Abstract
Purpose of Review
Abnormal accumulation of pericardial fluid is a common cardiac condition with different etiologies. Draining of the pericardial fluid (pericardiocentesis) is often indicated for diagnostic and therapeutic purposes and is performed in an elective or emergent setting. Echocardiography is the primary imaging method for diagnosing, localizing, and quantifying pericardial effusion as well as evaluating its hemodynamic effects, including the presence of cardiac tamponade. In this manuscript, we review the indications for pericardiocentesis and provide practical step-by-step guidance for echo-guided pericardiocentesis.
Recent Findings
Echo-guidance is an effective method to improve the safety and efficacy of pericardiocentesis. In experienced hands and with a stepwise approach, procedural outcomes are excellent, and complication rates are very low. Asymptomatic small idiopathic effusions have a benign course and can be left untreated. Prolonged drainage with an indwelling pericardial catheter is key for preventing fluid re-accumulation, and the use of colchicine to prevent fluid recurrence is encouraged whenever possible.
Summary
Understanding how to evaluate the significance of a pericardial effusion as well as the procedural steps in the performance of a pericardiocentesis are essential for optimal outcomes in treating patients with pericardial effusions and tamponade.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pericardium is a fibroelastic sac surrounding the heart which consists of an outer fibrous layer (parietal pericardium) and an inner serous membrane (visceral pericardium). The potential space between these two layers is the pericardial space, which contains 20–25 ml of fluid in normal conditions [1]. Accumulation of excess pericardial fluid can result from multiple causes that can be categorized into idiopathic, inflammatory, neoplastic, infectious, and traumatic. The prevalence of pericardial effusion in the general population is not well studied and has been reported to be between 0.8 and 5.7%, depending on the population demographic characteristics and comorbidities [2,3,4]. Pericardial effusion is not an uncommon finding in asymptomatic patients. An incidental small pericardial effusion is generally benign and, in most cases, resolves over time. However, in one study, the finding of a small pericardial effusion has been associated with increased mortality compared with patients with no effusion [3]. In patients with underlying systemic diseases, the frequency of pericardial effusion is much higher and is commonly a marker of disease severity. In a meta-analysis of 23 studies involving 17,022 patients with underlying pulmonary, infectious, cardiac, renal, or neoplastic diseases, 19.5% of patients had a pericardial effusion [5], and its presence was associated with an adverse outcome related to the underlying disease, compared with patients with the same condition but without pericardial effusion. In this study, there was insufficient data to assess the prognostic impact of idiopathic pericardial effusions.
Many conditions can lead to pericardial effusion including infectious pericarditis (viral, bacterial, tuberculosis), malignancy, inflammatory and autoimmune disorders, post-cardiac surgery, congestive heart failure, chest trauma (blunt or penetrating), ascending (type A) aortic dissection with extension into the pericardium, post-myocardial infarction, chronic renal failure, myxedema, radiation, and drug-induced. However, in most cases, and despite complete fluid analysis, the etiology of the effusion remains elusive and is categorized as idiopathic [6].
The development of echocardiography by Edler and Hertz in 1953 paved the way for noninvasive detection of pericardial effusion. Subsequently, Feigenbaum and colleagues found that they could identify experimentally induced pericardial effusions in a canine model [7]. The use of transthoracic echocardiography (TTE) revolutionized the diagnosis of pericardial effusion by making it safe, non-invasive, and highly accurate. Semiquantitative grading of the pericardial effusion is performed by measuring its size at end-diastole, which then can be further categorized into small (< 10 mm), moderate (10–20 mm), and large (> 20 mm) [8].
The pericardiocentesis procedure, while invasive, has been made much safer by utilizing TTE guidance. Currently, pericardiocentesis is a widely practiced procedure. When pericardiocentesis is done by experienced operators with echocardiographic guidance, it is generally highly successful and has a low risk for complications. In patients with malignancy, compared with a surgical subxiphoid window, ultrasound-guided percutaneous pericardiocentesis with prolonged drainage has a similar success rate, shorter hospital stay, and lower morbidity with no reduction in diagnostic accuracy [9].
The purpose of this review is to provide the reader with an understanding of how to diagnose pericardial tamponade and to present a practical step-by-step guidance for performing pericardiocentesis.
Indications for Pericardiocentesis
A pericardiocentesis can be elective, urgent, or emergent. Elective pericardiocentesis is usually performed to elucidate the etiology of the pericardial fluid, specifically to evaluate for malignancy or infection. It may also be done to clarify the hemodynamic significance of the effusion as in the evaluation for effusive-constrictive pericardial disease, which can present with an initial hemodynamic pattern typical of tamponade. Following pericardial drainage, the pressure waveform patterns change to those indicative of pericardial constriction with the persistence of elevated and equal pressures.
Elective pericardiocentesis may be indicated for diagnostic purposes when a malignancy is suspected. In the setting of sepsis or infective endocarditis with concomitant pericardial effusion, pericardiocentesis can be performed to exclude purulent pericarditis. Purulent pericarditis, an uncommon finding, usually occurs in the setting of bacteremia and immunosuppression such as in human immunodeficiency virus (HIV) infection, dialysis, or following cardiac or thoracic surgery. The diagnosis is generally established by pericardiocentesis, and a pericardial fluid sample is important for identifying the causal microorganism by culture and microscopy. Other findings suggestive of purulent pericarditis include elevated fluid leukocyte count as well as low pericardial to serum glucose ratio and high fluid protein content. In addition, the fluid is often turbid and malodorous. These patients are usually toxic and thus qualify for an urgent pericardiocentesis. Not infrequently, patients have clinical signs of tamponade, making the procedure both diagnostic and therapeutic. Empirical intravenous antibiotic treatment should be initiated promptly; however, in some cases, surgery is required to achieve complete drainage and removal of excessive fibrin deposits [10•]. Combining antibiotics and intrapericardial fibrinolysis has been reported as a less invasive treatment than surgical pericardiectomy to resolve purulent pericarditis and prevent pericardial constriction [11].
Emergent pericardiocentesis is indicated when the pericardial effusion creates hemodynamic compromise. This is an absolute clinical necessity when acute hemodynamic deterioration and hypotension develops. Acute iatrogenic tamponade can develop in the cardiac catheterization or electrophysiology (EP) laboratory if myocardial or coronary artery perforation occurs, leading to rapid accumulation of blood in the pericardial space. In these cases, as little as 50–100 ml of blood may lead to abrupt hemodynamic collapse with profound hypotension. Pericardial drainage needs to be performed immediately in this setting, preferably with echo-guidance; however, if time does not permit, a “blind” procedure should be done with the pericardiocentesis needle being inserted from the subxiphoid region. Most proceduralists angulate the pericardiocentesis needle toward the left shoulder since this should help avoid puncturing the lung. However, some advocate angling the needle towards the right shoulder to reduce the risk of lacerating the left anterior descending (LAD) coronary artery. In the catheterization laboratory, fluoroscopy is helpful for identifying the enlarged cardiac silhouette and for guiding pericardial puncture, although fluoroscopy may not precisely delineate the boundaries of the pericardial effusion or differentiate the epicardial surface from the limits of the pericardium. In cases of acute and rapid development of a pericardial effusion, as demonstrated in Fig. 1, less than 100 ml of blood may lead to a hemodynamically compromising pericardial effusion. Thus, the rate of fluid accumulation is very relevant to clinical presentation. Rapid accumulation of a relatively small effusion can lead to tamponade due to a non-compliant pericardium, whereas slowly accumulating effusions in a compliant pericardium may exceed 1000 ml without resulting in tamponade physiology.
Schematic pericardial pressure-volume curve. a In a rapidly accumulating pericardial effusion, pericardial compliance is low and small amount of fluid generates a large increase in intrapericardial pressure with rapid development of cardiac tamponade. b With a slowly accumulating effusion, pericardial stretching and adaptation occur leading to a small increase in pressure even with a large effusion volume. When the limit of pericardial stretch is reached, clinical tamponade develops
From our experience, moderate to large pericardial effusions are often detected as an incidental finding suggested by an increase in the cardiac silhouette on chest X-ray, or by computerized tomography (CT) scan or TTE performed as part of an unrelated diagnostic work-up. Patients may describe chest pain, palpitations, shortness of breath, or other non-specific symptoms. Asymptomatic large pericardial effusion can occur after cardiac surgery, especially if patients are receiving anticoagulation. Symptomatic effusions are generally those who develop rapidly or chronic effusions that are large enough to increase right atrial (RA) and systemic venous pressure or reduce cardiac output and systemic arterial pressure. Effusions that develop slowly over time may show echo findings of pre-tamponade or tamponade that precede symptoms or clinical signs of hypotension or hypoperfusion.
Pericardial Effusion in Specific Clinical Scenarios
Pericardial effusion can exacerbate left ventricular outflow tract (LVOT) gradients in patients with hypertrophic obstructive cardiomyopathy (HOCM). External compression by the effusion reduces the left ventricle (LV) cavity dimensions and LV filling, resulting in worsening in LVOT gradients. We have found that LVOT gradients drop following pericardiocentesis, even in patients not presenting with signs of pericardial tamponade such as hypotension or tachycardia. Conversely, in patients with aortic stenosis, the low cardiac output secondary to hemodynamic compromise from the pericardial effusion may reduce the generated transaortic gradients and mask the severity of aortic stenosis. Pericardial effusion is also a frequent finding in patients with pulmonary arterial hypertension [12]. In the setting of pulmonary hypertension, the chronic RV pressure overload and RV hypertrophy can prevent RV free wall collapse and mitigate some of the hemodynamic effects of tamponade [13]. Draining of a large effusion can result in a rapid increase in RV preload, which may not be well-tolerated in patients with chronic pulmonary hypertension and RV dysfunction. However, pericardiocentesis is generally safe in these patients [14, 15].
Echocardiographic Diagnosis of Tamponade
Cardiac tamponade is a life-threatening emergency defined as a low cardiac output state resulting from the accumulation of pericardial fluid, blood, blood clots, pus (empyema), or even gas under pressure. Common etiologies include pericarditis, post-cardiac surgery, trauma, or malignancy. Tamponade is considered a clinical diagnosis, classically presenting as the Beck’s triad of (1) hypotension, (2) jugular venous distention, and (3) diminished heart sounds [16]. However, the development of tamponade is a continuum that can manifest with subtle findings but can rapidly deteriorate and thus require a high index of suspicion and serial clinical and echocardiographic monitoring.
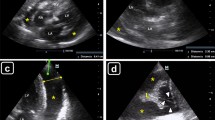
Echocardiography is the most useful diagnostic method to identify cardiac tamponade. When several echocardiographic parameters are consistent, the diagnosis of tamponade or its absence is straightforward. As seen in Fig. 2, findings suggestive of tamponade include a large pericardial effusion (> 20 mm in diastole) often with a “swinging heart”, markedly dilated (> 2.5 cm) inferior vena cava (IVC) without respiratory-phasic variations, late diastolic/early systolic collapse of the RA free wall, right ventricular (RV) early diastolic collapse, and exaggerated respiratory variations (> 25%) of the mitral inflow velocity (Fig. 3). When these findings are present, even in the absence of overt hemodynamic collapse, urgent pericardiocentesis should be performed, provided etiologies that may warrant surgical intervention such as acute aortic dissection, recent chest trauma, or post-myocardial infarction free wall rupture have been excluded.
Two-dimensional (2D) and M-mode echocardiographic signs of pericardial tamponade. a Parasternal long–axis view showing a large pericardial effusion encompassing the entire circumference of the heart (“swinging heart”) also seen in b short-axis view. c Apical four-chamber view showing right atrial early-systolic collapse (orange arrow). d Subcostal view showing right ventricular free-wall end-diastolic collapse (yellow arrow) also seen by e M-mode (blue arrows). f Dilated inferior vena cave without inspiratory collapse
Respiratory variations in flow velocities by spectral Doppler during pericardial tamponade. a Mitral inflow velocity and velocity-time integral (VTI) by pulsed-waved Doppler showing greater than 25% reduction in velocity during inspiration. b The same variation in the VTI is present in the left ventricular outflow tract (LVOT) Doppler tracing, reflecting respiratory changes in cardiac stroke volume (decrease with inspiration, increase with exhalation), consistent with pulsus paradoxus
Conversely, the combination of a small IVC with > 50% inspiratory collapse, no evidence of limited expansion of the RA and RV on multiple echocardiographic views, and absent respiratory mitral inflow variations, makes the diagnosis of tamponade unlikely, especially in patients with known, long-standing pericardial effusion. One caveat is when a patient is hypovolemic due to over-diuresis or bleeding, in which findings suggestive of elevated RA pressure (i.e., dilated, non-collapsing IVC) may be absent, despite a hemodynamically significant pericardial effusion. A repeat TTE should always be performed if clinical status worsens, as decompensation depends on the rate of fluid accumulation.
Echo-Guided Pericardiocentesis: Procedure Overview
Echo-guided pericardiocentesis significantly improves the feasibility and safety of pericardiocentesis. A high (> 95%) success rate for pericardiocentesis using echo-guidance has been reported by Tsang et al., on nearly 1000 patients, and complications were uncommon (< 2%) [17]. These results are much better than those reported for pericardiocentesis without echo guidance. Pericardiocentesis can readily be performed at the bedside in an intensive care unit (ICU) setting where continuous electrocardiographic (ECG) and hemodynamic (blood pressure and oxygen saturation) monitoring is available. This approach allows the procedure to be done in a stable environment, thus avoiding transferring a possibly unstable patient to the catheterization laboratory or procedure room. Moreover, as the patients remain in their ICU room, the potential for catheter dislodgement during patient transfer as well as infection risk are reduced.
Procedural Preparations
-
(A)
Check platelet count and coagulation status.
-
(B)
Perform a TTE at bedside to assess the fluid quantity, confirm the need for the procedure, and exclude the possibility of aortic dissection by assessing aortic size. In patients with dilated aorta whom there is suspicion or the possibility of an aortic dissection, transesophageal echocardiography (TEE) or CT should be performed prior to the pericardiocentesis to rule out aortic dissection. Draining pericardial effusion in the setting of aortic dissection can lead to worsening of the intrapericardial bleeding, as well as a possible increase in systemic blood pressure from a temporary increase in cardiac output and potentially worsening of the dissection and increased risk for aortic rupture.
-
(C)
Use TTE to identify the site where the effusion is largest and is easiest to access with a pericardiocentesis needle. Measure the distance from the chest wall to the fluid and the distance from the outer pericardium to the epicardium. Find the best needle access site to avoid trauma to the lung, liver, or other viscera. As shown in Fig. 4a, utilize the echo transducer to set the best angle of incidence for the needle and the chest wall. This angle should be used when inserting and advancing the needle into the thorax.
Procedural preparations for echo-guided pericardiocentesis. a TTE is used to locate the optimal site for puncture (transapical in this case). The probe is tilted to find the angle of incidence. b The puncture site is marked by ink or a small scratch of the skin (white arrow). c The pericardiocentesis site is prepared and draped in a usual sterile fashion. d The access site is anesthetized using 1% xylocaine without epinephrine. e A skin incision is made using a scalpel at the planned site of needle entry. f The entry site is dilated using a hemostat (reprinted with permission from Springer Nature: Siegel RJ, Arsanjani R: Echo Guided Pericardiocentesis. In Intraprocedural Imaging of Cardiovascular Intervention. Picard M, Passeri J, Dal-Bianco J, Editors. Springer International Publishing Switzerland; 2016:23–32) [26]
The Pericardiocentesis Procedure
-
(A)
Explain the procedure to the patient and instruct them to minimize their movements to reduce the risk of complications.
-
(B)
Have the patient lie in a comfortable supine position, as close as possible to the edge of the bed with the head elevated at 0–45° or higher if needed to avoid hypoxia and respiratory distress.
-
(C)
Mark the site of planned puncture and perform an antiseptic surgical prep of the skin to avoid introducing bacteria into the pericardiocentesis tract and the pericardial space. Use surgical drapes to keep the site sterile, as seen in Fig. 4 b and c.
-
(D)
Inject the skin and the tract of the puncture site liberally with 1% lidocaine (20–30 ml) without epinephrine (initially subcutaneously with a 25-gauge and then deeper with a 22-gauge needle). The pericardiocentesis should be relatively painless so that the patient is comfortable and does not move during the procedure. With adequate local analgesia, additional pain medication is not necessary. Moreover, in patients with hemodynamically significant effusions, intravenous sedation should be avoided as this may reduce adrenergic drive and lead to significant hypotension. Atropine should be available in case the patient experiences a vaso-vagal reaction manifested as bradycardia and hypotension.
-
(E)
Make a small skin incision (Fig. 4e) at the planned site of needle entry and use a hemostat to dilate the site (Fig. 4f). Various sites can be used for needle insertion, which includes subxiphoid, apical, left and right parasternal, apical lateral, and high lateral sites. The apical and subxiphoid approaches are the most common. We avoid the subcostal approach if the liver or other viscera are in the path of the pericardiocentesis needle.
-
(F)
Several needles or catheter types can be used for this procedure. We most often use an 18-gauge, 15 cm in length, pericardiocentesis Cook needle. A micropuncture needle or angiocath are also suitable.
-
(G)
Procedural monitoring:
-
(a)
Continuous ECG and oxygen saturation monitoring are important. Blood pressure should be cycled for measurement every 1–2 min.
-
(b)
TTE monitoring should be performed with a sterile ultrasound probe cover. If a sterile probe cover is not used, TTE windows outside the sterile field can be used to provide imaging.
-
(c)
When a transapical approach is used in female patients, avoid going through breast tissue. If the left breast obscures the sterile field and/or the site of pericardiocentesis, it can be taped cephalad to keep the breast out of the field during the procedure.
-
(d)
In cases with small or loculated effusion that needs to be sampled for diagnostic evaluation for cancer or infection, ECG monitoring can be useful to determine if the pericardial needle touches the epicardial surface of the heart. This can be done by attaching an ECG electrode to the pericardiocentesis needle. If the needle contacts the epicardial surface, there will be a current of injury with ST segment elevation appearing on the ECG tracing. If this occurs, the needle needs to be withdrawn until the ST changes disappear.
-
(H)
During needle insertion (Fig. 5 a and b), we advance the needle in 2–3 mm increments while injecting lidocaine and aspirating until pericardial fluid appears. It is imperative to maintain the pre-determined trajectory obtained by pre-procedure TTE.
-
(I)
If the fluid is not hemorrhagic, remove 10 to 30 ml to decrease intrapericardial pressure. Next, the syringe is detached from the needle without changing the needle location and/or angle. If there is a free flow of pericardial fluid, a j-tipped guidewire is inserted through the needle into the pericardial space as shown in Fig. 5c. The guidewire should advance easily and freely. If there is resistance, the guidewire should be withdrawn, and the needle redirected or advanced slightly or possibly pulled back. As soon as the pericardial fluid is easily aspirated, the guidewire is then re-inserted or advanced. With hemorrhagic effusions, expel 5 to 10 ml of fluid onto a gauze pad and examine it for small blood clots. If the fluid contains clots, the needle is either intracardiac or the clots are from fresh blood in the pericardial space resulting from aortic dissection or cardiac trauma due to cardiac laceration and/or perforation before or during the pericardiocentesis. Aspiration of blood clots is a critical finding. Further aspiration of bloody fluid needs to be immediately stopped as relief of tamponade in this setting will allow systemic pressure to rise and possibly promote worsening of the ventricular or aortic rupture or dissection. With non-clotting bloody fluid, to determine whether the source is intracardiac or from the pericardial space, inject agitated saline or agitated aspirate into the pericardial needle. This results in contrast, seen by TTE and allowing identification of whether the needle is in the heart or pericardial space. We like to use the aspirate and mix it between two syringes with a three-way stopcock and then re-inject it through the needle, resulting in a robust echo-contrast image. In the setting of aortic dissection or myocardial rupture, patients need to be transferred emergently to the operating room for thoracotomy and a TEE to confirm the diagnosis.
-
(J)
Once determining that the needle is in intra-pericardial, pass a dilator over the guidewire as seen in Fig. 5d. We generally dilate the space multiple times to allow for easy passage of the pericardial catheter.
-
(K)
In over 95% of cases, we leave a drain in the pericardial space, unless the pericardiocentesis tract went through the liver, in which the trans-hepatic pericardial drain can theoretically lead to hepatic hematoma, bleeding, fistula, or other complications.
-
(L)
A pig-tailed pericardial catheter is subsequently advanced into the pericardial space for at least 20 cm (Fig. 5e). It is our practice to suture the catheter in place so that it does not get inadvertently pulled out. We then aspirate pericardial fluid and empty it into a drainage bag until there is no further drainage.
-
(M)
A Jackson-Pratt (JP) bulb drain (Fig. 5f) is useful to induce light suction to the pericardial catheter.
-
(N)
Pericardial fluid analysis testing is patient specific. Namely, if it is a traumatic/iatrogenic etiology, we generally only send fluid for culture, gram stain, cell count, and glucose to ensure that no secondary infection is present. If malignancy is a possibility, we send most of the fluid for cytology. Differentiating between transudate and exudate by lactate dehydrogenase (LDH) levels or total protein has little clinical utility. Transudative fluid does not exclude a metastatic neoplasm as the cause. PCR and acid-fast stain for tuberculosis (TB) should be performed whenever a clinical suspicion for TB is present, specifically, in the elderly, immunocompromised patients, or patients from endemic areas.
-
(O)
We treat all patients with prophylactic anti-staphylococcal antibiotics until the pericardial catheter is withdrawn. If no contraindication is present, we put patients on empiric anti-inflammatory therapy with colchicine and add non-steroidal anti-inflammatory drugs (NSAIDs) if there is no contraindication such as renal insufficiency or gastrointestinal intolerance. We specifically discourage and do not use steroids as they increase the risk of recurrent pericarditis [10•].
-
(P)
After draining hemodynamically significant pericardial effusion, sinus tachycardia usually improves, and systolic blood pressure rises. In addition, improvement in RA pressure can be observed by IVC collapsibility and the abolishment of Doppler mitral inflow inspiratory variations (also evident on the pulse oximetry waveform). In addition, the resolution of pulsus paradoxus due to cardiac tamponade is expected. Re-expansion pulmonary edema (pericardial decompression syndrome) has been reported in up to 5% after removal of large effusions [18, 19•]; however, from our experience, this is uncommon and can likely be prevented by slow drainage through a pericardial catheter [19•].
Procedural steps for echo-guided pericardiocentesis. a A cook needle is used to gain access to the pericardial space. b The cook needle is slowly advanced until small amount of pericardial fluid is aspirated. c The guidewire is advanced through the needle into the pericardial space. d A dilator is passed over the guidewire to allow for easy passage of the pericardial catheter. e The pericardial catheter is advanced over the guidewire into the pericardial space for at least 20 cm. f The pericardial catheter is attached to a Jackson-Pratt (JP) drain and kept in place for generally more than 36 h (reprinted with permission from Springer Nature: Siegel RJ, Arsanjani R: Echo Guided Pericardiocentesis. In Intraprocedural Imaging of Cardiovascular Intervention. Picard M, Passeri J, Dal-Bianco J, Editors. Springer International Publishing Switzerland; 2016:23–32) [26]
We generally keep the intra-pericardial catheter in place for more than 36 h, and until both the drainage is less than 50 to 100 ml in a 24-h period and there is no more than a trivial pericardial effusion by TTE. We have found that with prolonged drainage, the pericardial effusion recurrence rate drops from 52% (without leaving a pericardial catheter in place) to 12% [20]. This was also reported by Tsang et al. from the Mayo Clinic, showing a reduction in fluid re-accumulation from 27 to 14% by using prolonged pericardial drainage [17]. Even in the setting of malignant effusions, prolonged drainage is as effective as a surgical window with a 12% recurrence after surgery and a 13% recurrence after prolonged pericardial catheter drainage [9].
Complications
The most common complications include arrhythmias, coronary artery or cardiac chamber puncture, hemothorax, pneumothorax, pneumopericardium, and hepatic injury. Pericardiocentesis can be technically demanding and should ideally be performed by a skilled clinician, as experience and volume affects procedural outcome. A minimum of five supervised pericardiocentesis procedures have been recommended for cardiologists and emergency medicine physicians in training [21], and several low-cost training simulators have been described [22, 23]. Coagulopathy or thrombocytopenia is considered a relative contra-indication for pericardiocentesis. However, in a large case series (N = 1127) from the Mayo Clinic, no excess bleeding risk was observed following echo-guided pericardiocentesis in patients with international normalized ratio (INR) > 2 or platelet count less than 50 × 109/L [24•]. However, these findings reflect the experience of a large volume center in which the procedure was done by cardiologists with extensive experience, and the overall complication rate (including bleeding) was very low. Ideally, the subxiphoid approach should be avoided in patients with coagulopathy if the liver or other viscera are in the way. However, Lindenberger et al. have shown no bleeding complications when pericardial access was obtained through the liver, even in patients with coagulopathy or thrombocytopenia [25]. Our > 30-year experience at Cedars-Sinai Medical Center, with over 700 patients has shown the major complication rate to be 1.1% [9, 20]. Major complications included pneumothorax (n = 1), myocardial laceration (n = 2), of which in one patient, the bleeding resolved after leaving the pericardial drain in for 4 days; however, the other patient required cardiothoracic surgery due to persistent hemorrhagic drainage secondary to the myocardial laceration which was confirmed at the time of surgery. Other major complications were breakage of the intrapericardial catheter during withdrawal (n = 1), requiring surgery for extraction of the retained catheter. Other than the complications mentioned above, there was no procedural mortality or need for emergent surgery for life-threatening complications. To avoid pericardial catheter fracture in cases when resistance is encountered during its removal, a light weight (for example, a 500 ml bag of saline) can be attached to the external portion of the catheter. From our experience, in the course of minutes to hours, the catheter will come out. This approach has not been associated with catheter breakage.
Conclusion
Echocardiographic guided pericardiocentesis is the standard of care when draining pericardial effusion unless the patient is planned for thoracic surgery for another reason. In experienced hands and when meticulous pre-procedural evaluation and planning is undertaken, procedural outcomes are excellent, and complication rates are very low. However, it is always important to evaluate the risk benefit of the procedure, as many small idiopathic effusions have a benign course and can be left untreated. Prolonged drainage with an indwelling pericardial catheter is key for preventing fluid re-accumulation, and the use of colchicine to prevent fluid recurrence is encouraged whenever possible. With an older patient population and the exponential growth in interventional cardiac therapies, the need for pericardiocentesis is likely to grow. Thus, accurate echocardiographic detection of pericardial effusion and signs of tamponade as well as performing echo-guided pericardiocentesis are all important skills for a clinical cardiologist.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Rodriguez ER, Tan CD. Structure and anatomy of the human pericardium. Prog Cardiovasc Dis. 2017;59(4):327–40. https://doi.org/10.1016/j.pcad.2016.12.010.
McKenna DA, Laxpati M, Colletti PM. The prevalence of incidental findings at cardiac MRI. Open Cardiovasc Med J. 2008;2:20–5. https://doi.org/10.2174/1874192400802010020.
Mitiku TY, Heidenreich PA. A small pericardial effusion is a marker of increased mortality. Am Heart J. 2011;161(1):152–7. https://doi.org/10.1016/j.ahj.2010.10.007.
Imazio M, Mayosi BM, Brucato A, Markel G, Trinchero R, Spodick DH, et al. Triage and management of pericardial effusion. Journal of cardiovascular medicine (Hagerstown, Md). 2010;11(12):928–35. https://doi.org/10.2459/JCM.0b013e32833e5788.
De Filippo O, Gatti P, Rettegno S, Iannaccone M, D’Ascenzo F, Lazaros G, et al. Is pericardial effusion a negative prognostic marker? Meta-analysis of outcomes of pericardial effusion. Journal of Cardiovascular Medicine. 2019;20(1):39–45.
Strobbe A, Adriaenssens T, Bennett J, Dubois C, Desmet W, McCutcheon K, et al. Etiology and long-term outcome of patients undergoing pericardiocentesis. J Am Heart Assoc. 2017;6(12). https://doi.org/10.1161/jaha.117.007598.
Feigenbaum H, Waldhausen JA, Hyde LP. Ultrasound diagnosis of pericardial effusion. JAMA. 1965;191:711–4. https://doi.org/10.1001/jama.1965.03080090025006.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. Journal of the American Society of Echocardiography. 2013;26(9):965–1012 e15.
Patel N, Rafique AM, Eshaghian S, Mendoza F, Biner S, Cercek B, et al. Retrospective comparison of outcomes, diagnostic value, and complications of percutaneous prolonged drainage versus surgical pericardiotomy of pericardial effusion associated with malignancy. Am J Cardiol. 2013;112(8):1235–9. https://doi.org/10.1016/j.amjcard.2013.05.066.
• Adler Y, Charron P. The 2015 ESC guidelines on the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36(42):2873–4. https://doi.org/10.1093/eurheartj/ehv479This is the most recenet guidelines for the mangment of pericardial disease with a comprehensive review of the evidence and management strategies of pericardial effusion.
Augustin P, Desmard M, Mordant P, Lasocki S, Maury JM, Heming N, et al. Clinical review: intrapericardial fibrinolysis in management of purulent pericarditis. Crit Care. 2011;15(2):220. https://doi.org/10.1186/cc10022.
Shimony A, Fox BD, Langleben D, Rudski LG. Incidence and significance of pericardial effusion in patients with pulmonary arterial hypertension. Can J Cardiol. 2013;29(6):678–82. https://doi.org/10.1016/j.cjca.2012.04.009.
Plotnick GD, Rubin DC, Feliciano Z, Ziskind AA. Pulmonary hypertension decreases the predictive accuracy of echocardiographic clues for cardiac tamponade. Chest. 1995;107(4):919–24.
Fenstad ER, Le RJ, Sinak LJ, Maradit-Kremers H, Ammash NM, Ayalew AM, et al. Pericardial effusions in pulmonary arterial hypertension: characteristics, prognosis, and role of drainage. Chest. 2013;144(5):1530–8.
Case BC, Yang M, Kagan CM, Yerasi C, Forrestal BJ, Tariq MU, et al. Safety and feasibility of performing pericardiocentesis on patients with significant pulmonary hypertension. Cardiovasc Revasc Med. 2019;20(12):1090–5. https://doi.org/10.1016/j.carrev.2019.09.022.
Beck CS. Two cardiac compression triads. J Am Med Assoc. 1935;104(9):714–6.
Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ et al., editors. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clinic Proceedings; 2002: Elsevier.
Ligero C, Leta R, Bayes-Genis A. Transient biventricular dysfunction following pericardiocentesis. Eur J Heart Fail. 2006;8(1):102–4. https://doi.org/10.1016/j.ejheart.2005.05.012.
• Sinnaeve PR, Adriaenssens T. A contemporary look at pericardiocentesis. Trends Cardiovasc Med. 2019;29(7):375–83. https://doi.org/10.1016/j.tcm.2018.10.016A comprehensive review on pericardiocentesis.
Rafique AM, Patel N, Biner S, Eshaghian S, Mendoza F, Cercek B, et al. Frequency of recurrence of pericardial tamponade in patients with extended versus nonextended pericardial catheter drainage. Am J Cardiol. 2011;108(12):1820–5.
Ristić AD, Imazio M, Adler Y, Anastasakis A, Badano LP, Brucato A, et al. Triage strategy for urgent management of cardiac tamponade: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2014;35(34):2279–84. https://doi.org/10.1093/eurheartj/ehu217.
Kalivoda EJ, Sullivan A, Bunting L. A cost-effective, rapidly constructed simulation model for ultrasound-guided Pericardiocentesis procedural training. J Emerg Med. 2019;56(1):74–9. https://doi.org/10.1016/j.jemermed.2018.09.010.
Baribeau Y, Bortman J, Khamooshian A, Laham R, Mahmood F, Mahmood F, et al. A 3-dimensionally printed, high-Fidelity ultrasound-guided pericardiocentesis training model. J Cardiothorac Vasc Anesth. 2020;34(1):245–7. https://doi.org/10.1053/j.jvca.2019.08.051.
• Ryu AJ, Kane GC, Pislaru SV, Lekhakul A, Geske JB, Luis SA, et al. Bleeding complications of ultrasound-guided pericardiocentesis in the presence of coagulopathy or thrombocytopenia. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2020;33(3):399–401. https://doi.org/10.1016/j.echo.2019.11.006A Large (>1000 patients) study on the bleeding complications of echo-guided pericardiocentesis.
Lindenberger M, Kjellberg M, Karlsson E, Wranne B. Pericardiocentesis guided by 2-D echocardiography: the method of choice for treatment of pericardial effusion. J Intern Med. 2003;253(4):411–7. https://doi.org/10.1046/j.1365-2796.2003.01103.x.
Siegel RJ, Arsanjani R: Echo guided pericardiocentesis. In Intraprocedural Imaging of Cardiovascular Intervention. Picard M, Passeri J, Dal-Bianco J, Editors. Springer International Publishing Switzerland; 2016:23–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nir Flint and Robert J. Siegel declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Structural Heart Disease
Rights and permissions
About this article
Cite this article
Flint, N., Siegel, R.J. Echo-Guided Pericardiocentesis: When and How Should It Be Performed?. Curr Cardiol Rep 22, 71 (2020). https://doi.org/10.1007/s11886-020-01320-2
Published:
DOI: https://doi.org/10.1007/s11886-020-01320-2