Abstract
Purpose of Review
Non-invasive molecular imaging is currently used as a research technique to better understand disease pathophysiology. There are also many potential clinical applications where molecular imaging may provide unique information that allows either earlier or more definitive diagnosis, or can guide precision medicine-based decisions on therapy. Contrast-enhanced ultrasound (CEU) with targeted microbubble contrast agents is one such technique that has been developed that has the unique properties of providing rapid information and revealing information only on events that occur within the vascular space.
Recent Findings
CEU molecular probes have been developed for a wide variety of disease states including atherosclerosis, vascular inflammation, thrombosis, tumor neovascularization, and ischemic injury. While the technique has not yet been adapted to clinical use, it has been used to reveal pathological processes, to identify new therapeutic targets, and to test the efficacy of novel treatments.
Summary
This review will explore the physical basis for CEU molecular imaging, its strengths and limitations compared to other molecular imaging modalities, and the pre-clinical translational research experience.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
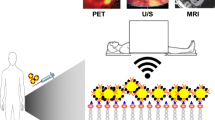
Over the past decade, there has been rapid development in molecular imaging techniques that are able to non-invasively assess molecular or cellular phenotype in animal models of disease and in humans. These techniques are advantageous in that they provide both spatial and temporal information on complex disease processes at the molecular level, and can be combined with traditional measurements provided by imaging of structure, function, and flow. Molecular imaging techniques can be applied in research settings to study cardiovascular disease pathophysiology and test novel therapies, as well as in clinical settings to detect pathological processes, risk-stratify patients, and guide precision treatment (Fig. 1) [1].
Methods for molecular imaging are diverse and span all forms of medical imaging including radionuclide, optical, magnetic resonance imaging (MRI), and ultrasound [2]. The general approaches to imaging a molecular profile in situ are diverse (Table 1) and rely primarily on the detector capabilities [1, 3]. One of the most common approaches is to use contrast agents that are functionalized to reveal a targeted molecular process. Some simple examples of this are the radiolabeling of antibodies or antibody fragments for radionuclide imaging of a disease process [4], or taking advantage of a metabolic process that uses a particular substrate such as the positron emission tomography (PET) imaging of the consumption rate of 18F-fluorodeoxyglucose (FDG), which enters the glycolysis pathway and becomes trapped in cells at a rate proportional to cellular glucose uptake [5]. However, given the expanding range of options in both probes and detectors, it is essential to carefully consider many of the factors that govern whether a technique is well suited to a specific clinical or research application.
Contrast-enhanced ultrasound (CEU) molecular imaging is a technique that relies on the ultrasound detection of encapsulated gas microbubbles (MBs) or other acoustically active micro- or nanoparticles that are retained in tissue on the basis of their ability to bind to molecules or cells of interest. CEU has several unique characteristics that differentiate it from other molecular imaging techniques. It is able to rapidly (within 5–10 min) obtain non-invasive measures of disease and can be done with portable and relatively inexpensive imaging technology, meaning that bedside molecular imaging is possible. From a research perspective, the rapid imaging protocols and the ability to “null” the signal from the agent provide an opportunity to examine multiple molecular processes in a short period of time. As a purely intravascular probe [6], MBs are an ideal tool for non-invasive interrogation of the endothelial-blood pool interface, but they are poorly suited for examining either extravascular or intracellular events. This review will focus on some of the principles underlying CEU molecular imaging and will highlight recent advancements in the application of targeted CEU to cardiovascular disease.
Principles of CEU Molecular Imaging
Contrast Agent Properties
CEU imaging relies on receiving signal generated by encapsulated gas MBs when exposed to ultrasound [7]. In the pressure fluctuations of an ultrasound field, MBs undergo volumetric oscillation. Steady expansion and contraction (stable cavitation) occurs at low pressure which can be non-linear with regard to the relationship between pressure and volume [8, 9]. Destruction of microbubbles can occur from exaggerated oscillation at high pressures, known as inertial cavitation [10]. Both inertial cavitation and non-linear stable cavitation result in emission of broad band ultrasound signals and ultrasound energy peaks at harmonic frequencies, thereby producing a unique acoustic signature that can be detected and isolated from background tissue signal [11,12,13]. Conventional MB agents used in humans are between 1 and 5 μm in diameter, have a core composed of a high-molecular weight inert gas such as perfluorocarbons or sulfur hexafluoride, and have a shell composed of lipids or proteins such as albumin. A wide variety of other acoustically active experimental ultrasound contrast agents has been explored, but are out of the scope of this review [14]. MB agents are smaller than erythrocytes and have a microvascular rheology similar to that of erythrocytes [6, 15]. Accordingly, they normally pass unimpeded through capillaries, which is important for both safety considerations and their use as flow tracers. Lipid MBs also often contain surface “coats” composed of biocompatible polymers such as polyethylene glycol (PEG), which prevent their interaction with cells and plasma proteins [16,17,18].
After intravenous injection, MBs remain in the vascular space, thereby opacifying the blood pool on ultrasound imaging. In clinical cardiovascular medicine, CEU is most often used to opacify the left ventricular (LV) cavity on echocardiography in order to better evaluate global or regional function, LV dimensions, and the presence of intracavitary masses such as thrombi [19]. Myocardial contrast echocardiography (MCE) for evaluation of regional myocardial perfusion can also be performed with conventional MB agents, during which blood flow is quantified by measuring the rate and extent of replenishment of MBs within the coronary circulation after a destructive ultrasound pulse [20, 21].
Molecular imaging with CEU is most commonly performed by imaging targeted MBs that have been retained by attachment to specific molecular processes within the vascular compartment. The retention of MBs can occur by two distinct approaches. Non-specific retention of MBs, by their binding to endothelial cells or to adherent and activated leukocytes, occurs through the endogenous ability of these cells to bind to MB shell constituents. There are many pathways involved in this binding, but the process that has been best defined is opsonization, whereby serum complement proteins bind to the MB surface and are then recognized by complement receptors on leukocytes or on endothelial cells [22, 23]. Choice of shell composition, charge, and the presence of PEG all play key roles in determining the degree of MB attachment to leukocytes and endothelial cells, as well as the rate of their removal from the reticuloendothelial system [17, 24, 25]. These non-specific interactions with MBs can be leveraged for detecting pathological processes that heighten opsonization or complement-mediated attachment, as is the case with MBs with phosphatidylserine shells, which are known to preferentially bind in regions of ischemic myocardium [26•].
An alternate and more specific strategy for targeting of MBs occurs via direct binding of a targeting ligand on the MB surface, often an antibody, peptide, or glycoprotein [27, 28]. Targeting ligands are attached to the MB surface via a variety of approaches, including biotin-streptavidin and direct covalent links. Conjugation is often at the end of the flexible PEG tethers in order to improve stoichiometry and reduce necessary bond force for retention [29]. In general, in excess of 25,000 ligands can be conjugated to each “multivalent” MB [30], and it is even possible to place several different ligands on each MB which may enhance their attachment efficiency [31].
Specificity of an MB probe for a target molecule is determined by factors such as off-target binding, specificity of the targeting ligand to the molecule of interest, bond kinetics, surface density of the targeting ligand, orientation of targeting ligands, and other factors shown in Fig. 2 [32]. Even with a highly specific probe, it is important to consider target molecule characteristics such as regional and temporal expression patterns, relevance of the target molecule to the disease pathway of interest, and bond kinetics under varying shear conditions.
In Vivo Protocol for Targeted CEU
There are several approaches used to detect targeted MB retention in tissue with ultrasound. Since circulating MBs are rapidly cleared by the reticuloendothelial system, it is possible to simply inject MBs as a venous bolus injection, then measure signal intensity for tracer retained in tissue 5–10 min later, after the majority of freely circulating MBs have been cleared from the blood pool. The efficacy of this approach is further enhanced by protocols to eliminate the signal attributable to any remaining freely circulating MBs [27]. An alternative and somewhat more complex method is to use transfer kinetics to evaluate the retention fraction (Fig. 3). With this method, video intensity is measured constantly after an intravenous injection which allows deconvolution of the two curves: one that represents freely circulating tracer which rises and decays and the one curve representing tracer that is retained which rises and plateaus [33]. This latter method is particularly helpful in research settings where tissue perfusion, which influences the number of microbubbles entering into tissue, changes substantially over time or between conditions.
Kinetic model for calculating retention fraction of microbubbles. Continuous measurement of contrast agent in a tissue is measured. Persistent elevation of signal represents agent that remains in tissue rather than is cleared from blood pool. The measured curve can then be deconvolved into two separate curves: (1) a transit curve for the population that transits freely through the tissue without retention and is cleared from blood pool after about 300–400 s (fit in this case to a γ-variate function) and (2) the fraction (F) of agent that is retained which can be fit to an integral of the transit function (γ-variate)
Strengths and Limitations of CEU Molecular Imaging
Whether using molecular imaging as a research tool or for a clinical application, the selection of the most appropriate modality for any given application requires one to consider the relative advantages and disadvantages for each approach. For molecular imaging approaches that use targeted contrast agents, one important consideration is the biodistribution of the targeted probe relative to the target pathway. A limitation of CEU molecular imaging is that the imaging probes are restricted to the vascular compartment. Hence, CEU is not an ideal method for finding abnormal cell types within atherosclerotic plaque or for detecting abnormal matrix composition in certain myocardial diseases. On the other hand, in conditions where it is desirable to limit the evaluation to endothelial or intraluminal phenotype (e.g., transplant vasculopathy, thrombosis), techniques that employ purely intravascular probes may offer advantage in terms of specificity. There are other key differences between small molecule contrast agents and larger multivalent particle-based agents. The former generally bind in a 1:1 ratio to a target receptor or are retained by virtue of a metabolic process and are well suited to precisely quantifying the degree of expression or enzymatic activity [34]. For the latter, many bond formations have to occur for retention, resulting in a threshold-effect with regard to target molecular expression before attachment is seen, and a greater influence of vascular shear [35]. However, particle-based agents provide a form of biomimicry where their behavior can provide a readout of the molecular environment that influences cellular or platelet adhesion.
There are also practical considerations in terms of detector performance. In general, techniques such as MRI and CT are considered to have the highest spatial resolution, while radionuclide imaging (PET, SPECT) tends to have the highest sensitivity and dynamic range for detecting tracer. Accordingly, one must consider whether it is more important to spatially localize an event or to be able to detect very low levels of abnormal molecular expression. In general, CEU molecular imaging offers a balance in that it provides moderate spatial resolution and sensitivity, and is suitable for applications where a balance of both traits is needed. If it is necessary to co-localize regional molecular imaging signal with anatomical features, CEU has the advantage of near-simultaneous structural and molecular imaging, while MR and hybrid PET-CT or SPECT-CT have the advantage of providing more detailed views of smaller anatomical features.
Temporal resolution is also essential to consider when selecting the most appropriate molecular imaging modality. CEU molecular imaging can be performed with rapid acquisition times of less than 10 min, while commonly used PET/SPECT and MR protocols require anywhere from just under an hour to a day between contrast injection and imaging [36,37,38]. Additionally, rapid clearance of MBs from the blood pool allows multiple different targeted contrast agents to be administered sequentially.
There are certain practical issues other than speed that are important when considering different molecular imaging modalities, particularly if their use is intended as an approach for disease screening in large populations or for rapid detection in common diseases (e.g., myocardial ischemia, atherosclerosis). Safety is one key issue. CEU contrast agents have an excellent safety profile, with serious pseudoanaphylactic reactions occurring in only 1 in 10,000 for conventional contrast agents [39,40,41,42]. CEU molecular imaging also does not require ionizing radiation. Other advantages include ease of use, portability of ultrasound which allows for bedside diagnosis, and low cost for both equipment and production of contrast agents.
Applications of Myocardial CEU Molecular Imaging
Ischemic Memory Imaging
In patients with acute coronary syndrome (ACS), the clinical diagnosis generally relies on clinical history, laboratory evaluation, and electrocardiogram (ECG). Unfortunately, many patients do not have classic angina symptoms [43], and many of those with ACS do not have diagnostic changes on the ECG [44]. Moreover, in those with unstable angina, ECG changes and even wall motion abnormalities detectable on point-of-care echocardiography can resolve before the initial evaluation by a health care provider. While high-sensitivity troponins have a high sensitivity for detecting non-ST-elevation MI [45], their performance in unstable angina is less certain, and they often lack specificity in certain populations [46]. Methods for rapidly evaluating both the presence and the spatial extent of recently resolved myocardial ischemia have a high likelihood of improving the quality and efficiency of clinical care in those presenting with symptoms suspicious for ACS.
CEU molecular imaging is capable of assessing ongoing or recent, but resolved, myocardial ischemia at the patient bedside and provides a spatial readout of the affected area. Ischemic memory imaging has been achieved both with MBs bearing targeting ligands for the endothelial selectin family of adhesion molecules and with phosphatidylserine-containing MBs (PS-MB) (Fig. 4) [26•, 47, 48]. Targeting of selectins on the endothelial surface, which has been validated in murine and non-human primate models of myocardial infarction [48, 49], is based on early and late endothelial response to ischemia. In the first minutes following ischemia, P-selectin stored in endothelial Weibel-Palade bodies is mobilized to the cell surface where it can remain for many hours, whereas E-selectin expression requires approximately 1 h to be expressed due to its dependence on transcriptional upregulation [50]. Agents targeted to selectins are thereby retained in the microcirculation of the post-ischemic risk area, even in the absence of any significant necrosis, for hours after resolution of hypoperfusion. Alternatively, ischemic memory imaging can be performed without a targeting ligand using PS-MBs, as validated in murine and canine models of resolved myocardial ischemia [26•]. The selective retention of phosphatidylserine MBs in areas of recent ischemia is mediated in part through opsonization and binding to complement receptors. The simplicity of this approach will likely accelerate its clinical translation.
Molecular imaging of ischemic memory. a Examples of CEU molecular imaging with MB-PS (left panels) and triphenyltetrazolium chloride (TTC) staining (right panels) in a canine model of ischemia reperfusion of the LAD (top panels) or left circumflex coronary artery (bottom panels). Images demonstrate contrast enhancement that encompasses the entire risk area which extends beyond the region of infarction. The color-coded scale for CEU is provided at the bottom (modified from Christiansen JP et al. Circulation 2002 Apr 16;105(15):1764–7) [75]. b Graph illustrating signal enhancement on CEU molecular imaging from a murine model of brief ischemia-reperfusion injury without infarction. Signal enhancement in the post-ischemic risk area was greater than in the remote area and was similar for MB-PS and P-selectin-targeted MBs and persisted for > 6 h after ischemic injury. PS phosphatidylserine, PSGL-1 p-selectin glycoprotein ligand-1 (modified from Mott B, et al. Cardiovasc Imaging. 2016;9(8):937–46, with permission from Elsevier) [26•]
Detection of Transplant Rejection and Myocarditis
Orthotopic heart transplantation is an important therapeutic option for eligible candidates with severely symptomatic heart failure. However, there have been a relatively flat number of allografts available for transplant in the USA each year. Approximately one-quarter of all allografts have evidence for rejection in the first year [51]. This figure together with the problem of sampling error for detecting rejection by endomyocardial biopsy has resulted in a need for better surveillance. Molecular imaging for this application would require a technique that ideally could be done rapidly and without repetitive exposure to ionizing radiation. The ability to detect transplant rejection has been demonstrated in a rat model using MBs targeted to ICAM-1 [52].This study was predicated on the notion that rejection involved not only a T lymphocytic response to myocytes but also to the allograft endothelial cells resulting in endothelial activation and adhesion molecule expression. More recently, CEU molecular imaging has been used to directly assess T lymphocytes in a model of orthotopic heart transplantation [53•]. This study relied on MBs bearing a CD3-targeted antibody that allowed them to assess lymphocytes in the process of intravascular recruitment.
The pathophysiology of myocarditis involves many common pathways with heart transplant rejection, and the most important of which involves recruitment of cells involved in both adaptive and innate immunity. Accordingly, CEU with MBs targeted to selectins, CD4, and phagocytic cells have been used in a rodent model of myocarditis and shown to detect not only severe fulminant disease but also more moderate involvement as well [54•]. Because of the rapid and quantitative nature of CEU, and its ability to provide information immediately to the clinician, there is hope that it may provide unique diagnostic opportunities for all of these forms of myocardial inflammation in the future.
Atherosclerosis
Atherosclerosis is a process that develops over decades and involves vascular inflammation, including expression of endothelial cell adhesion molecules (ECAMs) and secondary recruitment of leukocytes and platelets [55, 56]. Often, atherosclerosis is clinically silent for decades of plaque progression. Although biomarkers and other techniques have been developed for risk-stratifying patients [57, 58], many patients with severe atherosclerotic disease will not have major risk factors and many will experience MI as their first manifestation of disease [59]. Often, patients are diagnosed only once disease progression has reached the point of critical stenosis, which results in angina [60]. The ability to non-invasively detect the early molecular signatures of aggressive atherosclerosis may provide opportunity for early stratification to therapies that can arrest disease development.
CEU is just one of many molecular imaging techniques that have been used to detect atherosclerotic plaque growth in the early stages of disease. CEU probes targeted against ECAMs including selectins, ICAM-1, and VCAM-1 have been shown to detect early and late stage atherosclerosis in rodent models of atherosclerosis [61,62,63,64,65]. In non-human primates on a Western diet, CEU molecular imaging of VCAM-1 and P-selectin has been shown to detect the very earliest stages of endothelial activation prior to any changes in intima media thickness (Fig. 5) [66]. Recently, CEU molecular imaging of endothelial phenotype has provided in vivo evidence for the role of platelet-endothelial interactions in early disease progression [67, 68]. This process involves abnormal regulation of endothelial-associated ultra-large multimers of VWF that mediate platelet attachment through ligation of platelet GPIbα and increase atherosclerotic progression [56]. While all of these probes have been developed for early clinical detection of disease, they have also played an important role in identifying new therapeutic targets and for assessing the effects of either new or established therapies [63, 69, 70•].
Molecular imaging data from the carotid artery of rhesus macaques after starting a Western-style high-fat diet. a CEU molecular imaging signal for control non-targeted MBs and MBs targeted to VCAM-1 or P-selectin from the carotid arteries at baseline (BL) and every 4 months after starting Western. b Non-contrast 2-D image of the carotid artery at the bifurcation (top) and a background-subtracted color-coded image obtained after injecting P-selectin-targeted MBs demonstrating enhancement of the vascular endothelium (bottom) (modified from Chadderdon S et al., Circulation 2014;129(4):471–8) [66]
Thrombus Detection
There are many potential applications for a technique that can detect the presence of thrombus in cardiovascular disease. Some of the most common include need to diagnosis acute venous or peripheral arterial thrombus, detection of high-risk carotid or coronary arterial plaque, detection of left atrial or left ventricular thrombus, and detection of microvascular thrombosis in post-MI no-reflow phenomenon. Molecular imaging of thrombus has been performed with a variety of targeted ultrasound contrast agents [71]. The targeting moiety employed depends on the intended role of molecular imaging. MBs have been targeted by surface conjugation of ligands that recognize the platelet GPIIb/IIIa receptor, fibrin, or tissue factor [28, 72,73,74]. As mentioned above, MBs have also been targeted to platelet GPIbα and VWF using peptide ligands for each. The relative value of each of these agents depends on (1) whether the thrombotic process of interest is platelet-rich or fibrin-rich; (2) the shear forces in the chamber/vessel involved; and (3) whether the process involves disruption of the endothelial barrier or not. An additional consideration is whether or not MBs need to outcompete endogenous ligands for the intended target. For example, MBs need to outcompete fibrinogen for GPIIb/IIIa, but little competition exists for GPIbα.
Conclusions
CEU molecular imaging is a versatile technique that has many potential applications in clinical cardiovascular medicine, and is already being used as a valuable research tool to understand cellular biology in disease. The technique is well suited to evaluating pathophysiologic events that occur at the interface between the blood pool and the vessel wall. Accordingly, the most common cardiovascular applications have involved imaging of endothelial activation, endothelial adhesion molecule expression, thrombus formation, and interactions between leukocytes or platelets and the vascular endothelium. However, clinical adoption will rely on both the development and testing of human-ready targeted contrast agents, and demonstration that CEU molecular imaging provides incremental value to conventional paradigms of care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc Imaging. 2013;6(12):1327–41. https://doi.org/10.1016/j.jcmg.2013.09.014.
Chen Z-Y, Wang Y-X, Lin Y, Zhang J-S, Yang F, Zhou Q-L, et al. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. Biomed Res Int. 2014;2014:1–12. https://doi.org/10.1155/2014/819324.
Lindner JR, Sinusas A. Molecular imaging in cardiovascular disease: which methods, which diseases? J Nucl Cardiol. 2013;20(6):990–1001. https://doi.org/10.1007/s12350-013-9785-0.
Keelan ET, Harrison AA, Chapman PT, Binns RM, Peters AM, Haskard DO. Imaging vascular endothelial activation: an approach using radiolabeled monoclonal antibodies against the endothelial cell adhesion molecule E-selectin. J Nucl Med : official publication, Society of Nuclear Medicine. 1994;35(2):276–81.
Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation--current and emerging clinical applications. Clin Radiol. 2015;70(7):787–800. https://doi.org/10.1016/j.crad.2015.03.010.
Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15(5):396–403. https://doi.org/10.1067/mje.2002.117290.
Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol. 2007;32(2):51–96. https://doi.org/10.1016/j.cpcardiol.2006.10.004.
Jong ND, Frinking P, Cate FT, Wouw PVD, editors. Characteristics of contrast agents and 2D imaging. IEEE Ultrasonics Symposium. Proceedings; 1996 3–6 1996.
Overvelde M, Garbin V, Sijl J, Dollet B, de Jong N, Lohse D, et al. Nonlinear shell behavior of phospholipid-coated microbubbles. Ultrasound Med Biol. 2010;36(12):2080–92. https://doi.org/10.1016/j.ultrasmedbio.2010.08.015.
Shi WT, Forsberg F, Tornes A, Ostensen J, Goldberg BB. Destruction of contrast microbubbles and the association with inertial cavitation. Ultrasound Med Biol. 2000;26(6):1009–19.
Burns PN. Harmonic imaging with ultrasound contrast agents. Clin Radiol. 1996;51(SUPPL. 1):50–5.
Burns PN, Powers JE, Simpson DH, Brezina A, Kolin A, Chin CT et al., editors. Harmonic power mode Doppler using microbubble contrast agents: an improved method for small vessel flow imaging. 1994 Proceedings of IEEE Ultrasonics Symposium; 1994 31 1994.
Simpson DH, Chien Ting C, Burns PN. Pulse inversion Doppler: a new method for detecting nonlinear echoes from microbubble contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46(2):372–82. https://doi.org/10.1109/58.753026.
Paefgen V, Doleschel D, Kiessling F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front Pharmacol. 2015;6:197. https://doi.org/10.3389/fphar.2015.00197.
Ismail S, Jayaweera AR, Camarano G, Gimple LW, Powers ER, Kaul S. Relation between air-filled albumin microbubble and red blood cell rheology in the human myocardium. Influence of echocardiographic systems and chest wall attenuation. Circulation. 1996;94(3):445–51.
Du H, Chandaroy P, Hui SW. Grafted poly-(ethylene glycol) on lipid surfaces inhibits protein adsorption and cell adhesion. Biochim Biophys Acta Biomembr. 1997;1326(2):236–48. https://doi.org/10.1016/S0005-2736(97)00027-8.
Fisher NG, Christiansen JP, Klibanov A, Taylor RP, Kaul S, Lindner JR. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. J Am Coll Cardiol. 2002;40(4):811–9. https://doi.org/10.1016/S0735-1097(02)02038-7.
Chen CC, Borden MA. Ligand conjugation to bimodal poly(ethylene glycol) brush layers on microbubbles. Langmuir. 2010;26(16):13183–94. https://doi.org/10.1021/la101796p.
Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, et al. Clinical practice of contrast echocardiography: recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging. 2017;18(11):1205–af. https://doi.org/10.1093/ehjci/jex182.
Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–83.
Kaul S, Kelly P, Oliner JD, Glasheen WP, Keller MW, Watson DD. Assessment of regional myocardial blood flow with myocardial contrast two-dimensional echocardiography. J Am Coll Cardiol. 1989;13(2):468–82. https://doi.org/10.1016/0735-1097(89)90528-7.
Lindner JR, Song J, Xu F, Klibanov AL, Singbartl K, Ley K, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000;102(22):2745–50.
Anderson DR, Tsutsui JM, Xie F, Radio SJ, Porter TR. The role of complement in the adherence of microbubbles to dysfunctional arterial endothelium and atherosclerotic plaque. Cardiovasc Res. 2007;73(3):597–606. https://doi.org/10.1016/j.cardiores.2006.11.029.
Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33(2):318–25. https://doi.org/10.1016/j.ultrasmedbio.2006.08.008.
Chen CC, Borden MA. The role of poly(ethylene glycol) brush architecture in complement activation on targeted microbubble surfaces. Biomaterials. 2011;32(27):6579–87. https://doi.org/10.1016/j.biomaterials.2011.05.027.
• Mott B, Packwood W, Xie A, Belcik JT, Taylor RP, Zhao Y, et al. Echocardiographic ischemic memory imaging through complement-mediated vascular adhesion of phosphatidylserine-containing microbubbles. JACC Cardiovasc Imaging. 2016;9(8):937–46. https://doi.org/10.1016/j.jcmg.2015.11.031 This study provides validation for the use of a clinically approved CEU agent (Sonazoid) in detection of prior ischemia in a canine model of closed-chest myocardial infarction.
Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107–12. https://doi.org/10.1161/hc4201.097061.
Unger EC, McCreery TP, Sweitzer RH, Shen D, Wu G. In vitro studies of a new thrombus-specific ultrasound contrast agent. Am J Cardiol. 1998;81(12, Supplement 1):58G–61G. https://doi.org/10.1016/S0002-9149(98)00055-1.
Klibanov AL, Gu H, Wojdyla JK, Wible JH, Kim DH, Needham D et al. Attachment of ligands to gas-filled microbubbles via PEG spacer and lipid residues anchored at the interface. Proceedings of 26th International Symposium on Controlled Release of Bioactive Materials. 1999;124–5.
Takalkar AM, Klibanov AL, Rychak JJ, Lindner JR, Ley K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J Control Release : official journal of the Controlled Release Society. 2004;96(3):473–82. https://doi.org/10.1016/j.jconrel.2004.03.002.
Ferrante EA, Pickard JE, Rychak J, Klibanov A, Ley K. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J Control Release : official journal of the Controlled Release Society. 2009;140(2):100–7. https://doi.org/10.1016/j.jconrel.2009.08.001.
Behm CZ, Lindner JR. Cellular and molecular imaging with targeted contrast ultrasound. Ultrasound Q. 2006;22(1):67–72.
Carr CL, Qi Y, Davidson B, Chadderdon S, Jayaweera AR, Belcik JT, et al. Dysregulated selectin expression and monocyte recruitment during ischemia-related vascular remodeling in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31(11):2526–33. https://doi.org/10.1161/ATVBAHA.111.230177.
Truillet C, Oh HLJ, Yeo SP, Lee CY, Huynh LT, Wei J, et al. Imaging PD-L1 expression with immunoPET. Bioconjug Chem. 2018;29(1):96–103. https://doi.org/10.1021/acs.bioconjchem.7b00631.
Weller GER, Villanueva FS, Klibanov AL, Wagner WR, editors. Shear modulates adhesion of ultrasound contrast microbubbles targeted to dysfunctional endothelium. Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society] [Engineering in Medicine and Biology; 2002 23–26 2002.
Alie N, Eldib M, Fayad ZA, Mani V. Inflammation, atherosclerosis, and coronary artery disease: PET/CT for the evaluation of atherosclerosis and inflammation. Clin Med Insights Cardiol. 2014;8(Suppl 3):13–21. https://doi.org/10.4137/cmc.S17063.
Klein C, Schmal TR, Nekolla SG, Schnackenburg B, Fleck E, Nagel E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J Cardiovasc Magn Reson : official journal of the Society for Cardiovascular Magnetic Resonance. 2007;9(4):653–8. https://doi.org/10.1080/10976640601105614.
Winter PM, Caruthers SD, Lanza GM, Wickline SA. Quantitative cardiovascular magnetic resonance for molecular imaging. J Cardiovasc Magn Reson : official journal of the Society for Cardiovascular Magnetic Resonance. 2010;12:62. https://doi.org/10.1186/1532-429x-12-62.
Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53(9):802–10. https://doi.org/10.1016/j.jacc.2009.01.005.
Goldberg YH, Ginelli P, Siegel R, Ostfeld RJ, Schaefer M, Spevack DM. Administration of perflutren contrast agents during transthoracic echocardiography is not associated with a significant increase in acute mortality risk. Cardiology. 2012;122(2):119–25. https://doi.org/10.1159/000338731.
Platts DG, Luis SA, Roper D, Burstow D, Call T, Forshaw A, et al. The safety profile of perflutren microsphere contrast echocardiography during rest and stress imaging: results from an Australian multicentre cohort. Heart Lung Circ. 2013;22(12):996–1002. https://doi.org/10.1016/j.hlc.2013.05.637.
Liu YN, Khangura J, Xie A, Belcik JT, Qi Y, Davidson BP, et al. Renal retention of lipid microbubbles: a potential mechanism for flank discomfort during ultrasound contrast administration. J Am Soc Echocardiogr. 2013;26(12):1474–81. https://doi.org/10.1016/j.echo.2013.08.004.
Brieger D, Eagle KA, Goodman SG, Steg PG, Budaj A, White K, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126(2):461–9. https://doi.org/10.1378/chest.126.2.461.
Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–70. https://doi.org/10.1056/nejm200004203421603.
Neumann J, Sörensen N, Schwemer T, et al. Diagnosis of myocardial infarction using a high-sensitivity troponin i 1-hour algorithm. JAMA Cardiol. 2016;1(4):397–404. https://doi.org/10.1001/jamacardio.2016.0695.
Sandoval Y, Apple FS, Smith SW. High-sensitivity cardiac troponin assays and unstable angina. Eur Heart J Acute Cardiovasc Care. 2016;7(2):120–8. https://doi.org/10.1177/2048872616658591.
Davidson BP, Kaufmann BA, Belcik JT, Xie A, Qi Y, Lindner JR. Detection of antecedent myocardial ischemia with multiselectin molecular imaging. J Am Coll Cardiol. 2012;60(17):1690–7. https://doi.org/10.1016/j.jacc.2012.07.027.
Villanueva FS, Lu E, Bowry S, Kilic S, Tom E, Wang J, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007;115(3):345–52. https://doi.org/10.1161/circulationaha.106.633917.
Davidson BP, Chadderdon SM, Belcik JT, Gupta S, Lindner JR. Ischemic memory imaging in nonhuman primates with echocardiographic molecular imaging of selectin expression. J Am Soc Echocardiogr : official publication of the American Society of Echocardiography. 2014;27(7):786–93.e2. https://doi.org/10.1016/j.echo.2014.03.013.
Jones SP, Trocha SD, Strange MB, Granger DN, Kevil CG, Bullard DC, et al. Leukocyte and endothelial cell adhesion molecules in a chronic murine model of myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;279(5):H2196–201.
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant : the official publication of the International Society for Heart Transplantation. 2014;33(10):996–1008. https://doi.org/10.1016/j.healun.2014.08.003.
Weller GE, Lu E, Csikari MM, Klibanov AL, Fischer D, Wagner WR, et al. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation. 2003;108(2):218–24. https://doi.org/10.1161/01.Cir.0000080287.74762.60.
• Liu J, Chen Y, Wang G, Lv Q, Yang Y, Wang J, et al. Ultrasound molecular imaging of acute cardiac transplantation rejection using nanobubbles targeted to T lymphocytes. Biomaterials. 2018;162:200–7. https://doi.org/10.1016/j.biomaterials.2018.02.017 This study demonstrates the use of CEU molecular imaging in detecting early cases of cardiac transplant rejection by targeting infiltrating T lymphocytes.
• Steinl DC, Xu L, Khanicheh E, Ellertsdottir E, Ochoa-Espinosa A, Mitterhuber M, et al. Noninvasive contrast-enhanced ultrasound molecular imaging detects myocardial inflammatory response in autoimmune myocarditis. Circ Cardiovasc Imaging. 2016;9(8):e004720. https://doi.org/10.1161/circimaging.116.004720 This article describes CEU detection of endothelial activation and leukocute recruitment in a murine model of myocarditis using P-selectin and CD4 targeted as well as phosphatidylserine microbubbles.
Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27(1):165–97. https://doi.org/10.1146/annurev.immunol.021908.132620.
Wu MD, Atkinson TM, Lindner JR. Platelets and von Willebrand factor in atherogenesis. Blood. 2017;129(11):1415–9. https://doi.org/10.1182/blood-2016-07-692673.
Fernández-Ortiz A, Jiménez-Borreguero LJ, Peñalvo JL, Ordovás JM, Mocoroa A, Fernández-Friera L, et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J. 2013;166(6):990–8. https://doi.org/10.1016/j.ahj.2013.08.024.
Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121(22):2388–97. https://doi.org/10.1161/circulationaha.109.901413.
Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306(19):2120–7. https://doi.org/10.1001/jama.2011.1654.
Falk E, Sillesen H, Muntendam P, Fuster V. The high-risk plaque initiative: primary prevention of atherothrombotic events in the asymptomatic population. Curr Atheroscler Rep. 2011;13(5):359–66. https://doi.org/10.1007/s11883-011-0193-0.
Kaufmann BA, Carr CL, Belcik JT, Xie A, Yue Q, Chadderdon S, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30(1):54–9. https://doi.org/10.1161/ATVBAHA.109.196386.
Moguillansky D, Leng X, Carson A, Lavery L, Schwartz A, Chen X, et al. Quantification of plaque neovascularization using contrast ultrasound: a histologic validation. Eur Heart J. 2011;32(5):646–53. https://doi.org/10.1093/eurheartj/ehq197.
Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, Inaba Y, et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis: effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging. 2013;6:74–82.
Kee PH, Kim H, Huang S, Laing ST, Moody MR, Vela D, et al. Nitric oxide pretreatment enhances atheroma component highlighting in vivo with intercellular adhesion molecule-1-targeted echogenic liposomes. Ultrasound Med Biol. 2014;40(6):1167–76. https://doi.org/10.1016/j.ultrasmedbio.2013.12.013.
Moccetti F, Weinkauf CC, Davidson BP, Belcik JT, Marinelli ER, Unger E, et al. Ultrasound molecular imaging of atherosclerosis using small-peptide targeting ligands against endothelial markers of inflammation and oxidative stress. Ultrasound Med Biol. 2018;44(6):1155–63. https://doi.org/10.1016/j.ultrasmedbio.2018.01.001.
Chadderdon SM, Belcik JT, Bader L, Kirigiti MA, Peters DM, Kievit P, et al. Proinflammatory endothelial activation detected by molecular imaging in obese nonhuman primates coincides with onset of insulin resistance and progressively increases with duration of insulin resistance. Circulation. 2014;129(4):471–8. https://doi.org/10.1161/circulationaha.113.003645.
Shim CY, Liu YN, Atkinson T, Xie A, Foster T, Davidson BP, et al. Molecular imaging of platelet-endothelial interactions and endothelial von Willebrand factor in early and mid-stage atherosclerosis. Circ Cardiovasc Imaging. 2015;8(7):e002765. https://doi.org/10.1161/CIRCIMAGING.114.002765.
Brown E, Belcik JT, Hodovan JM, Moccetti F, Ozawa K, Bader LA et al. Platelet-endothelial interactions in atherosclerosis-prone arteries in a non-human primate model of obesity and insulin resistance. Vascular Discovery: From Genes to Medicine Scientific Sessions 2018; San Francisco 2018. p. 17.
Khanicheh E, Mitterhuber M, Xu L, Haeuselmann SP, Kuster GM, Kaufmann BA. Noninvasive ultrasound molecular imaging of the effect of statins on endothelial inflammatory phenotype in early atherosclerosis. PLoS One. 2013;8(3):e58761. https://doi.org/10.1371/journal.pone.0058761.
• Moccetti F, Brown E, Xie A, Packwood W, Qi Y, Ruggeri Z, et al. Myocardial infarction produces sustained proinflammatory endothelial activation in remote arteries. J Am Coll Cardiol. 2018;72(9):1015–26. https://doi.org/10.1016/j.jacc.2018.06.044 This study describes how CEU molecular imaging was used to better understand the roles of platelet adhesion and endothelial activation in response to myocardial ischemia, and showed a potential use for the targeted anti-oxidant apocynin in reducing risk for future ischemic events.
Lindner JR. Molecular imaging of thrombus: technology in evolution. Circulation. 2012;125(25):3057–9. https://doi.org/10.1161/CIRCULATIONAHA.112.112672.
Lanza GM, Wallace KD, Scott MJ, Cacheris WP, Abendschein DR, Christy DH, et al. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1996;94(12):3334–40.
Alonso A, Della Martina A, Stroick M, Fatar M, Griebe M, Pochon S, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke. 2007;38(5):1508–14. https://doi.org/10.1161/strokeaha.106.471391.
Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR. Diagnostic ultrasound combined with glycoprotein 2b/3a targeted microbubbles improve microvascular recovery following acute coronary thrombotic occlusions. Circulation. 2009;119(10):1378–85. https://doi.org/10.1161/CIRCULATIONAHA.108.825067.
Christiansen JP, Leong-Poi H, Klibanov AL, Kaul S, Lindner JR. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation. 2002 Apr 16;105(15):1764–7.
Funding
Mr. Brown is supported by grant 18PRE33960532 from the American Heart Association; Dr. Lindner is supported by grants R01-HL078610, R01-HL120046, and P51-OD011092 from the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Eran Brown grants from American Heart Association (Grant: 18PRE33960532).
Jonathan R. Lindner reports grants from GE Lifesciences.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Echocardiography
Rights and permissions
About this article
Cite this article
Brown, E., Lindner, J.R. Ultrasound Molecular Imaging: Principles and Applications in Cardiovascular Medicine. Curr Cardiol Rep 21, 30 (2019). https://doi.org/10.1007/s11886-019-1117-9
Published:
DOI: https://doi.org/10.1007/s11886-019-1117-9