequent is calcified nodule (5%) (Fig. 1) [5, 6]. Our CVPath Registry of coronary artery disease and sudden coronary death is now nearly 30 years old and the number of cases collected is over 1,200 cases [7•], and 1,176 cases have information of sex and age. Table 1 summarizes the prevalence of acute thrombosis from 1,176 cases stratified by age and sex. The mean age of the total population was greater in women than men (women 54.1 ± 16.1, men 50.9 ± 12.3, years, p = 0.001). To date, the result thus far is consistent with our previous reports; 44.5% of cases have acute thrombus; plaque rupture is the most prevalent type of underlying lesion (63.1%) followed by plaque erosion (32.1%), and calcified nodule (4.8%). The prevalence of plaque rupture is greater than plaque erosion in men in both older (76.6% vs. 16.9%) and younger (64.7% vs. 34.5%) groups (≥ 50 years and < 50 years). Women ≥ 50 years old have a higher incidence of plaque rupture compared to plaque erosion (50.0% vs. 22.9%), whereas plaque erosion is more common in women < 50 years compared to plaque rupture (77.1% vs. 21.7%). Calcified nodule is rarely seen in both men and women < 50 years. The incidence of organized thrombus (i.e., chronic total occlusion or CTO) is similar in men and women but more frequent in older groups in both men and women compared to the younger group. The presence of severe coronary artery disease without any thrombus as a cause of death was slightly higher in women than men (37.0% vs. 30.9%).
Morphologic features of lesions with acute thrombi and no acute thrombi. A An image of plaque rupture. B A high-power image of the red boxed area in image A. The absence of the fibrous cap and the thrombus (Th) directly attaching to the necrotic core (NC) are seen. C An image of plaque erosion with the underlying pathological intimal thickening. D A high-power image of the red boxed area in image C. There is no fibrous cap disruption and fibrin-rich thrombus is attached to the luminal surface. E An image of calcified nodule. F An image of underporalized picrosirius red stain of the same section of image E. The presence of collagen is not demonstrated in the necrotic core calcification (red arrow) while is demonstrated in the collagen calcification (blue arrows). G Image of healed plaque rupture (single layer). H A high-power image of the red boxed area in G showing healed thrombus (red double arrow) overlying ruptured fibrous cap (black arrow) and necrotic core. J Image of chronic total occlusion. K A high-power image of the red boxed area in J. Black arrowheads indicate neoangiogenesis, and brown cells are macrophages. Images A to C and E to K are stained with Movat Pentachrome. Image D is stained with Hematoxylin and Eosin. (Reproduced with permission from: Yahagi et al. Nat Rev Cardiol 2016 [17••]; and Torii et al. J Am Coll Cardiol 2021 [7•], with permission from Elsevier.)
Plaque Rupture
Plaque rupture is defined by an area of fibrous cap disruption where the luminal thrombus is in continuity with the underlying necrotic core (Fig. 1) [6]. The lesion typically has a large necrotic core and the disrupted cap is heavily infiltrated by macrophage and T-lymphocytes [6]. The fibrous cap consists mainly of type I collagen with very few smooth muscle cells. The luminal thrombus in plaque ruptures may or may not be obstructive [8]. The thrombus at the site of rupture consists predominantly of platelets (grossly appearing as “white thrombus”), while the thrombus in the proximal and distal to the site of rupture may propagate consisting of layers of fibrin with interspersed red blood cells (grossly giving the appearance of “red thrombus”). Multiple factors play an important role in fibrous cap disruption; macrophages in the fibrous cap release matrix metalloproteinase (MMP) which causes collagenolysis and weaken the fibrous cap [9], high shear stress may also contribute to the destabilization [10], and the presence of macrophage calcification and iron deposition in the cap also contributes to rupture of the cap [11]. The media in most of these lesions are focally destroyed, i.e., absence of internal elastic lamina (IEL) and smooth muscles with replacement by collagen [12].
Plaque Erosion
Plaque erosion is defined as an acute luminal thrombus in direct contact with the intima in an area devoid of endothelial cells with a thick fibrous cap (no disruption) (Fig. 1) [13]. Erosion is observed in lesions with a thick intima that is rich in smooth muscle cells and proteoglycan matrix with an absence of endothelial lining [14]. The underlying plaque morphology in erosion consists of pathologic intimal thickening (16%), early or late fibroatheroma in 50% and 34% of patients, respectively [15]. Compared to rupture, artery wall shows negative remodeling, less inflammation, less calcification, and less plaque burden in erosion [16]. Distinct morphological features of erosion-prone plaques have not been identified i.e., which atheromas (fibroatheroma pathologic intimal thickening lesions) will go and form an erosive lesion remains unknown. The mechanisms of erosion also need further understanding, but it is most likely related to vasospasm [17••]. One notable feature of plaque erosion is that the vessel wall media is intact, with a well-formed IEL and external elastic lamina (EEL) and intact smooth muscle cells with minimal collagen and proteoglycans, suggesting that vasospasm resulting from excessive contraction of medial smooth muscle cells as a possible underlying mechanism [18]. This is unlike plaque rupture, wherein the IEL is often disrupted and the underlying media is thinned and disorganized [12].
Calcified Nodule
The least frequent cause of acute coronary thrombosis is calcified nodule (CN) which was first reported by our group in 2000 [6]; however, no detailed histopathological observation was reported due to its low prevalence. In 2021, we reported the first detailed histopathological study of CNs including 26 culprit lesions from 25 autopsy individuals [7•]. CN is defined as a lesion with fibrous cap disruption from eruptive calcific nodules associated with an occlusive or non-occlusive platelet/fibrin thrombus (Fig. 1) [7•]. CNs occur in older individuals with a similar prevalence in both sexes and are associated with comorbidities such as chronic kidney disease and diabetes mellitus [7•]. A lesion with CNs has a non-occlusive luminal thrombus and multiple nodular fragments of calcification penetrating the overlying fibrous cap and protruding into the luminal space with loss of endothelial cells. The culprit lesions of CN are highly calcified and predominantly located in the proximal to the middle part of right coronary artery where a hinge motion or excessive torsion are seen during the cardiac cycle [7•]. The evaluation of histologic serial sections from regions proximal and distal to the culprit section revealed that a greater amount of collagen calcification is observed in the adjacent proximal and distal sections compared to the culprit sections. Also, the culprit sections have a greater amount of necrotic core calcification which does not have tensile strength due to the lack of structural components (i.e., collagen) compared to the franking proximal and distal sections where the calcification overlies collagen-rich lesions. We assume that the potential mechanism of CNs is related to heavily calcified coronary segments lying directly adjacent to more flexible regions (necrotic core calcium), and these less calcified regions are more susceptible to external mechanical forces due to greater movement of the coronary artery during the cardiac cycle, thus leading to CN. Given the aging population and the increasing prevalence of diabetes due to obesity, the incidence of CN is also expected to rise. Therefore, there is need to increase awareness of this lesion and its pathogenesis in order to prevent fatal thrombotic complications.
Healed Lesions
Healed lesions include healed plaque rupture, healed plaque erosion, and healed calcified nodule, and are composed of smooth muscle cells, proteoglycans, and collagen type III with or without underlying disrupted fibrous cap, necrotic core, or nodular calcification (Fig. 1) [19]. The concept of healed plaque rupture was introduced by Mann and Davies in the 1990s [20]. On average, individuals who died from unstable angina or acute myocardial infarction had 2.4 episodes of coronary thrombosis described as minor non-occlusive thrombi, which contribute to lesion progression [20]. Any plaque greater than 50% cross-sectional area narrowing had an incidence of previous healed plaque rupture in 71% of lesions [20]. Silent luminal thrombi are usually non-occlusive, but if occlusive, these lesions become chronic total occlusions with or without silent healed myocardial infarction, depending upon the presence of collateral circulation (Fig. 1) [17••].
Plaque Stability and Coronary Artery Calcification
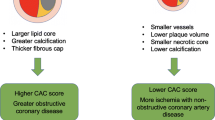
It has been debated whether coronary arterial calcification represents plaque instability (or synonymously vulnerability, thrombus-prone, or high-risk plaque) or is merely a marker of plaque burden. However, pathological studies suggest that severely calcified plaques with little to no necrotic core are stable, and less calcified plaques are unstable [21•]. All clinical studies have a crucial limitation that is the resolution of the current imaging techniques is not as good as histology. It is clear that unstable lesions (i.e., rupture, thin-cap fibroatheroma, and erosion) do not show severe calcification while stable lesions (i.e., healed rupture and fibrocalcific plaque) show severe calcification. These results suggest that screening for coronary artery calcium may not be effective especially in young women for two reasons: plaque erosions, which are highly prevalent in this population, are usually non-calcified (80%); and healed erosions do not show significant calcification compared to healed ruptures, may be due to smaller areas of necrotic core [22]. Given histopathologic findings, it is possible that risk stratification for CAD by CTA-derived coronary artery calcification in asymptomatic women is not fruitful [23]. Application of calcium scoring alone has been shown to underestimate women’s risk for cardiovascular events compared to men [24], this creates a dilemma to assess risk stratification methods for women. Raggi et al. studied 10,377 asymptomatic individuals (40% women) and found that women had lower coronary artery calcification (CAC) scores in spite of higher all-cause mortality than men. In women, all coronary calcification scores (CAC score of 101–400, 401–1000, and > 1000), most notably in groups with high CAC scores of 401–1000 and > 1000 (HR = 5.5, p < 0.0001), had a low predictive value for mortality [25]. Considering these results, sex- and age-adjusted CAC cutoffs scores for women are essential [24].
The sex differences in calcification have been evaluated for a long time. Similar to the incidence of coronary artery disease in women, the prevalence of calcification is 10 to 15 years behind that of men and equalized only a decade or so after menopause, and is attributed to the protective effect of estrogen [26, 27]. We evaluated the extent of calcification in 108 human hearts from sudden cardiac death victims (70 men and 38 women) by histology. The semi-quantitative score of calcification was greater in males than in females up to the sixties. However, in the seventies, the difference between males and females disappeared, suggesting a likely effect of the menopausal state promoting rapid progression of calcification and atherosclerosis in females. When comparing pre- and post-menopausal women, the degree of calcification was three times greater in the post-menopausal state than in the pre-menopausal state [22]. The secondary analysis of the Women’s Health Initiative (WHI) trial which enrolled 50–59 years old women who had undergone hysterectomy and were randomized to receive estrogen therapy or placebo underwent computed tomography (i.e., Agatston score for coronary calcium) 8.7 years after the completion of the trial. The extent of coronary artery calcification was significantly less in the estrogen therapy than in the placebo group [28].
Non-atherosclerotic Coronary Artery Disease in Women
In both sexes, the predominant cause of CVD is atherosclerosis; however, non-atherosclerotic spontaneous coronary dissection as an underlying cause of ACS should not be forgotten as this entity is very prevalent in women, especially young women.
Spontaneous coronary artery dissection (SCAD) is defined as separation of the medial layers of epicardial coronary artery wall by intramural hemorrhage with or without intimal tear. SCAD occurs in both sexes but 90% of cases occurring in women in the age range 47 to 53 years [29]. The etiology is unknown; however, recently fibromuscular dysplasia (FMD) has been observed with greater frequency in SCAD. FMD is more common in women involving most commonly renal arteries, extracranial carotid artery, vertebral, mesenteric, and lower extremity, intracranial carotid, and is associated with SCAD in 10.5% of cases. On the other hand, SCAD patients have a much higher incidence of FMD, and this association ranges from 51 to 91% [30]. Also, SCAD accounts for about 15 to 20% of myocardial infarctions during pregnancy and postpartum period [29]. At autopsy, SCAD is commonly seen in the proximal segments. The dissection occurs in the outer 2/3 of the media and adventitia. Eosinophile infiltration in adventitia is observed; however, this is thought to be secondary to the dissection [30]. Although conservative treatment is recommended for patients presenting with SCAD, those with high risk should undergo PCI with stenting.
Takayasu arteritis (TA) is also seen in young women and often involves coronary artery [31]. Although TA primarily affecting Asian women, disease distribution is worldwide and prevalence varies by geographical locations [32]. The average age at diagnosis is 25–30 years old, with 75–97% of patients being female [33]. In an autopsy study, three main abnormal features of coronary artery were observed in patients with TA: stenosis of the coronary ostia, diffuse or focal coronary arteritis, and coronary aneurysm [34]. Pathologic findings observed in the acute phase are edema, focal necrosis, chronic inflammation, scattered giant cells in the outer two-thirds of the aortic wall, intimal proliferation, and obliteration of the vasa vasorum [35]. In the late phase, intimal and adventitial thickening, and stenosis at the ostia of the aortic arch vessels are seen [35].
Risk Factors
Contributions of Risk Factors to the Underlying Mechanisms of Coronary Artery Thrombosis
We have reported two autopsy studies that determined the associations between coronary risk factors and morphologic characteristics of underlying mechanism of thrombosis by sex [36, 37]. We evaluated 113 hearts of men dying of sudden coronary artery disease with atherosclerosis and analyzed their coronary risk factors [36]. Fifty-nine cases showed acute coronary thrombus (i.e., plaque rupture and erosion) and 54 cases showed severe stenosis by atherosclerotic stable plaques. Of the case with acute thrombus, 41 cases had plaque rupture and 18 had plaque erosion. On the other hand, we evaluated the hearts of 51 women dying of SCD with atherosclerosis and risk factors and compared these to 15 women who died of trauma as control [37]. Coronary death was further divided by mechanisms of death: plaque rupture (N = 8), plaque erosion (N = 18), and stable plaque (N = 18) and of these eleven had healed infarction. In men, cigarette smoking was associated with acute coronary thrombosis, both plaque rupture and erosion (75% of cases), compared to stable plaque (41% of cases). A ratio of total cholesterol/HDL cholesterol was significantly higher in cases with plaque rupture, compared to plaque erosion, and stable plaque. Other risk factors, such as age, cigarette smoking, hypertension, and glycosylated hemoglobin were not different between plaque rupture and erosion. In women, elevated serum total cholesterol level was associated with plaque rupture but not with plaque erosion, while cigarette smoking was associated with plaque erosion but not with plaque rupture. Eighty-seven percent of plaque ruptures were observed in women age over the age of 50 years, whereas plaque erosion was observed only in 17%. When the age of 50 years was considered approximately the time of onset of menopause, plaque erosion was more frequent in younger (premenopausal) women who smoked, while plaque rupture was more common in older (postmenopausal) women with dyslipidemia.
These studies revealed that there are similarities and differences between men and women in the underlying cause of acute thrombosis and coronary risk factors. The link between hypercholesterolemia and plaque rupture was seen in men and women. Plaque erosions were more common in women than in men suggesting that there is a protective effect of estrogen on the occurrence of plaque rupture. Smoking was associated with acute thrombosis irrespective of sex.
Traditional Risk Factors
The INTERHEART study identified potentially modifiable risk factors, such as smoking, hypertension, diabetes mellitus, plasma apolipoproteins, obesity, dietary patterns, physical activity, alcohol consumption, and psychosocial factors, which account for 96% of the population-attributable risk of myocardial infarction in women [38]. Men and women share similar traditional risk factors for CAD; however, certain risk factors contribute more to women than men. Also, the menopausal effects on the manifestation of the risk factors for coronary disease have been underestimated.
Smoking
Despite a general decline in tobacco use in the US population, this trend in recent decades has been less pronounced for women than for men [39]. Smoking is the single most modifiable risk factor for CVD in women age < 55 years, increasing their risk 7 times [40]. Compared to men, prolonged smoking is significantly more hazardous for women [41–43]. Although the mechanisms underlying these differences remain unknown, one possible mechanism is the association of plaque erosion to smoking and younger age, which may lead to increased risk of acute coronary thrombosis in women.
Hypertension
In premenopausal women, endogenous estrogens maintain vasodilation and contribute to blood pressure control. Therefore, women develop hypertension about a decade later than men and the prevalence of hypertension becomes higher in women than men above the age of 60 years [39, 44]. Poor blood pressure control is more often seen in women than men [45]. Compared to men, blood pressure levels in healthy adulthood are lower in women, and therefore, it has been shown that women have a higher risk for CVD and events at “normal” systolic blood pressure (SBP) [46, 47], suggesting the possible need for a lower sex-specific definition of optimal SBP for women. Thus, there is room to correct for sex differences in outcomes of CVD in women for hypertension [47].
Diabetes Mellitus
Diabetes mellitus is an important predictor of CAD risk in men and women. Considerable evidence supports that diabetes mellitus is a more potent risk factor for CAD and stroke in women compared to men [48–50]. A meta-analysis showed that the relative risk of fatal coronary heart disease associated with diabetes was about 50% higher in women than it is in men [48]. The mechanisms of this increased risk for CAD in women with diabetes compared to men remain unclear. However, a sex disparity in the management and treatment of cardiovascular risk factors in individuals with diabetes to the detriment of women is possibly involved [41].
Pathological autopsy studies have shown that diabetes mellitus is associated with stable plaques (i.e., no acute thrombi) rather than lesions with acute thrombi in both sexes [36, 37], larger plaque calcification burden, and healed plaque rupture lesions [51].
Dyslipidemia
Cholesterol profiles of men and women vary by age. In women in premenopausal states, concentrations of total cholesterol, LDL-cholesterol, and triacylglycerols are lower and HDL is higher compared to men [52]. In women, following menopause, serum levels of low-density lipoproteins (LDL) cholesterol increase, and serum levels of high-density lipoproteins (HDL) cholesterol decrease [53]. This change in cholesterol profiles may help explain the increase of CAD in older women [54]. Epidemiologic studies have confirmed that high total cholesterol is a risk factor for CAD in women [38]. However, this greater CVD risk is typically not observed before menopause, even if cholesterol levels are elevated [39]. To date, no systematic assessment has been performed for sex-specific effects of major lipid profiles on cardiovascular risk. Results from meta-analyses involving large-scale individual participants generally suggest that the associations between several major lipids and the risk of CHD or stroke are similar between men and women [55–57]. These findings support previous pathological studies which showed that dyslipidemia was associated with high prevalence of TCFA in both sexes [36, 37].
Non-traditional Risk Factors and Comorbidities Associated with CVD in Women
Menopause
The role of menopause in atherosclerosis is unique to women. Coronary artery disease in women is delayed by 10 to 15 years compared with men likely related to the protective effect of estrogen on atherosclerosis and a lower prevalence of traditional risk factors in younger women [22]. Therefore, CAD is uncommon in premenopausal women, particularly in the absence of other risk factors [58]. On the other hand, postmenopausal women have a similar risk of CVD as men and the incidence of MI in women noticeably increases after menopause [58]. A meta-analysis showed that age at menopause of less than 50 years was independently associated with a RR of 1.38 of CVD after controlling for age and smoking [59]. Also, early menopause due to surgical removal of ovaries was associated with a RR of 4.55 for CVD [59]. The hormonal status also affects CAD risk in men. Men with the common genetic variation in estrogen receptor alpha (homozygous) have 3 times greater odds of myocardial infarction compared to those without the variant [60].
Other Comorbidities or Specific Factors in Women
Other female-specific conditions and factors are associated with an increased risk of CVD in women. Pregnancy-associated complications can affect mother’s cardiovascular outcomes at a remote period [19, 61–63]. Autoimmune diseases, polycystic ovary syndrome, breast artery calcification, and radiation therapy for breast cancer are also reported as risk factors for CAD in women [64–66].
Conclusions
Although CVD is the leading cause of death in both men and women worldwide, CVD in women has been underestimated for a long time. It is necessary to understand sex-based differences in plaque morphology between both sexes in order to better manage CAD. Plaque erosion is frequently seen in young women who smoke, while plaque rupture is seen in old women and men who have dyslipidemia. The prevalence and degree of coronary artery calcification are also different by sex and less calcification is observed in premenopausal women, which makes coronary artery calcification score-based risk stratification in this population challenging. A deeper understanding of the sex differences in the plaque morphology of coronary atherosclerosis, tied to different presentation of cardiac risk factors, may lead to improved outcomes of CVD in women.
Abbreviations
- CVD:
-
Cardiovascular disease
- CAD:
-
Coronary artery disease
- CTO:
-
Chronic total occlusion
- MMP:
-
Matrix metalloproteinase
- IEL:
-
Internal elastic lamina
- EEL:
-
External elastic lamina
- CN:
-
Calcified nodule
- CAC:
-
Coronary artery calcification
- OR:
-
Odds ratio
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Laslett LJ, Alagona P Jr, Clark BA 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25 Suppl):S1-49.
Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults Especially Women. Circulation. 2015;132(11):997–1002.
Izadnegahdar M, Singer J, Lee MK, et al. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Womens Health (Larchmt). 2014;23(1):10–7.
Nedkoff LJ, Briffa TG, Preen DB, et al. Age- and sex-specific trends in the incidence of hospitalized acute coronary syndromes in Western Australia. Circ Cardiovasc Qual Outcomes. 2011;4(5):557–64.
Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239(1):260–7.
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–75.
• Torii S, Sato Y, Otsuka F, et al. Eruptive calcified nodules as a potential mechanism of acute coronary thrombosis and sudden death. J Am Coll Cardiol. 2021;77(13):1599–1611. The first detailed histopathological study of calcified nodule which is the least frequent cause of acute coronary thrombosis.
Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103(7):934–40.
Sukhova GK, Schönbeck U, Rabkin E, et al. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99(19):2503–9.
Gijsen FJ, Wentzel JJ, Thury A, et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am J Physiol Heart Circ Physiol. 2008;295(4):H1608-1614.
Vengrenyuk Y, Carlier S, Xanthos S, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103(40):14678–83.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66.
Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93(7):1354–1363.
Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34(10):719–28.
Yahagi K, Zarpak R, Sakakura K, et al. Multiple simultaneous plaque erosion in 3 coronary arteries. JACC Cardiovasc Imaging. 2014;7(11):1172–4.
Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105(3):297–303.
•• Yahagi K, Kolodgie FD, Otsuka F, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13(2):79–98. A comprehensive review paper of histopathology of coronary artery atherosclerosis.
Hao H, Gabbiani G, Camenzind E, Bacchetta M, Virmani R, Bochaton-Piallat ML. Phenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesion. Arterioscler Thromb Vasc Biol. 2006;26(2):326–32.
Gavin JR III, Alberti K, Davidson MB, DeFronzo RA. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183.
Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart. 1999;82(3):265–8.
• Jinnouchi H, Sato Y, Sakamoto A, et al. Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis. 2020;306:85–95. A recent comprehensive review paper of histopatholog of coronary artery calcification.
Burke AP, Farb A, Malcom G, Virmani R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J. 2001;141(2 Suppl):S58-62.
Bigeh A, Shekar C, Gulati M. Sex differences in coronary artery calcium and long-term CV mortality. Curr Cardiol Rep. 2020;22(4):21.
Hoffmann U, Massaro JM, Fox CS, Manders E, O'Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102(9):1136–1141, 1141.e1131.
Raggi P, Shaw LJ, Berman DS, Callister TQ. Gender-based differences in the prognostic value of coronary calcification. J Womens Health (Larchmt). 2004;13(3):273–83.
Burke AP, Virmani R, Galis Z, Haudenschild CC, Muller JE. 34th Bethesda Conference: Task force #2–What is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41(11):1874–86.
Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81(5):1680–7.
Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–602.
Kim ESH. Spontaneous Coronary-Artery Dissection. N Engl J Med. 2020;383(24):2358–70.
Jinnouchi H, Finn AV, Virmani R. Nonatherosclerotic vascular disease in women. Tex Heart Inst J. 2018;45(4):233–5.
Subramanyan R, Joy J, Balakrishnan KG. Natural history of aortoarteritis (Takayasu’s disease). Circulation. 1989;80(3):429–37.
Kim ESH, Beckman J. Takayasu arteritis: challenges in diagnosis and management. Heart. 2018;104(7):558–65.
Gornik HL, Creager MA. Aortitis. Circulation. 2008;117(23):3039–51.
Matsubara O, Kuwata T, Nemoto T, Kasuga T, Numano F. Coronary artery lesions in Takayasu arteritis: pathological considerations. Heart Vessels Suppl. 1992;7:26–31.
Stone JR, Bruneval P, Angelini A, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I Inflammatory diseases. Cardiovasc Pathol. 2015;24(5):267–78.
Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336(18):1276–82.
Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97(21):2110–6.
Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52.
Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273–93.
Njølstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation. 1996;93(3):450–456.
Appelman Y, van Rijn BB, ten Haaf ME, Boersma E, Peters SAE. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241(1):211–8.
Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–305.
Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ. 1998;316(7137):1043–7.
Norris CM, Yip CYY, Nerenberg KA, et al. State of the science in women's cardiovascular disease: a Canadian perspective on the influence of sex and gender. J Am Heart Assoc. 2020;9(4):e015634.
Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294(4):466–72.
Wills AK, Lawlor DA, Matthews FE, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8(6):e1000440.
Ji H, Niiranen TJ, Rader F, et al. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation. 2021;143(7):761–3.
Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–8.
Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383(9933):1973–80.
Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27(12):2898–904.
Yahagi K, Kolodgie FD, Lutter C, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37(2):191–204.
Williams CM. Cardiovascular risk factors in women. Proc Nutr Soc. 1997;56(1b):383–91.
Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–6.
Williams CM. Lipid metabolism in women. Proc Nutr Soc. 2004;63(1):153–60.
Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000.
Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–39.
Zhang X, Patel A, Horibe H, et al. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol. 2003;32(4):563–72.
Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111(2):383–90.
Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–79.
Shearman AM, Cupples LA, Demissie S, et al. Association between estrogen receptor α gene variation and cardiovascular disease. JAMA. 2003;290(17):2263–70.
Canoy D, Beral V, Balkwill A, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131(3):237–44.
Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol. 2014;180(1):29–40.
Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013;209(4):368.e361-368.
Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98.
Daan NM, Louwers YV, Koster MP, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril. 2014;102(5):1444-1451.e1443.
Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2015;36(8):482–489c.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
CVPath Institute have received institutional research support from NIH-HL141425, Leducq Foundation Grant, 4C Medical, 4Tech, Abbott Vascular, Ablative Solutions, Absorption Systems, Advanced NanoTherapies, Aerwave Medical, Alivas, Amgen, Asahi Medical, Aurios Medical, Avantec Vascular, BD, Biosensors, Biotronik, Biotyx Medical, Bolt Medical, Boston Scientific, Canon,Cardiac Implants, Cardiawave, CardioMech, Cardionomic, Celonova, Cerus, EndoVascular, Chansu Vascular Technologies, Childrens National, Concept Medical, Cook Medical, Cooper Health, Cormaze, CRL, Croivalve, CSI, Dexcom, Edwards Lifesciences, Elucid Bioimaging, eLum Technologies, Emboline, Endotronix, Envision, Filterlex, Imperative Care, Innovalve, Innovative, Cardiovascular Solutions, Intact Vascular,,Interface Biolgics, Intershunt Technologies, Invatin, Lahav, Limflow, L&J Bio, Lutonix, Lyra Therapeutics, Mayo Clinic, Maywell, MDS, MedAlliance, Medanex, Medtronic, Mercator, Microport, Microvention, Neovasc, Nephronyx, Nova Vascular, Nyra Medical, Occultech, Olympus, Ohio Health, OrbusNeich, Ossio, Phenox, Pi-Cardia, Polares Medical, Polyvascular, Profusa, ProKidney, LLC, Protembis, Pulse Biosciences, Qool Therapeutics, Recombinetics, Recor Medical, Regencor, Renata Medical, Restore Medical, Ripple Therapeutics, Rush University, Sanofi, Shockwave, SMT, SoundPipe, Spartan Micro, Spectrawave, Surmodics, Terumo Corporation, The Jacobs Institute, Transmural Systems, Transverse Medical, TruLeaf, UCSF, UPMC, Vascudyne, Vesper, Vetex Medical, Whiteswell, WL Gore, Xeltis. A.V.F. has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; CSI; Lutonix Bard; Sinomed; Terumo Corporation; and is a consultant to Amgen; Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Lutonix Bard; Sinomed. R.V. is a consultant/scientific advisory board member of Abbott Vascular; Bosten Scientific; Celonova; Cook Medical; CSI; Edwards Lifesciences; Bard BD; Medtronic; OrbusNeich Medical; ReCor Medical; SinoMedical Sciences Technology; Surmodics; Terumo Corporation; W. L. Gore; Xeltis. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Sato, Y., Kawakami, R., Sakamoto, A. et al. Sex Differences in Coronary Atherosclerosis. Curr Atheroscler Rep 24, 23–32 (2022). https://doi.org/10.1007/s11883-022-00980-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-022-00980-5