Abstract
In this chapter we discuss the indications, as well as the echocardiographic/Doppler diagnosis of pericardial tamponade. We provide an in depth description of how to perform an echocardiographic guided pericardiocentesis. A detailed description is given on how to prepare for performing pericardiocentesis, equipment needed, use of local analgesia, exclusion criteria, and the optimal site for pericardial puncture as dictated by echocardiographic guidance. We explain different approaches for pericardial access, how to identify the angle for needle insertion and to determine the depth of needle penetration that is both safe and required. We describe the use of pericardial catheter drainage, laboratory studies that should be performed on the pericardial fluid and management of pericardial drain, as well as when the drain should be removed. We outline our use of medications before (local analgesia), and after the procedure (anti-staphlococcal coverage while the pericardial catheter is in place and the use of colchicine and non-steroidal anti-inflammatory agents). Detailed imaging is provided for the echocardiographic findings as well as step-by-step photo demonstration of the pericardiocentesis procedure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pericardial effusion
- Pericardial tamponade
- Ultrasound guidance
- Echocardiography
- Doppler
- Pericardial drainage
Historical Perspective
Hippocrates first identified the pericardium as a sac around the heart containing a small amount of yellow fluid. Some 600 years later Galen, the Greek physician to Roman gladiators, was the first to describe pericardial effusion due to cardiac trauma. The first open drainage of a traumatic pericardial effusion was performed by the surgeon Dominque Larrey in 1810. A soldier in the French army had stabbed himself in the chest and was progressively deteriorating. Forty-days after the self – inflicted wound, Larrey surgically drained more than a liter of fluid and clot. Initially the patient made a remarkable recovery, only to succumb to infection about 3 weeks later. The first percutaneous drainage of the pericardium was done in Vienna by Frank Schuh in 1840, to treat a 24 year old woman suffering from severe dyspnea. Schuh inserted a trocar between her ribs and removed over a liter of blood tinged effusion. His patient had prompt improvement in her breathing, but succumbed to an intra-thoracic neoplasm. In 1896, the German physician Ludvig Rehn performed the first successful treatment of pericardial tamponade by draining the effusion and suturing an RV laceration due to a stab wound. In 1911 Antoine Marfan in France described the subcostal approach for pericardiocentesis. This became the standard approach for decades, and is still used as the primary site for access when the pericardiocentesis procedure is done without echocardiographic guidance.
Indications for Pericardiocentesis
The indications for pericardiocentesis can be categorized as elective, urgent, or emergent. Elective pericardiocenteses are often indicated for diagnostic purposes in the setting of an effusion to assess for metastatic disease or to diagnose purulent pericarditis. Emergent pericardiocentesis is usually performed when there is acute hemodynamic compromise with hypotension. This may occur in the cardiac catheterization and electrophysiology (EP) laboratories when there is a cardiac perforation resulting in the rapid accumulation of 50–100 cc of blood in the pericardial space. Even with echo equipment readily available for guidance, deterioration in some cases may so be rapid that an immediate subcostal needle puncture is required to relieve possible tamponade to prevent complete cardiovascular collapse.
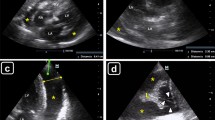
The most frequent clinical setting in which a significant pericardial effusion is detected in our hospital are: an increase in the cardiac silhouette on chest X-ray, post cardiac procedures such as open heart surgery or percutaneous cardiac intervention, hypotension and/or tachycardia. In these settings an echocardiogram is done to confirm the clinical suspicion of a pericardial effusion. In addition, some effusions are found “incidentally”. These patients often have shortness of breath, tachycardia, chest pain or other non-specific symptoms. Post-cardiac surgical patients may develop asymptomatic and large pericardial effusions – especially if they are receiving anticoagulation. Effusions that develop rapidly generally manifest clinically with symptoms and/or hemodynamic compromise. With effusions that develop slowly, the echo findings of tamponade or “pre- tamponade” moderate to large effusions often precede symptoms or clinical signs of hypotension or hypoperfusion. As shown in Fig. 2.1, a large pericardial effusion is circumferential and greater than 1 cm [1]. Multiple echocardiographic signs of cardiac tamponade are shown in the following Figs. 2.2, 2.3, and 2.4. Right atrial systolic collapse (LV systole) as shown in Fig. 2.2 – is an early sign of tamponade. However when there are signs of an elevated right atrial pressure as demonstrated in Fig. 2.3 – (a dilated and non-collapsing IVC) or diastolic collapse of the RV seen in Fig. 2.4 – signs and symptoms of tamponade are generally present. Additionally, Doppler inspiratory variation of the tricuspid and mitral inflow as well as LVOT pulsed wave (PW) Doppler could indicate tamponade physiology. It should be kept in mind however that certain clinical findings will prevent the development of tamponade physiology and may thus delay the diagnosis. These findings include intra-cardiac shunts such as an ASD, elevated LV filling pressures that preclude the RVEDP equalizing with the LVEDP (i.e. >20 mmHg), and aortic regurgitation that allows LV filling, independent of respiratory hemodynamic changes. Very large effusions in excess of 2000 ml can accumulate if the fluid build-up is slow. Thus the hemodynamic changes as a consequence of the effusion – emergent vs. urgent and even elective pericardiocentesis is generally more related to the rate of fluid accumulation than to effusion size.
Pericardiocentesis Without Echocardiographic Guidance
Pericardiocentesis without echocardiographic guidance is usually done in the emergent setting, and almost always employs a subcostal approach to try to enter the pericardial space. It may be preferable to angulate the needle toward the left shoulder as this is away from the lung and closer to the heart, however the needle can also be pointed towards the right shoulder to avoid puncturing the left anterior descending coronary artery. Some interventional cardiologists utilize fluoroscopy which allows visualization of the enlarged cardiac silhouette but does not permit precise delineation of the pericardial effusion or differentiate the epicardial surface from the limits of the pericardium. Consequently, using fluoroscopy is limited for finding the safest and easiest site to enter the pericardial effusion. Without echo guidance, complication rates range between 20 % and 50 % and the mortality is 6 % [2]. Complications include cardiac penetration, coronary artery laceration, pneumothorax, and penetration or laceration of viscera in the path of the pericardiocentesis needle [3].
Echocardiographic Guided Pericardiocentesis
The Mayo Clinic group, especially Callahan, Seward, Tsang and Nishimura [4] were early proponents for echo guided pericardiocentesis.
Their publications demonstrated that echo guidance improves the safety and feasibility of percutaneous pericardiocentesis. Over a 21 year period of performing pericardiocentesis on nearly 1,000 patients (n = 977), their success rate of obtaining fluid and draining the effusion was 97 %; major complications were only 1.2 % and minor complications occurred in 3.5 %. Their results reflect a substantial improvement over pericardiocentesis without echo guidance [2]. In our experience at Cedars Sinai Medical Center over the past 15 years with more than 500 patients, the major complication rate was 0.2 % with 1 patient having a pneumothorax and no mortality or need for urgent surgery [5, 6].
We prefer to perform pericardiocentesis in the ICU at the patient’s bedside which allows for continuous monitoring of heart rate, blood pressure, and oxygen saturation. This avoids moving a potentially hemodynamically unstable patient to and from the catheterization laboratory or procedure room. In addition, keeping the patient in the same room reduces infection risk and the potential for catheter dislodgement during patient transfers (Tables 2.1a and 2.1b)
Preparations Prior to Pericardiocentesis
-
1.
Obtain patient consent for the pericardiocentesis procedure.
-
2.
Check platelet count and coagulation status.
-
3.
Perform a 2D echo to find the largest effusion site, which is easiest to access.
-
4.
Exclude patients with aortic dissection and/or a dilated aorta which suggests the possibility of an aortic dissection. While pericardiocentesis in the setting of a dissection may alleviate hypotension, it may also extend the dissection and/or promote aortic rupture.
-
5.
Determine the optimal site for the pericardial puncture, avoiding lung, liver or other viscera. In addition the distance from the skin to the effusion should be measured and it should be confirmed that the needle length is sufficient to access the effusion (Figs. 2.5a, b).
-
6.
Measure the distance from the chest wall (Fig. 2.5a) or subcostal space to the effusion (Fig. 2.5b). A transapical access may be preferable, but this decision is dictated by effusion size and location. We use the ultrasound transducer to determine the optimal entry site into the thorax, and also to set the angle of incidence for the needle and the chest wall that will be used when entering the chest and advancing the needle. Thus the operator performing the pericardiocentesis should image the pericardial effusion from the planned site of needle entry to set the best angle of needle insertion. The ultrasound transducer should be tilted medial and laterally as well as in the anterior and posterior directions to see where there is sufficient amount of pericardium for safe needle access to the pericardial effusion.
The Pericardiocentesis Procedure
-
1.
Explain each aspect of the procedure to the patient. This helps to reassure the patient and keeps patient movement to a minimum which is important to avoid complications.
-
2.
Position the patient in the supine position close to the edge of the bed with the head at 0–30° and even as high as 45° to avoid respiratory distress. Positioning is important to facilitate ease of the procedure.
-
3.
Mark the puncture site. This may be done with “permanent ink” however this sometimes disappears with the sterile preparation solution. It can be preferable to gently scratch the skin to create a mark which is easily seen as in Fig. 2.6a. This mark is not affected by the skin prep solution.
Fig. 2.6 (a) The puncture site could be marked by permanent ink or by scratching the skin as shown below. In this female patient the breast tissue is taped to hold the breast out of the plane of access for pericardiocentesis. (b) The pericardiocentesis site is prepared and draped in usual sterile fashion. (c) The access site is anesthesized using 1 % xylocaine without epinephrine. (d) A skin incision is made using a scalpel at the planned site of needle entry. (e) The entry site is dilated using a hemostat
-
4.
Prepare the puncture site with a very thorough and meticulous skin preparation to avoid the introduction of any bacteria into the pericardial space. Drape the area as seen in the Fig. 2.6b.
-
5.
Infiltrate the site with 1 % xylocaine without epinephrine (1st with a 25 gauge and then with a 22 gauge needle) as shown in Fig. 2.6c. It has become our habit to use 20–30 ml of xylocaine so that the procedure is painless, the patient is comfortable, and does not move during the procedure. With adequate local xylocaine analgesia additional pain medication is not necessary. A patient with a hemodynamically significant effusion should never receive sedation as this may reduce adrenergic drive and result in profound hypotension
-
6.
Make a skin incision (Fig. 2.6d) at the planned site of needle entry and dilate that site with a hemostat (Fig. 2.6e). Figure 2.7 shows the various sites used at our institution for needle insertion which include sub-xiphoid, trans-apical, parasternal, apical lateral and high lateral sites. The trans apical and subxiphoid approaches are most common. We avoid the subxiphoid approach if the liver or other viscera are in the way. However, a report by Lindenberger describes pericardial access through the liver. They reported no complications even in anti-coagulated patients [7]. As we almost uniformly leave a pericardial drain in place, this could be problematic with a trans-hepatic stick potentially leading to a hepatic fistula, bleeding or other problems.
-
7.
Monitoring:
-
(a)
During advancement of the 18 gauge 15 cm in length pericardiocentesis needle as shown in Fig. 2.8a, ECG is monitored, along with blood pressure every 1–2 min
Fig. 2.8 (a) A Cook needle is used to gain access to the pericardial space. (b) The Cook needle is slowly advanced until small amount of pericardial fluid is aspirated. (c) The guide wire is advanced thru the needle into the pericardial space. (d) A dilator is passed over the guidewire to allow for easy passage of the pericardial catheter. (e) The pericardial catheter is advanced over the guidewire into the pericardial space for at least 20 cm. (f) The pericardial catheter is attached to a Jackson Pratt drain and kept in place for generally more than 36 h
-
(b)
A surface 2D echo using a regular probe from a location outside of the surgical field to prevent contamination (e.g. parasternal view, apical view for a subcostal approach and a subcostal view for an apical approach). Alternatively, a sterile sheath or ultrasound probe cover may be placed over a 2D transthoracic transducer, using standard ultrasonic gel with in the sheath and sterile gel on the surface of the probe cover. In addition, there are specially manufactured acoustically reflective procedure needles that may be used to visualize the needle with ultrasound imaging during insertion.
-
(c)
The sub-xiphoid approach is monitored by echo from a parasternal or apical window. Measuring the distance from the skin to the effusion is important in this approach because the distance to the effusion is generally greater than with the transthoracic puncture.
-
(d)
With a trans-thoracic puncture care should be taken to ensure that there is no intervening lung between the chest wall and puncture site. As shown in Fig. 2.5a, this is readily confirmed by echo showing the effusion extending to the chest wall.
-
(e)
For transapical approaches in women, one should not go through the breast tissue. If the left breast is in the surgical field, we securely tape it upwards as demonstrated in Fig. 2.6a so that the breast remains out of the sterile field during the procedure.
-
(f)
In cases in which there is a relatively small or loculated effusion in which the pericardiocentesis is being done for diagnostic evaluation (cancer or infection) ECG monitoring via the pericardiocentesis needle itself can be helpful to avoid myocardial puncture. To do this, an alligator clamp is attached to the pericardial needle and to an ECG electrode. The electrode is then attached to an ECG machine or the bedside ECG monitor. If during the procedure the needle comes into contact with the ventricular epicardium ST segment elevation will appear on the ECG tracing. If this occurs, the needle should be pulled back slowly until ST segment elevation disappears.
-
(a)
-
8.
During needle insertion (Fig. 2.8b) we advance in 2–3 mm movements at the angle determined by the pre-procedure bedside echo.
-
9.
When non-sanguinous fluid is aspirated we generally remove 10 cc of fluid to decompress the pericardial sac, and then carefully detach the syringe from the needle without changing the needle location and/or angle. A guide wire is then advanced thru the needle into the pericardial space as shown in Fig. 2.8c. The guide wire should advance easily; if it does not, the wire should be removed and the needle redirected. When the pericardial aspirate is free flowing the guide wire should be re-advanced. If the effusion is bloody, discharge some of the aspirate on to a gauze pad and check for the presence of small blood clots. If the aspirate clots, the needle is not in the pericardial space or it is very fresh blood from a ruptured heart or aortic dissection with leakage of blood into the pericardial space. This is an emergent situation and aspiration of blood should be immediately stopped as relief of tamponade in this setting will allow systemic pressure to rise and lead to further ventricular or aortic rupture or dissection. Such patients should be taken urgently to the OR and a TEE done to confirm the diagnosis.
If the bloody fluid is non-clotting one needs to determine if the source of the blood is the heart or the pericardial space. This may be done by injecting agitated saline into the pericardial space. As demonstrated in Fig. 2.9. our preference is to aspirate the patient’s blood admix it between two syringes with a three way stopcock and re-inject it through the catheter, resulting in a robust echo-contrast image allowing determination of whether the needle is intra-pericardial as in Fig. 2.9 or intra-cardiac if the echo contrast would be seen within the heart itself.
-
10.
After confirmation that the needle is in the pericardial space in which case the dilator is passed over a guidewire as seen in Fig. 2.8d. We generally dilate the space three times to allow for easy passage of the pericardial catheter.
-
11.
The pericardial catheter is then advanced into the pericardial space at least 20 cm (Fig. 2.8e). The catheter is sutured in place and the pericardial fluid is aspirated and transferred to drainage bag until there is no further drainage.
-
12.
We attach the pericardial catheter to a Jackson Pratt drain (Fig. 2.8f) and keep the intra-pericardial catheter in place for generally more than 36 h and until the drainage is less than 50 ml in a 24 h period and there is concomitantly no more than a trivial pericardial effusion on echo.
-
13.
Pericardial fluid is sent for analysis. The tests which are ordered depend on the clinical setting. When it is clearly a traumatic etiology, we generally only send for culture, gram stain, cell count and glucose to ensure that no secondary infection is present. If there is a potential for a neoplastic process, in addition to the above, we send most of the fluid for cytology. We have found that distinguishing between transudate and exudate by LDH or total protein has little diagnostic or prognostic value. A transudate does not exclude a neoplastic etiology [8]. When there is a potential for infection especially in elderly or immune compromised patients, we assess not only for bacteria but also for fungi and for TB by PCR and AFB stain.
-
14.
Patients are treated with an antibiotic prophylaxis that provides anti- staphylococcal coverage until the pericardial catheter is removed. If there are no contraindications, it is also our practice to place the patients on anti-inflammatory therapy with colchicine and NSAIDS or ASA.
-
15.
Following removal of a large pericardial effusion, the heart rate usually improves (Fig. 2.10). In addition, echocardiographic images demonstrate improvement in right atrial pressure (IVC collapsibility), resolution of inspiratory variation of the tricuspid and mitral inflow (Fig. 2.11), as well as the respiratory variation seen on a pulse oximeter, these all reflect the resolution of pulsus paradoxus which is caused by cardiac tamponade.
Fig. 2.10
References
Weitzman LB, Tinker WP, Kronzon I, Cohen ML, Glassman E, Spencer FC. The incidence and natural history of pericardial effusion after cardiac surgery – an echocardiographic study. Circulation. 1984;69(3):506–11.
Tsang TSM, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, Bailey KR, Seward JB. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77(5):429–36.
Silvestry FE, Kerber RE, Brook MM, Carroll JD, Eberman KM, Goldstein SA, Herrmann HC, Homma S, Mehran R, Packer DL, Parisi AF, Pulerwitz T, Seward JB, Tsang TSM, Wood MA. Echocardiography-guided interventions. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2009;22(3):213–31; quiz 316–317.
Callahan JA, Seward JB, Nishimura RA, Miller FA, Reeder GS, Shub C, Callahan MJ, Schattenberg TT, Tajik AJ. Two-dimensional echocardiographically guided pericardiocentesis: experience in 117 consecutive patients. Am J Cardiol. 1985;55(4):476–9.
Patel N, Rafique AM, Eshaghian S, Mendoza F, Biner S, Cercek B, Siegel RJ. Retrospective comparison of outcomes, diagnostic value, and complications of percutaneous prolonged drainage versus surgical pericardiotomy of pericardial effusion associated with malignancy. Am J Cardiol. 2013;112(8):1235–9.
Rafique AM, Patel N, Biner S, Eshaghian S, Mendoza F, Cercek B, Siegel RJ. Frequency of recurrence of pericardial tamponade in patients with extended versus nonextended pericardial catheter drainage. Am J Cardiol. 2011;108(12):1820–5.
Lindenberger M, Kjellberg M, Karlsson E, Wranne B. Pericardiocentesis guided by 2-D echocardiography: the method of choice for treatment of pericardial effusion. J Intern Med. 2003;253(4):411–7.
Atar S, Chiu J, Forrester JS, Siegel RJ. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. 1999;116(6):1564–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Siegel, R.J., Arsanjani, R. (2016). Echo Guided Pericardiocentesis. In: Picard, M., Passeri, J., Dal-Bianco, J. (eds) Intraprocedural Imaging of Cardiovascular Interventions. Springer, Cham. https://doi.org/10.1007/978-3-319-29428-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-29428-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29426-1
Online ISBN: 978-3-319-29428-5
eBook Packages: MedicineMedicine (R0)