Abstract
Purpose of Review
To provide a functional review for practicing clinicians on the current and emerging treatment considerations for transthyretin (TTR) cardiac amyloidosis (ATTR-CA).
Recent Findings
Current treatment considerations are characterized as those silencing TTR translation, stabilizing TTR tetramers, and disrupting amyloid fibril deposition.
Summary
Historically considered a rare disease state, ATTR-CA is increasingly recognized as an important mediator of heart failure morbidity and mortality. The emergence of widely available therapies for ATTR-CA has developed hope for patients where little was previously present. Thus, it is important that all cardiology clinicians have a functional understanding of the disease state and treatment options. This review will discuss agents within each of the above classes with expanded discussion on tafamidis given its favorable efficacy, safety, and availability. ATTR-CA diagnostic considerations are reviewed with regard to the identification of potential tafamidis candidates, and practical economic considerations are also reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyloidosis is an umbrella term referring to a broad group of heterogeneous diseases characterized by the tissue deposition of β-sheets of amyloid fibrils [1]. These fibrils are derived from the aggregation of misfolded proteins whose accumulation leads to eventual organ or tissue dysfunction. The manifestations of the various amyloid diseases are determined by the amyloidogenic protein source involved and the location of fibril deposition. Cardiac amyloidosis (CA) is primarily manifested through two different etiologies, transthyretin amyloidosis (ATTR) and immunoglobulin light chain amyloidosis (AL). Recognition of the CA source is key for several reasons. The most critical reason is that, though clinical presentations may be similar, the prognosis of AL-CA is grim with untreated survival periods of less than 6 months in patients presenting with heart failure symptoms whereas ATTR-CA survival can be measured in years [2, 3]. Another consideration is that AL-CA, given its clonal cell source, is principally treated within the hematology community whereas ATTR-CA management will generally be managed within cardiology. As such, multi-disciplinary cooperation within the treatment of AL-CA is important for effective and efficient management. A full discussion of nuances of AL-CA versus ATTR-CA presentation is beyond the scope of this review, and readers are directed to more detailed reviews on this topic [4, 5].
ATTR-CA can be diagnostically separated into wild-type/senile (wtATTR) and variant/mutant (vATTR) subtypes based on whether mutations in the transthyretin (TTR) protein gene are present. Here also, presentation, prevalence, and penetrance differences exist between the forms which had been described in detail previously [4]. Through continued investigation of cardiac amyloidosis, wtATTR is emerging as the more prevalent form of ATTR-CA [3]. Notably, recent data with expanded diagnostic strategies found the presence of ATTR-CA in 13% of patients hospitalized with heart failure and a preserved ejection fraction (HFpEF) and in 16% of patients referred for transcatheter aortic valve replacement (TAVR) [6, 7]. Given the potentially large group of patients impacted by ATTR-CA, the role of effective therapies is critical to improve global outcomes. The focus of this review will be to examine the present pharmacological treatments of ATTR-CA and evaluate emerging treatments to aid clinicians in the effective management of ATTR-CA.
Body
Therapeutic Targets
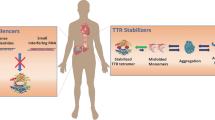
The pathophysiology of ATTR-CA and resulting heart failure symptoms progresses mechanistically in similar fashions regardless of ATTR subtype. The key steps in ATTR-CA progression include (1) TTR release from the liver, (2) disassociation of the TTR tetramer into monomers, (3) monomer aggregation into amyloid fibrils, and (4) fibril infiltration into cardiac tissue leading to restrictive cardiomyopathy (see Fig. 1) [8]. Given this sequence, current therapeutic targets can be classified into TTR silencers, TTR stabilizers, and finally agents which attempt to disrupt deposited amyloid fibrils.
TTR Silencers
Ceasing the production of TTR from the liver is attractive as an upstream rate-limiting step in amyloid fibril production and deposition. Currently, inotersen and patisiran are FDA approved for the treatment of polyneuropathy associated with vTTR and serve as targets of interest for use in ATTR-CA therapy [9, 10]. Inotersen and patisiran, as an antisense oligonucleotide (ASO) and a small interfering RNA (siRNA) respectively, bind to and facilitate cleavage of wt- and vTTR mRNA thus preventing gene translation and ultimately, TTR production [11]. In clinical trials, inotersen and patisiran therapy resulted in a 74% and 81% decrease in TTR production, respectively [12, 13].
Inotersen and patisiran have been evaluated as treatments for vTTR polyneuropathy [12, 14]. In NEURO-TTR, inotersen, as a weekly subcutaneous injection, primarily slowed neuropathy progression versus placebo based on functional and quality of life (QOL) outcomes; however, it did not reverse the disease process [12]. A significant concern regarding thrombocytopenia and glomerulonephritis was raised during the trial which led the FDA to recommend weekly platelet level, biweekly serum creatinine, estimated glomerular filtration rate (eGFR), urinalysis, and urine protein creatinine ratio, and liver function tests every 4 months during therapy [9, 12]. Patisiran, as an intravenous infusion every 3 months, also slowed functional decline versus placebo in the APOLLO study; however, in contrast to inotersen, 56% of patients had an improvement in functional outcomes from baseline [14]. Adverse effects were similar among patients receiving patisiran or placebo with only mild to moderate peripheral edema (30% vs. 22%) and injection-site reactions (20% vs. 9%) occurring more frequently with patisiran than placebo.
Currently, no prospective data on cardiovascular endpoints with inotersen or patisiran in patients with ATTR-CA are available, but observational and post-hoc data is available for review. Specifically, with inotersen, review of the NEURO-TTR cohort data showed that therapy failed to suggest any effect on longitudinal strain or other echocardiographic variables versus placebo [12]. In contrast, a small evaluation of inotersen in patients without neuropathy showed a reduction in LV wall thickness by 8.4% and 11.5% in 10 and 8 patients respectively at 2 and 3 years and a reduction in septal thickness at 3 years [15]. Inotersen therapy was generally well tolerated with the exception of platelet reductions in 15% of patients during drug therapy.
Post-hoc analyses of a subpopulation of vATTR patients with cardiac manifestations in the APOLLO study revealed that patients treated with patisiran saw significantly reduced NT-proBNP levels and all-cause mortality in comparison with placebo at 18 months [14, 16]. Treatment with patisiran was also associated with significantly reduced left ventricular wall thickness and longitudinal strain, indicating that patisiran therapy may improve cardiac structure and function [16].
Based on the current limited evidence for TTR silencers in ATTR-CA and safety concerns with inotersen, patisiran seems to be the more promising of these agents but its overall role in ATTR-CA remains unclear. Future studies will provide more information on the potential role of TTR silencers in ATTR-CA. These include a small single-center phase 2 study of inotersen (NCT03702829) with surrogate endpoints expected March 2022 and the APOLLO-B trial (NCT03997383) with an expected completion date of November 2022 [17, 18]. The APOLLO-B trial will primarily evaluate the impact of patisiran versus placebo on the 6-min walk test in both wt- and vATTR-CA patients. It will also include a secondary composite endpoint of mortality and hospitalizations. This will provide data that is critical in our understanding of the potential impact of TTR stabilization on ATTR-CA outcomes.
TTR Stabilizers
Stabilizing the TTR tetramer is the most therapeutically advanced method of decreasing amyloid fibril deposition and related consequences in ATTR-CA. At this point, four agents have been shown to stabilize TTR: diflusinil, tafamidis, epigallocatechin-3-gallate (EGCG), and AG 10 [19,20,21,22,23]. AG10 and EGCG are still being evaluated with regard to clinical outcomes (ATTRIBUTE-CM (NCT03860935), AG10, completion expected 2023) based on positive surrogate marker data in ATTR patients; however, full discussion here is beyond the scope of this review [23,24,25,26]. Current options available to clinicians are diflusinil and tafamidis. Diflusinil has limited clinical outcomes data and off-target, non-steroidal anti-inflammatory-related side effects that make therapy unattractive for majority of patients with HF symptoms [19, 20]. Tafamidis is currently approved for use in ATTR polyneuropathy in Europe, Asia, and Latin America and has recently gained approval in the USA for use in ATTR-CA based on data from the ATTR-ACT trial [27, 28]. The trial enrolled patients with clinical evidence of heart failure or recent heart failure hospitalization and evidence of cardiac amyloid involvement on biopsy related to either vATTR or wtATTR [28]. Patients were randomized in a 2:1:2 fashion to tafamidis 80 mg, tafamidis 20 mg, or placebo daily for 30 months. The 20-mg dose of tafamidis in ATTR-ACT was derived from historic success in clinical evaluations of amyloid-related polyneuropathy and pre-clinical data in ATTR-CA, whereas the 80-mg daily dose was exploratory and based on suggestion of greater TTR stabilization [28]. Outcomes were primarily analyzed through pooled analyses with both tafamidis doses. Tafamidis significantly reduced the hazard ratio for all-cause mortality by 30% and relative risk ratio for frequency of cardiovascular-related hospitalization by 32% [29•]. Functional and quality of life measures, assessed by a 6-min walk test and Kansas City Cardiomyopathy Questionnaire-Overall Summary (KCCQ-OS) score, declined in all patients, but the decline was reduced significantly in the pooled tafamidis group as compared with placebo. Overall, adverse event rates were similar between tafamidis and placebo with rates of discontinuation being higher in the placebo arm. On subgroup analysis, rates of hospitalization were reduced with the pooled tafamidis arm in patients with New York Heart Association (NYHA) class I or II symptoms while it was increased in subjects with class III symptoms. Mortality rates were reduced similarly in both subgroups. Reductions in mortality and hospitalization were consistent across vATTR and wtATTR subgroups and with the use of both tafamidis doses. In summary, tafamidis reduced rates of mortality and hospitalization in patients with ATTR-CA regardless of subtype and significantly reduced the decline in functional and quality of life measurements. When analyzing the data between the two doses of tafamidis used in the trial, there were no differences in outcomes. This would suggest that greater TTR stabilization rates do not correlate to an improved clinical outcome.
Application of the ATTR-ACT trial results to clinical practice warrants review of two aspects of the trial: patient identification and dose selection. To be included within the trial, patients were required to have confirmed ATTR deposits through biopsy, which may hinder real-world application [28, 29]. The evolution and adoption of diagnostic strategies for ATTR-CA that may obviate the need for biopsy in select ATTR-CA patients would lower a potential barrier to the clinical use of tafamidis. One noninvasive strategy evaluated for the diagnosis of ATTR-CA was the combination of radionuclide bone scintigraphy and studies for monoclonal proteins to aid in ruling out AL-CA. A finding of grade 2 or 3 myocardial radiotracer uptake on radionuclide bone scintigraphy combined with negative studies for monoclonal proteins (defined as the absence of a band on immunofixation electrophoresis of serum and urine, and a serum free light chain ratio between 0.26 and 1.65) provided 100% specificity and 70% sensitivity when compared with results from ATTR-CA biopsy [30•]. This data supports that it is reasonable to use this strategy as a diagnostic surrogate for cardiac biopsy, which has been adopted by expert consensus diagnosis recommendations [5, 31••]. However, failing to appreciate the presence of monoclonal protein could overlook the diagnosis of AL-CA which could lead to withholding appropriate treatment and quickening mortality. Clinicians should be cautioned against using positive scintigraphy studies without negative tests for monoclonal protein as the specificity of these tests alone for the positive biopsy of ATTR-CA presence was only 68% based on grade 1–3 uptake and 87% when using grade 2–3 uptake [30•]. It was also noted that 41% of patients with either grade 0–1 scintigraphy result or testing suggestive for monoclonal protein ultimately had positive ATTR-CA deposits on biopsy. Thus, the combination of scintigraphy and monoclonal testing for ATTR-CA diagnosis has a clear role and place in therapy but has not eliminated the need for biopsy.
Tafamidis has been approved by the FDA as two equivalent oral dosage forms, tafamidis meglumine (Vyndaqel®) at 80 mg once daily and tafamidis (Vyndamax®) at 61 mg once daily [27]. The tafamidis meglumine dosed at 80 mg once daily is the dosage form used in the ATTR-ACT trial and is dispensed as four 20-mg capsules, whereas the tafamidis 61 mg once daily dosage form is a single capsule providing an equivalent dose of 80 mg of tafamidis meglumine. Despite serving as a novel, successful target for the treatment of ATTR-CA, tafamidis therapy adoption may be challenged by financial considerations. Based on 2019 costs for tafamidis and a US health care perspective, tafamidis was found cost-ineffective, requiring a 92.6% reduction in cost to reach a cost-effectiveness threshold of $100,000 per quality-adjusted life year [32]. With appreciation of similar clinical outcomes between the 80-mg and 20-mg doses in the ATTR-ACT study, consideration could be given to the use of tafamidis meglumine 20 mg daily in an effort to provide a 75% reduction in raw drug price. Titration from 20 to 80 mg daily of tafamidis would not be expected to stop disease progression in ATTR-CA given ATTR-ACT showed no significant differences when evaluating the clinical outcomes between the two doses studies [29•].
Currently, TTR stabilizer therapy, primarily tafamidis, has a role in patients with symptomatic ATTR-CA. A point of consideration to be addressed by future studies would be whether earlier initiation of TTR stabilizer therapy would serve to improve clinical outcomes by limiting amyloid fibril deposition. This notion of improved outcomes resulting from earlier intervention has been suggested from data on tafamidis in polyneuropathy [33]. Specifically, patients with polyneuropathy initially treated with tafamidis versus placebo for 12 months followed by conversion to open-label tafamidis were less likely to progress for up to 6 years of follow-up, suggesting a durable effect with early intervention. Improved recognition of ATTR-CA spurred by new diagnostic strategies and availability of treatment options will aid clinicians in providing effective management of ATTR-CA. However, further clinical evaluation is needed to determine the optimal point at which the balance of therapy provision versus resource utilization will favor patient outcomes.
Fibril Removal/Disruption
Amyloid fibril disruption is a therapeutic target aimed at halting ATTR disease progression and possibly reversing amyloid aggregation. Emerging therapies have mixed mechanisms of action impacting amyloid fibrils themselves or other ancillary targets associated with amyloid deposits. Doxycycline, a tetracycline antibiotic, was studied in familial amyloid polyneuropathy (FAP) mouse models and found to disrupt ATTR deposits and lower markers associated with amyloid affected tissues, matrix metalloproteinase-9 (MMP-9) and serum amyloid P component (SAP), suggesting a positive clinical impact [34]. Tauroursodeoxycholic acid (TUDCA), an endogenous bile acid, significantly reduced fibril aggregation and decreased biomarkers for apoptosis and oxidation [35]. The combination of doxycycline and TUDCA, in FAP mice, was synergistic, lowering ATTR deposition and associated tissue markers, MMP-9 and SAP, greater than with either agent independently [36]. The identification of doxycycline/TUDCA synergy led to a phase II open-label trial of doxycycline 100 mg orally twice and TUDCA 250 mg orally thrice daily in 20 patients with ATTR amyloidosis [37]. Over 12 months, NYHA functional class, mean left ventricular wall thickness, and neuropathic involvement remained stable. Medication side effects were mild with events leading to therapy discontinuation including nausea and gastric pain.
Currently, a randomized phase III study comparing doxycycline/TUDCA to supportive therapy is underway with an expected completion date of April 2021 [38]. This trial utilizes 18-month survival as an endpoint and is of significant interest due to the low cost of doxycycline/TUDCA compared with other therapies, such as tafamidis, which may facilitate clinical application. Ursodeoxycholic acid (UDCA) is a secondary bile acid with efficacy similar to TUDCA when combined with doxycycline and has been examined in 2 studies of patients with ATTR-CA [39, 40]. First, a phase II 18-month prospective study of 28 patients treated with doxycycline 200 mg/day, with 2 weeks of intermittent discontinuation after 4 weeks of therapy, and consistent UDCA 750 mg/day was studied [39]. Disease stability was not achieved based on worsening prognostic markers at 12 and 18 months into treatment. Lack of efficacy in the study was possibly due to a high dropout rate of 14% and the study design of intermittent doxycycline discontinuation. Conversely, a larger, uncontrolled evaluation of 53 patients treated with consistent doxycycline/UDCA found no significant change in NYHA functional class, cardiac troponins, or echocardiographic parameters over 22 months suggesting stabilization of disease progression [40]. Patient-reported side effects included rash and gastrointestinal complaints, but larger controlled studies are needed to gain a better understanding of both safety and efficacy.
Monoclonal antibodies are another therapeutic option under development as fibril disruptors. Targeting ATTR amyloid fibrils, and related substance targets, via antibody interaction facilitates local complement activation and phagocytosis of fibril deposits. Dezanizumab is an IgG1 antibody targeting SAP which exists as a protective agent against native amyloid degradation [41]. A phase I trial examined the administration of dezanizumab to 16 patients with systemic amyloidosis after (R)-1-[6-[(R)-2-carboxy-pyrrolidin-1yl]-6-oxo-hexanoyl]pyrrolidine-2-carboxylic acid (CPHPC) was administered to remove serum SAP, thus allowing the antibody to target SAP within amyloid deposits thus making the fibrils susceptible to natural clearance [42]. Amyloid load was reduced in the liver, spleen, and kidney following dezanizumab administration in AL and ATTR amyloid patients [42]. However, as there were few ATTR participants in the study and a lack of clinical outcomes, application of this data is limited and future clinical evaluations are needed to understand dezanizumab’s potential role in therapy.
Finally, PRX004 is an investigational monoclonal antibody designed to target a novel epitope on ATTR fibrils and deposits [43]. An in vitro study resulted in PRX004 inhibition of fibrillogenesis and encouraged antibody-induced phagocytosis of ATTR deposits [43]. Currently, PRX004 via intravenous infusion is undergoing a phase I clinical trial with an anticipated 36 participants and expected completion date of November 2021; however, future evaluations with clinical outcomes will still be needed [44].
Conclusion
At present, tafamidis is the most effective and clinically relevant TTR-directed therapy for ATTR-CA given mortality risk reduction over its study period. As therapeutic innovations progress, the emergence of useful treatments for ATTR-CM will give options for patients suffering from this condition. One important challenge associated with TTR-directed therapies, at least in the early years of treatment availability, will be balancing cost effectiveness from a societal and healthcare system perspective. With new potential therapeutic targets, clinicians are apt to have a greater appreciation for the presence of ATTR-CA and the potential to improve patient outcomes.
Change history
28 September 2020
On page 2 of the original publication, in the section on TTR Silencers dosing of patisiran in the APOLLO study was stated as being given every 3��months; this is inaccurate as patisiran was dosed every 3��weeks in the APOLLO study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387:2641–54.
Perry BW, Ikram A, Hachamovitch R, et al. Efficacy of chemotherapy for light-chain amyloidosis in patients presenting with symptomatic heart failure. J Am Coll Cardiol. 2016;67:2941–8.
Ruderg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2872–91.
Donnelly JP, Hanna M. Cardiac amyolidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84(12 Suppl 3):12–26.
Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12(9):e006075. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006075.
Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94.
Castano A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–87.
Ruberg FL, Berk JL. Transthyretin cardiac amyloidosis. Circulation. 2012;126:1286–300.
Tegsedi [package insert]. Boston: Akcea Therapeutics, Inc; 2019.
Onpattro [package insert]. Cambridge: Alnylam Pharmaceuticals, Inc; 2020.
Ackermann EJ, Guo S, Benson MD, Booten S, Freier S, Hughes SG, et al. Suppressing transthyretin production in mice, monkeys and humans using 2nd-generation antisense oligonucleotides. Amyloid. 2016;23:148–57.
Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31.
Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109.
Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21.
Dasgupta NR, Rissing SM, Smith J, Jung J, Benson MD. Inotersen therapy of transthyretin amyloid cardiomyopathy. Amyloid. 2020;27:52–8.
Solomon SD, Adams D, Kristen A, Grogan M, González-Duarte A, Maurer MS, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis analysis of the APOLLO Study. Circulation. 2019;139:431–43.
24 Month open label study of the tolerability and efficacy of an antisense oligonucleotide (inotersen) in patients with transthyretin (ttr) amyloid cardiomyopathy – full text view- ClinicalTrials.gov [Internet]. [cited 2020 April 28]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03702829. Accessed 28 Apr 2020
APOLLO-B: a phase 3, randomized, double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of patisiran in patients with transthyretin amyloidosis with cardiomyopathy (ATTR amyloidosis with cardiomyopathy) – full text view- ClinicalTrials.gov [Internet]. [cited 2020 April 28]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03997383. Accessed 28 Apr 2020
Berk JL, Dyck PJ, Obici L, Zeldenrust SR, Sekijima Y, Yamashita T, et al. The diflunisal trial: update on study drug tolerance and disease progression. Amyloid. 2011;18(Suppl 1):196–7.
Sekijima Y, Tojo K, Morita H, Koyama J, Ikeda S. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid. 2015;22:79–83.
Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109:9629–34.
Ferreira N, Cardoso I, Domingues MR, Vitorino R, Bastos M, Bai G, et al. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett. 2009;583:3569–76.
Judge DP, Heitner SB, Falk RH, Maurer MS, Shah SJ, Witteles RM, et al. Transthyretin stabilization by AG10 in symptomatic transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. 2019;74(3):285–95.
A phase 3, randomized, double-blind, placebo-controlled study of the efficacy and safety of AG10 in subjects with symptomatic transthyretin amyloid cardiomyopathy (ATTRIBUTE-CM) – full text view- ClinicalTrials.gov [Internet]. [cited 2020 April 28]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03860935. Accessed 28 Apr 2020
aus dem Siepen F, Bauer R, Aurich M, et al. Green tea extract as a treatment for patients with wild-type transthyretin amyloidosis: an observational study. Drug Des Devel Ther. 2015;9:6319–25.
Kristen AV, Lehrke S, Buss S, Mereles D, Steen H, Ehlermann P, et al. Green tea halts progression of cardiac transthyretin amyloidosis: an observational report. Clin Res Cardiol. 2012;101:805–13.
Vyndaqel [package insert]. New York: Pfizer, Inc; 2020.
Maurer MS, Elliott P, Merlini G, et al. Design and rationale of the phase 3 ATTR-ACT clinical trial (tafamidis in transthyretin cardiomyopathy clinical trial). Circ Heart Fail. 2017;10:e003815.
Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–16 Randomized trial of tafamidis showing reduction in mortality with therapy creating foundation of current ATTR-CA therapy.
Gillmore DJ, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–12 Study highlighting role for expanded non-biopsy ATTR-CA diagnosis strategy; includes processes for ruling out AL-CA as part ATTR-CA diagnosis.
Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;141:00–00. https://doi.org/10.1161/CIR.0000000000000792. Contemporary scientific statement from the American Heart Association providing clinicians with updated diagnostic and treatment strategies.
Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, et al. Cost-effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation. 2020;141:1214–24.
Barroso FA, Judge DP, Edebe B, et al. Long-term safety and efficacy of tafamidis for the treatment of hereditary transthyretin amyloid polyneuropathy: results up to 6 years. Amyloid. 2017;24:194–204.
Cardoso I, Saraiva MJ. Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. FASEB J. 2006;20:234–9.
Macedo B, Batista AR, Ferreira N, Almeida MR, Saraiva MJ. Anti-apoptotic treatment reduces transthyretin deposition in a transgenic mouse model of familial amyloidotic polyneuropathy. Biochim Biophys Acta. 1782;2008:517–22.
Cardoso I, Martins D, Ribeiro T, Merlini G, Saraiva MJ. Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med. 2010;8:74.
Obici L, Cortese A, Lozza A, Lucchetti J, Gobbi M, Palladini G, et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid. 2012;19(Suppl 1):34–6.
A study of doxycycline and tauroursodeoxycholic acid (Doxy/TUDCA) plus standard supportive therapy versus standard supportive therapy alone in cardiac amyloidosis caused by transthyretin – full text view- ClinicalTrials.gov [Internet]. [cited 2020 April 28]. Available from: https://clinicaltrials.gov/ct2/show/NCT03481972. Accessed 28 Apr 2020
Wixner J, Pilebro B, Lundgren HE, Olsson M, Anan I. Effect of doxycycline and ursodeoxycholic acid on transthyretin amyloidosis. Amyloid. 2017;24:78–9.
Karlstedt E, Jimenez-Zepeda V, Howlett JG, White JA, Fine NM. Clinical experience with the use of doxycycline and ursodeoxycholic acid for the treatment of transthyretin cardiac amyloidosis. J Card Fail. 2019;25:147–53.
Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93–7.
Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med. 2015;373:1106–14.
Higaki JN, Chakrabartty A, Galant NJ, Hadley KC, Hammerson B, Nijjar T, et al. Novel conformation-specific monoclonal antibodies against amyloidogenic forms of transthyretin. Amyloid. 2016;23:86–97.
A study of PRX004 in subjects with amyloid transthyretin (ATTR) Amyloidosis – full text view- ClinicalTrials.gov [Internet]. [cited 2020 April 20]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03336580. Accessed 20 Apr 2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on New Therapies for Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Hough, A., Wearden, J., de Almeida, K. et al. Review of Transthyretin Silencers, Stabilizers, and Fibril Removal Agents in the Treatment of Transthyretin Cardiac Amyloid. Curr Cardiol Rep 22, 106 (2020). https://doi.org/10.1007/s11886-020-01374-2
Published:
DOI: https://doi.org/10.1007/s11886-020-01374-2