Abstract
Assessing left ventricular diastolic and regional function is a crucial part of the cardiovascular evaluation. Diastolic function is as important as systolic function for left ventricular performance because it is the determinant of the ability of the left atrium and ventricle to fill at relatively low pressures. Additionally, diastolic function plays an important role in the management and prognosis of patients with symptoms and signs of heart failure. Technical advances in the imaging modalities have allowed a comprehensive noninvasive assessment of global and regional cardiac mechanics and precise estimation of cardiovascular hemodynamics. In this review, we will discuss and compare clinically available techniques and novel approaches using echocardiography, cardiac magnetic resonance, and computed tomography for the assessment of diastolic and regional left ventricular function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A comprehensive left ventricular (LV) performance evaluation requires the assessment of diastolic function as it provides essential information about the LV relaxation and restoring forces, chamber compliance, and LV filling pressures [1, 2]. Diastolic function assessment is critical in the management of patients presenting with shortness of breath [3]. Diastolic function is an independent predictor of functional capacity and prognosis among patients with heart failure [4, 5]. The main noninvasive tool for the assessment of diastolic function is echocardiography (Echo). Echo can reliably assess LV diastolic function through the determination of well-validated, feasible parameters [6••]. Technical advances in the noninvasive imaging modalities have allowed the assessment of LV mechanics which has resulted in the development of novel parameters that can play a promising role in the quantification of LV diastolic function [7•].
Additionally, LV regional function has recently attracted significant attention given that it offers a feasible and reliable method for the assessment of myocardial diseases [8•]. Moreover, regional LV function parameters can provide incremental prognostic value over conventional LV performance parameters. In Part 2, we review the role of parameters for assessing LV diastolic function and LV regional function [9].
Diastolic Function
It is important to emphasize that diastolic function is as essential as systolic function in the assessment of the global LV performance. Diastolic function encompasses the ability of the LV to fill at low left atrial (LA) pressure, at rest and during exercise, so as to generate the required stroke volume and cardiac output during systole [10]. Diastolic dysfunction is a consequence of (1) impaired LV relaxation with or without reduced restoring forces (a consequence of recoil of the myocardial twisting that occurs during systole, which is a major contributor to establishing base-to-apex intraventricular pressure gradient or diastolic suction), and/or (2) impaired LV compliance resulting in elevated LV filling pressures [7•]. Based on the current American Society of Echocardiography and European Association of Cardiovascular Imaging (ASE/EACVI) recommendations, the determination of presence or absence of elevated LV filling pressure should be the first step in the grading of diastolic function among patients presented with signs and symptoms of heart failure [6••]. This initial focus on the determination of presence or absence of elevated LV filling pressure becomes more important among patients with heart failure with reduced ejection fraction (HFrEF), as they always suffer from concomitant diastolic dysfunction.
Echocardiographic Parameters of Diastolic LV Function
Indices of Left Ventricular Relaxation and Restoring Forces
LV relaxation is determined by LV inactivation, LV afterload, and LV dyssynchrony. LV inactivation is an active process that consumes energy for actin-myosin detachment by reducing the level of calcium in the sarcoplasm. An increase in LV afterload results in increased LV wall stress and subsequently a delayed LV relaxation and loss of restoring forces. Similarly, LV dyssynchrony (as in those with aortic stenosis or those with hypertension) results in LV relaxation impairment. Invasively, LV relaxation is measured by the time constant of LV pressure decay, tau (τ) [7•, 10, 11]. Various echo parameters have been suggested as a surrogate for τ. It is important to know that there is no clinical method to separate between impairment in LV relaxation versus restoring forces.
The regional LV lengthening velocity, measured by pulse-wave (PW) to obtain tissue Doppler imaging (TDI)—derived mitral annular early diastolic (e′) velocity, is the fundamental parameter for LV relaxation and restoring forces and is inversely related to τ [6••, 12]. Because e′ velocity is also determined by the filling pressure—i.e., it is partially load-dependent—and because of the limitation associated with extrapolating information from a regional parameter (e′) that can be affected by dysfunction in the sampled region (septal or lateral), and in an attempt to obtain a less angle-dependent parameter that is less influenced by tethering and cardiac translational motion from adjacent segments, global LV longitudinal diastolic strain rate during isovolumetric relaxation (SRIVR) and early diastole (SRE) using the speckle tracking technique have been suggested as novel indices to replace TDI-derived e′[13, 14]. SRIVR does not appear to exhibit significant changes with variations in preload, and SRIVR has demonstrated significant correlations with τ (r = − 0.74, P < 0.001); similarly, SRE has demonstrated significant but lower correlation with τ (r = −0.56, P = 0.001) [13]. However, because of the technical difficulty in obtaining these measurements (especially SRIVR) and their measurement variability, these parameters are still considered experimental measures of LV relaxation [6••].
Additionally, the advent of speckle tracking-derived circumferential strain has allowed the measurement of the twisting movement of the LV, with counterclockwise rotation of the apex and clockwise rotation of the base around the long axis when viewed from the apex direction [8•]. Torsion is the result of the contraction of the oppositely directed myocardial fibers, left-handed helix at the subepicardium, and right-handed helix at the subendocardium [10, 15]. The reference values of LV net twist angle are variable depending on the technique used, age of the subject, and loading condition of the LV [16]. Takeuchi et al. [17] measured the LV twist in 118 asymptomatic volunteers using 2-dimensional (2D) strain software (Echopac PC, version 4.03, GE Healthcare). Defining LV twist was as the apical rotation relative to the base, the mean value of peak twist was 7.7 ± 3.5°. LV twist profiles were significantly higher as the age of the participants increased [17]. Moreover, the recoil of the LV twist (LV untwisting) is a novel measure that reflects the LV active relaxation and the passive restoring forces that contribute to the diastolic suction and early LV filling [8•]. However, it is not independent of LA pressure and LV systolic function [18]. Untwisting rate has been correlated with τ only among those patients with HFrEF [18]. It was observed that untwisting rate increased significantly among those with mild diastolic dysfunction but, later on, it normalized or even decreased with the progression of diastolic dysfunction [19]. The timing of peak untwisting rate, rather than the rate itself, has been suggested as a better parameter among patients with preserved ejection fraction (EF) [18, 20].
The isovolumetric relaxation time (IVRT) and the deceleration time (DT) of the early transmitral flow velocity (E) are important parameters of LV relaxation and restoring forces [6••, 7•]. IVRT is obtained by placing continuous-wave (CW) Doppler sample volume at the LV outflow tract (in either the apical 5-chamber or 3-chamber view) to display simultaneously the aortic outflow and the transmittal inflow waves at a sweep of 100 mm/s. IVRT is the measured time interval between aortic closure and mitral valve opening [6••]. DT is obtained at the apical 4-chamber view by placing the PW Doppler sample volume at tips of the mitral leaflets. DT is the time measured from E to the zero-velocity baseline [6••]. Prolongation of the IVRT (>70 ms) and DT (>160 ms) is consistent with impaired relaxation of the LV [6••].
Another parameter for LV relaxation is M-mode flow propagation velocity (Vp), which is obtained by directing the M-mode cursor from the LV apex through the mitral inflow, and has been suggested to reflect LV diastolic suction or apex-to-base intraventricular pressure gradient [10, 21]. Due to its low feasibility and reproducibility, however, the current ASE/EACVI LV diastolic function guidelines recommend against the routine assessment of this parameter [6••].
Other surrogates of decreased LV relaxation are an impaired relaxation pattern on transmitral flow velocities (decreased E/A peak velocity ratio) and an increased time difference between E and e′ [6••, 7•, 10].
Indexes of Left Ventricular Compliance
LV compliance is defined as the change in LV volume per change in LV pressure change during diastole. It is defined not only by the myocardial stiffness (determined at the cellular level by titin, collagen isoform I, and matrix metalloproteinase degradation rate) but also by the LV geometry and the elastic properties of the extraventricular structures, such as pericardium, and right ventricular pressure. Therefore, the term chamber compliance is a better term to use [7•, 11].
The transmitral flow velocities measured at the mitral tips using PW (mitral E velocity and A velocity) and the DT of the E wave are the main parameters for the assessment of LV compliance. Having a high E/A ratio (≥2) with a deceleration time of <160 ms is consistent with restrictive (decreased compliance) physiology, except among those with E >120 cm/s in which the DT can exceed 160 ms [6••, 7•].
Other surrogates for reduced LV chamber compliance are a short mitral valve A-duration (measured at the level of the mitral annulus), low A-wave amplitude with concomitant long duration, and a high amplitude of the pulmonary venous reversed A-wave (Ar). Ar-A duration >30 ms is consistent with elevated LVEDP and reduced LV chamber compliance [6••].
Parameters of Elevated Left Ventricular Filling Pressure
LA pressure and LV filling pressure—LV pre-A pressure, mean LV diastolic pressure, and LVEDP—can be measured directly and invasively. Additionally, mean pulmonary capillary wedge pressure (PCWP) and diastolic pulmonary artery pressure are indirect invasive measures useful for estimating the mean LA pressure rather than LVEDP.
Noninvasively, indirect parameters can be utilized for the determination of LV filling pressure [7•, 11]. LV filling pressure is mainly determined noninvasively using the PW Doppler—derived transmitral flow velocities (E and A velocities). Transmitral flow velocities are a reflection of the transmitral pressure gradient with the E velocity representing the LA-LV pressure gradient during early diastole (determined mainly by the rate of LV relaxation and the LA pressure) and the A velocity reflecting the LA-LV pressure gradient during late diastole (mainly determined by LV compliance and LA contractile function). E/A ratio and early diastolic deceleration time can be used to identify LV filling patterns: normal, impaired relaxation, pseudonormal, and restrictive filling [6••].
The expert consensus in the current ASE/EACVI LV Diastolic Function guidelines emphasized that the initial step in the assessment of diastolic function is to review clinical information and 2D and Doppler data, as the guidelines might not be applicable in certain clinical situations [6••]. Afterward, the assessment of LV filling pressure pattern would be the next step in the determination of diastolic function (in the absence of atrial fibrillation, significant mitral valve disease, LV assist devices, left bundle branch block, and ventricular paced rhythm) [6••]. A transmitral inflow E/A ratio <0.8 with a peak E velocity of ≤50 cm/s (indicating mean LA pressure either normal or low) corresponds with grade I diastolic dysfunction. When the mitral inflow pattern shows an E/A ratio ≥2 (except in the setting of recent cardioversion of atrial fibrillation or flutter to sinus rhythm), the LA pressure is elevated and corresponds to grade III diastolic dysfunction. If the E/A is ≤0.8 with an E velocity of >50 cm/s or an E/A is between 0.8–2, the guidelines recommend the use of E/e′, tricuspid regurgitation velocity, and LA volume index for the determination of whether the LV filling pressure is elevated or not (Fig. 1).
Algorithm for the assessment of diastolic function modified from the current ASE/EACVI recommendation. A transmitral peak A velocity, E transmitral peak E velocity, HFpEF heart failure with preserved ejection fraction, HFrEF heart failure with reduced ejection fraction, LA left atrium, LVEF left ventricular ejection fraction, TR tricuspid regurgitation. (Reprinted from: Nagueh et al. J Am Soc Echocardiogr, 2016, 29(4):277–314, with permission from Elsevier) [6••]
The mitral E/e′ ratio has the advantage of adjusting the E velocity for LV relaxation and, therefore, it better reflects the LV filling pressure. An average E/e′ >14 is highly specific for elevated LV filling pressure, regardless of the age, and it provides convincing evidence of diastolic dysfunction [6••].
In the absence of pulmonary artery disease, pulmonic valve stenosis, or significant mitral valve regurgitation, an elevated pulmonary artery pressure (assessed by either tricuspid regurgitation systolic jet velocity or pulmonary regurgitation end-diastolic velocity) is highly consistent with elevated LA pressure. This parameter has the advantage of being age-independent [6••]. The maximum LA volume index (>34 ml/m2) is another important parameter for the evaluation of LV filling pressure. In the absence of atrial fibrillation or significant mitral valve disease, LA enlargement can be a consequence of chronically elevated LV pressure [6••].
Another parameter that has been suggested for the determination of LV filling pressure, especially in the setting of HFrEF, is the pulmonary venous flow velocity pattern assessed by PW Doppler. Pulmonary venous flow consists of systolic (S) and diastolic (D) waves. Peak S velocity is determined by the LA pressure, LA contractility, and LV and right ventricle (RV) contractilities, while peak D velocity is influenced by early diastolic LV filling and the LV compliance. An S/D ratio of <1 is indicative of increased LA pressure in the setting of HFrEF [6••].
The novel parameters of global longitudinal diastolic strain, SRIVR, and SRE have been shown to have better predictive ability in comparison to TDI-derived e′ for the LV filling pressure [13, 22, 23]. The mitral E/SRIVRT has shown a good correlation with PCWP in both normal EF and reduced EF (r = 0.91, P < 0.01 and r = 0.74, P < 0.01, respectively) [13]. Global longitudinal early diastolic strain has also been observed to be an independent predictor of outcomes. For example, patients with atrial fibrillation, an increase of 10 cm in E/SRE was associated with hazard ratio of 1.258 for all-cause mortality and hospitalization for heart failure (P < 0.001) (median follow-up of 20 months) [22]. Similarly, after an acute myocardial infarction, E/SRE cut-off value of >1.25 was associated with hazard ratio of 4.38 (P < 0.0001) for composite outcome of all-cause mortality, hospitalization for heart failure, new onset atrial fibrillation, or stroke (median follow-up of 29 months) [23]. In both studies, E/SRE outperformed E/e′ in predicting adverse cardiac events [22, 23].
Another promising parameter for assessment of LV filling pressure is the LA longitudinal strain rate during systole, which has been shown to correlate with PCWP among patients with HFrEF [24] and can potentially identify asymptomatic patients with diastolic heart failure [25].
CMR Parameters of LV Diastolic Function
CMR can provide accurate LV volume change measurements over time which can be converted to an LV volume curve to estimate the peak filling rate and time to peak filling, both parameters of diastolic function [7•]. CMR can provide analogous measurements to Doppler echocardiographic blood flow and tissue velocities [7•]. The flow patterns through the mitral valve and pulmonary vein can be obtained using velocity-encoded cine magnetic resonance imaging, and they have shown significant linear correlations with corresponding Echo Doppler-derived values [26]. Likewise, tissue velocity assessment is feasible utilizing phase-contrast magnetic resonance imaging with CMR measures correlating significantly with analogous echo DTI-derived parameters [27].
In addition, CMR can precisely and reproducibly quantify LV deformation in systole and diastole. Ambale-Venkatesh et al. [28] utilized a CMR tissue tagging technique to calculate strain relaxation index (SRI)—by dividing the difference between post-systolic and systolic times of strain peaks by the early diastolic strain rate. SRI, a new index of relaxation, was an independent predictor of heart failure and atrial fibrillation over an 8-year follow-up period [24].
More recently, the utilization of CMR imaging with pre- and post-gadolinium contrast T1 mapping has been investigated as a novel indirect method for the assessment of diastolic dysfunction. T1 mapping analyzes the longitudinal proton relaxation times after an electromagnetic pulse and represents them as pixel intensities [7•]. Post-contrast T1 mapping imaging permits the quantification of the extracellular volume (ECV), a marker of fibrosis. The post-contrast T1 mapping time and ECV correlated significantly with β, the load-independent passive LV stiffness constant (r = 0.71 and r = 0.58, respectively), among 20 cardiac transplant recipients [29]. Similar findings were observed among patients with heart failure and preserved EF (HFpEF): ECV was significantly correlated with β (r = 0.75, P < 0.001). ECV values above the median correlated with higher LV stiffness while ECV values below the median identified patients with prolonged active LV relaxation [30•]. Additionally, Su et al. [31] showed that ECV was significantly correlated with diastolic dysfunction only among those with HFp EF but not among those with HFrEF or healthy participants.
Computed Tomography Parameters of Diastolic LV Function
MDCT can provide information about LV volumes, but because of the radiation exposure and the availability of other modalities, the role of MDCT in the evaluation of LV diastolic function has been very limited [7•].
Assessment of Regional Function
For a better understanding of the LV regional function, it is essential to have a good knowledge of LV structure, myocardial architecture, and the contribution of the different layers of the myocardium to LV mechanics. The normal LV is a prolate ellipsoid structure with a long-to-short axis ratio of 2:1. Its inlet (mitral valve) and outflow (aortic valve) are at a 30° angle to each other, creating a V shape structure that is only separated by the anterior leaflet of the mitral valve [8•, 15].
The myocardial fibers are arranged as a continuum of two helical geometries, with three different orientations: longitudinal with a right-handed helix angle of +60–80°at the subendocardium, circumferential with a 0° orientation at the mid-wall, and oblique with a left-handed helix angle of −60° at the subepicardium (Fig. 2) [10, 32]. The different orientations of the myocardial fibers are essential for the formation of shearing and torsion mechanics of the myocardium that contribute to the amplification of the 15% shortening of the myocytes into more than 40% thickening and more than 60% ejection fraction of the normal LV [8•]. Based on the described myocardial fibers’ orientation, the subendocardial region mainly contributes to the longitudinal mechanics while the midwall and subepicardial regions predominantly generate the circumferential mechanics.
a Myocardial fiber arrangement in three different orientations: longitudinal to the LV long axis at the subendocarium (Endo), circumferential at the mid-wall, and oblique at the subepicardium (Epi). b The different orientations of the myocardial fibers are essential results in the amplification of the 15% shortening of the myocytes into more than 40% thickening and more than 60% ejection fraction of the left ventricle. (Reprinted from: Thomas et al. J Am Coll Cardiol, 2006, 48(10):2012–25, with permission from Elsevier) [10]
Additionally, the two-helical geometry of the LV myocardium produces the twisting rotation during the cardiac cycle. The larger radius (further away from the centerline) of the subepicardial fibers results in a higher torque of this region in comparison to the subendocardial region [8•, 10]. Consequently, the subepicardial fibers dominate the twisting motion of the LV during ejection with counterclockwise rotation of the LV apex and clockwise rotation of the LV base when viewed from the apex perspective (Fig. 3) [10].
a Diagram representing the continuum of two helical geometries of the myocardiaum: the subendocardial fibers (green arrow) with right-handed helix angle of +60–80° and the subepicardial fibers (red arrow) with left-handed helix angle of −60°. b Relationship between shear strain (ϵxy) and torsion (θ). Shear strain is mainly generated by the left-handed subepicardial fibers (red arrow), being the more abundant and having a larger radius in comparison to subendocardial fibers. Torsion is determined proportionally by the shear strain and ventricular length and is inversely proportional to short-axis radius. (Reprinted from: Thomas et al. J Am Coll Cardiol, 2006, 48(10):2012–25, with permission from Elsevier) [10]
Segmentation of the Left Ventricle
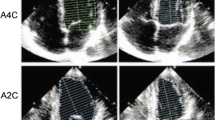
For assessment of regional LV function, the LV is divided into segments of comparable myocardial mass which allow for standardized evaluation, reporting, and communication of abnormalities in regional wall motion across all cardiovascular imaging modalities [33••, 34]. The American Heart Association, American College of Cardiology, ASE, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance recommend the 17-segment model for consistent and uniform reporting of regional left ventricular myocardial and cavity findings [33,35,••, 34–36]. The model divides the LV cavity into basal, mid, and apical thirds that correspond to 35, 35, and 30% of myocardial mass, respectively, closely matching autopsy data. The basal third corresponds to the LV cavity (and corresponding myocardium) from the mitral annulus to the tip of papillary muscles. The mid cavity is the region of the entire length of papillary muscles while the apical part is the area beyond the papillary muscles to just before the end of the cavity. Myocardium just beyond the end of LV cavity is the true apex. When viewed circumferentially, the basal and mid have six segments while the apical part has four apical segments and one true apex (Fig. 4). Regional function of the basal, mid, and apical segments can be assessed in the short-axis or using three orthogonal views. The true apex is best evaluated on a vertical and/or a horizontal long-axis view across all cardiac imaging modalities [34]. An abnormality is typically present in at least two contiguous segments or is seen in one segment on two different views.
The 17-segment model of the left ventricle. Top row shows actual echo images from the apical views, the center image represents the bull’s-eye display of the LV segments, and the bottom row of panels depicts schematically the LV wall segments in each view. (Reprinted from: Lang et al. Journal of the American Society of Echocardiography 2015, 28:1–39.e14, with permission from Elsevier) [33••]
Visual Assessment
In Echo, MDCT and CMR, the regional myocardial function can be assessed based on the visually observed wall motion and thickening. A semiquantitative wall motion score can be assigned to each segment based on the corresponding visualized wall motion: (1) normal or hyperkinetic, (2) hypokinetic, (3) akinetic, and (4) dyskinetic. The wall motion scores can then be averaged, with all segments visualized to calculate the LV wall motion score.
Regional Function Using Doppler or Speckle Tracking
Quantitative assessment of LV regional myocardial function can be performed using TDI or speckle-tracking techniques [37,38,39]. Both techniques provide similar data; however, Echo DTI is dependent on the Doppler angle at which the images were acquired and may underestimate motion that is not parallel to the ultrasound beam [33••].
Regional Function Analysis by MDCT
MDCT is an established technique for evaluation of coronary heart disease in appropriately selected patients, as well for assessment of global cardiac function, but its utility for regional function evaluation is not yet proven. Regional function by MDCT is most commonly assessed subjectively on cine images in different cardiac planes. The limiting factors for MDCT assessment of regional cardiac function are relatively poor temporal resolution, lack of markers for detection of myocardial strain, and increasing use of dose modulation techniques for radiation dose reduction [40]. As compared to Echo and CMR, the inherent temporal resolution is low, but improvements in MDCT technology have allowed faster gantry rotation and shorter scan time that translates into improved temporal resolution. The temporal resolution of single-source MDCT scanners is approximately 165–175 msec. Hence, there is no utility of cardiac phase reconstruction at any more than 10% increments (the difference between each phase is approximately 100 msec at a heart rate of 60 beats per minute). With DSCT scanners, the temporal resolution can be as low as 65 msec; therefore, reconstruction at 5% increments (20 phases) is recommended for global and regional functional analysis [41]. In one of the earlier studies on objective regional functional analysis by MDCT, Cerqueiraet et al. [42] performed gated blood pool tomography using floating axis (defining a different LV geometric center on end-diastolic and end-systolic images) and fixed axis (use of only end-diastolic LV geometric center) systems and calculated the regional LV ejection fraction in healthy subjects. A study using the floating axis system showed that MDCT-based regional ejection fraction analysis is comparable to SPECT for predicting ischemia and an even better predictor of significant stenosis than is SPECT [43]. Unlike echocardiography and MRI that utilize myocardial “speckles” and distortion of “tagging lines” on myocardium, respectively, the conventional MDCT lacks a surrogate marker for detection of myocardial strain. With the inherent advantage of high spatial resolution, volumetric MDCT images may be used to define endocardial trabeculae. The relative motion of trabeculae (with calculation of a metric known as Stretch Quantifier of Endocardial Engraved Zones, SQUEEZ), as well as newer biomedical LV deformational models on 4D-cardiac CT, has allowed myocardial strain and regional cardiac function assessment on MDCT [40, 44, 45•]. Regional cardiac function assessment using SQUEEZ technique has been shown to be comparable to the reference standard tagged MRI in animal models [46].
Regional Function and Strain Analysis by CMR
CMR is currently the reference standard for regional cardiac function analysis and myocardial strain imaging. As compared to echocardiography (which is the most commonly used modality for regional function analysis), it is currently a relatively cumbersome and technically difficult procedure, with less availability, the need for multiple breath-holds, and lengthy post-processing time. Myocardial strain is a very well-studied marker for evaluation of regional cardiac dysfunction. Myocardial strain is a dimensionless entity and refers to a change in length of myocardium relative to its original length [47]. As evident from the definition, myocardial lengthening will give positive numbers and myocardial shortening will give negative numbers. With respect to the frame of reference, myocardial strain can be defined as Eulerian and Lagrangian. Lagrangian strain displacement is calculated by taking the deforming myocardium as a reference while there is a fixed frame of reference for calculation of Eulerian strain. Most of the available literature regarding myocardial strain evaluation calculates Lagrangian strain in longitudinal, circumferential, and radial orientation [32].
The LV myocardium is helically arranged with subendocardial myocardium oriented as a right-hand helix that changes into a left-hand helix in the subepicardium. The subendocardial myocardium is responsible for the long-axis function of LV; impairment of subendocardial function translates into abnormalities in longitudinal strain, whereas the midmyocardial and subepicardial fibers predominantly contribute to short-axis function and mainly translate into abnormalities in circumferential and radial strain. Since ischemic heart disease involves subendocardium initially, abnormalities in longitudinal strain can predict early myocardial dysfunction before global dysfunction sets in; thus, changes in LS can help determine patient prognosis and response to therapy [47]. Various sequences and techniques—e.g., regional myocardial thickening analysis on segmented cine images, myocardial tagging, tissue phase mapping (TPM), feature-tracking MRI (FT-MRI), displacement encoding with stimulated echoes (DENSE), and strain-encoded imaging (SENC)—have been described for assessment of regional myocardial function by MRI. These techniques are briefly reviewed here, along with their advantages and disadvantages.
Regional Myocardial Thickening and Feature-Tracking at Balanced Steady-State Free Precession Imaging
SSFP is the most common bright blood cine sequence used for quantitative or qualitative cardiac function analysis. The image acquisition in SSFP can be segmented (preferably with retrospective gating) or ECG-triggered real-time cine. Real-time images are mostly used in patients with frequent ectopy or inability to do breath-hold. Since the data need to be acquired in a single-shot acquisition time of 50–80 milliseconds, the real-time images suffer from relatively poor spatial resolution. If there is frequent ectopy or atrial fibrillation, there are inconsistent R-R intervals that cause spatial and temporal blurring. Due to these factors, real-time cine images are not suitable for quantitative cardiac functional analysis [32]. Qualitative regional functional assessment can be performed on both segmented and real-time cine SSFP images, where the regional wall motion is visually graded on a scale from normal to dyskinetic.
The quantitative functional analysis on SSFP can be done either by measuring regional myocardial thickening or by feature tracking. Regional myocardial thickening assessment requires determination of endocardial and epicardial contours, which is followed by measurement of change in regional myocardial thickness between diastole and systole [32]. Feature tracking (FT-MRI) is a relatively newer optical flow technique, which is analogous to echocardiographic speckle tracking and allows assessment of tissue voxel motion on cine-MRI images [48]. FT-MRI also allows detection of myocardial strain, strain rate, displacement, and torsion on SSFP cine images. The feature tracking technique is not limited to evaluation of blood-myocardium junction, but can also be used to track mid-myocardial features (Fig. 5). FT-MRI has good intra-observer and inter-observer agreement, and the image post-processing is less cumbersome as compared to other techniques that enable regional function and strain analysis [49, 50]. Currently, the evidence does not support the use of FT-MRI for segmental strain analysis due to its inherent strain heterogeneity [32]. Since segmented SSFP images are routinely acquired for CMR exams, regional myocardial thickening and FT-MRI can be done without addition of sequences.
A patient with a recent non-transmural ST-elevation myocardial infarction in the left anterior descending territory. Feature-tracking CMR images show abnormal peak regional radial (a), circumferential (b), and longitudinal 3-chamber (c) and 4-chamber (d) Lagrangian strain values in mid anterior septum and inferior wall. Corresponding SSPP and late gadolinium enhancement CMR images in 3-chamber (e) and 4-chamber (f) view. Peak global strain-time curves (g) show significantly reduced circumferential and longitudinal strains. (Reprinted from: Collins JD. Radiol Clin North Am, 2015, 53(2):369–95, with permission from Elsevier) [32]
Myocardial Tagging
Myocardial tagging is the most studied technique for regional cardiac function and myocardial strain analysis [32]. Grid or parallel lines are superimposed on the myocardium (Fig. 6) at the start of the R-wave using a preparatory pulse and deformation of these lines or grids studied over the cardiac cycle. These taglines are superimposed on myocardium by a radiofrequency prepulse known as spatial modulation of magnetization (SPAMM) or delays alternating with nutation for transient excitation (DENSE). SPAMM tags fade with time, making diastolic assessment difficult. As compared with 1.5T scanners, this effect is less prominent with 3T MR scanners with a T1 relaxation time of approximately 1100 msec. C-SPAMM (complementary SPAMM) is an improvement over SPAMM with increased tag contrast, but with a trade-off of double acquisition time [32, 51]. To obtain sufficient tags over the myocardium, intertag distances of 6–7 mm are typically used. Due to its ability to analyze the perpendicular components of myocardial deformation, grid tagging is preferred over line tagging [51]. These tags can be qualitatively interpreted visually, with abnormal tag deformation representing regional wall motion abnormality. Quantitative tag analysis can be cumbersome and time-consuming. Techniques of quantitative tag analysis include tag following (manual or semiautomated segmentation of tag lines), optical flow techniques (tracks individual pixels from one time point to other), and harmonic phase analysis (HARP, tracks phase change from one of the off-center spectral peaks in the k-space domain) [32]. HARP analysis is automatic, analyzes Lagrangian and Eulerian strain, and is more time efficient than conventional tag analysis, but with limitations of lower spatial resolution and decreased signal-to-noise ratio [32].
Example of CMR tagging with a spatial modulation of magnetization (SPAMM) technique showing a short-axis image of the left and right ventricles in a patient with hypertrophic cardiomyopathy. Tag lines oriented in a grid pattern are superimposed and track the myocardium. Deformation of the grid lines during ventricular systole and diastole is analyzed and intramyocardial wall motion appreciated
Tissue Phase Mapping
TPM is based on acquiring three-directional velocity vector fields that are encoded to the phase using a bipolar gradient [32, 52]. A flow-compensated echo with velocity encoding in-plane and through-plane is recorded in a single acquisition. The regional function is then measured using myocardial velocities. This technique offers a relatively higher spatial resolution of functional information (1–3 mm) as compared to the tagging technique (4–8 mm), which is limited by intertag distance and tag density [52]. Motion artifacts limit TPM, so it is performed with black blood preparation to decrease ghosting in the direction of the phase-encoding gradient [32].
Displacement Encoding with Stimulated Echoes
DENSE is a phase-based MR technique that encodes the tissue velocity of each pixel in three directions. This technique encodes displacement on the pixel level and does not depend on interpolation methods such as tagging (Fig. 7) [32, 51, 53]. With navigator, DENSE can be obtained during free breathing. Cine DENSE acquisition is possible by using the echo-planar imaging technique or a spiral readout trajectory [54]. Due to T1 recovery, the encoding does not last throughout the cardiac cycle, which limits analysis of diastolic tissue displacement. Since the technique is based on stimulated echo, DENSE also has an inherently low SNR, but the post-processing is relatively quick, and there is a low inter- and intraobserver variability [32].
Systolic image at base, mid-chamber, and apex showing displacement (left) and E2 strain maps (right) using displacement encoding with stimulated echoes (DENSE). (Reproduced from: Kim et al. NMR Biomed 2007, 20(6):591–601, with permission from John Wiley and Sons) [53]
Strain-Encoded Imaging
SENC is a relatively new technique that has tag planes oriented parallel to the imaging as compared to perpendicular orientation on conventional tagging [32, 54, 55]. As with conventional tagging, the tags fade with T1 recovery, limiting late diastolic functional assessment. SENC measures through-plane strain, which is directly related to pixel intensity, enabling efficient processing (Fig. 8). Since it measures through-plane strain, the longitudinal strain is measured on short-axis plane and the circumferential strain is measured on long-axis plane. This technique is also limited by low SNR and tag fading, limiting diastolic function analysis [54].
Color-coded composite images showing the local frequency, v (a), tag frequency, w (b), tilt angle (c), and Eulerian longitudinal strain (d), all reproduced from 17 Strain-Encoded MRI images with varying z phase encode values. (Reproduced from: Osman et al. Magn Reson Med 2001, 46(2):324–34, with permission from John Wiley and Sons) [55]
Conclusion
In summary, noninvasive assessment of the diastolic function is an integral part of the LV performance evaluation. Moreover, the regional LV function has emerged as a feasible tool for subclinical detection of myocardial diseases and it provides incremental prognostic value. Echo, CMR, and MDCT are rapidly evolving noninvasive tools that have significantly improved our understanding of LV myocardial function by providing qualitative and quantitative hemodynamic indices of the LV function, including, LV deformation mechanics, LV relaxation, and filling pressure parameters. In addition, these imaging modalities have also offered noninvasive techniques that have enhanced our knowledge about fascinating aspects of LV myocardial fiber architecture. Recently, multiple investigations have assessed the clinical application of regional LV parameters such as strain, stain rate, shearing, twisting, and torsion. These novel parameters offer promise in the management of cardiovascular disease by providing sensitive subclinical markers of the disease process. Additional studies are needed to further delineate the specific practical applications of these different modalities and their unique capabilities within the clinical spectrum of LV function assessment.
Abbreviations
- ASE:
-
American Society of Echocardiography
- CMR:
-
Cardiac magnetic resonance imaging
- CW:
-
Continuous-wave
- D:
-
Pulmonary venous diastolic flow wave
- DENSE:
-
Displacement encoding with stimulated echoes
- DSCT:
-
Dual source computed tomography
- DT:
-
Deceleration time
- E:
-
Early transmitral flow velocity
- e′:
-
Early diastolic velocity of the mitral annulus
- EACVI:
-
European Association of Cardiovascular Imaging
- Echo:
-
Echocardiography
- ECV:
-
Extracellular volume
- EF:
-
Ejection fraction
- FT-MRI:
-
Feature-tracking magnetic resonance imaging
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- IVRT:
-
Isovolumetric relaxation time
- LA:
-
Left atrial
- LV:
-
Left ventricular
- LVEDP:
-
Left ventricular end-diastolic pressure
- MDCT:
-
Multidetector computed tomography
- PW:
-
Pulsed-wave
- S:
-
Pulmonary venous systolic flow wave
- SENC:
-
Strain-encoded imaging
- SPAMM:
-
Spatial modulation of magnetization
- SRE :
-
Strain rate during early diastole
- SRIVR :
-
Strain rate during isovolumetric relaxation
- SSFP:
-
Balanced steady-state free precession
- SQUEEZ:
-
Stretch Quantifier of Endocardial Engraved Zones
- TDI:
-
Tissue Doppler imaging
- TPM:
-
Tissue phase mapping
- Vp:
-
M-mode flow propagation velocity
- β:
-
Load-independent passive LV stiffness constant
- τ:
-
Tau or time constant of left ventricular pressure decay
- 2D:
-
2-Dimensional
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105(11):1387–93.
Yamada H, Klein AL. Diastology 2010: clinical approach to diastolic heart failure. J Echocardiogr. 2010;8(3):65–79.
Yancy CW et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239.
Gardin JM et al. Relationship of Doppler-echocardiographic left ventricular diastolic function to exercise performance in systolic heart failure: the HF-ACTION study. Am Heart J. 2009;158(4 Suppl):S45–52.
Cavalcante JL et al. Diastolic function improvement is associated with favourable outcomes in patients with acute non-ischaemic cardiomyopathy: insights from the multicentre IMAC-2 trial. Eur Heart J Cardiovasc Imaging. 2016;17(9):1027–35.
•• Nagueh SF et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. This document provides a comprehensive practical approach for the assessment of LV diastolic function utlitizing Echo.
• Flachskampf FA et al. Cardiac imaging to evaluate left ventricular diastolic function. J Am Coll Cardiol Img. 2015;8(9):1071–93. This study provides a thorough review about the current and experimental multimodality immaging parameters for the assessment of the LV diastolic function.
• Mor-Avi V et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24(3):277–313. This document provides a detailed information about the echocardiographic techniques utilized for the assessment of cardiac mechanics and the clinical applications of these techniques.
Kinno M, et al. Comparison of echocardiography, cardiac magnetic resonance, and computed tomographic imaging for the evaluation of left ventricular myocardial function: part 1 (global assessment). Curr Cardiol Rep. 2016.
Thomas JD, Popovic ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol. 2006;48(10):2012–25.
Lang RM, et al. ASE’s Comprehensive echocardiography. 2nd ed. Philadelphia, PA: Elsevier Saunders. 879; 2016. p. 19103–2899
Opdahl A et al. Determinants of left ventricular early-diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation. 2009;119(19):2578–86.
Wang J et al. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115(11):1376–83.
Dokainish H et al. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101(10):1504–9.
Adhyapak SM, Parachuri VR. Architecture of the left ventricle: insights for optimal surgical ventricular restoration. Heart Fail Rev. 2010;15(1):73–83.
Sengupta PP et al. Twist mechanics of the left ventricle: principles and application. J Am Coll Cardiol Img. 2008;1(3):366–76.
Takeuchi M et al. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr. 2006;19(9):1077–84.
Wang J et al. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation. 2007;116(22):2580–6.
Park SJ et al. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr. 2008;21(10):1129–37.
Notomi Y et al. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol. 2008;294(1):H505–13.
Brun P et al. Left ventricular flow propagation during early filling is related to wall relaxation: a color M-mode Doppler analysis. J Am Coll Cardiol. 1992;20(2):420–32.
Hsu PC et al. The ratio of early mitral inflow velocity to global diastolic strain rate as a useful predictor of cardiac outcomes in patients with atrial fibrillation. J Am Soc Echocardiogr. 2014;27(7):717–25.
Ersboll M et al. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two-dimensional speckle-tracking study. Eur Heart J. 2014;35(10):648–56.
Wakami K et al. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22(7):847–51.
Kurt M et al. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2(1):10–5.
Hartiala JJ et al. Velocity-encoded cine MRI in the evaluation of left ventricular diastolic function: measurement of mitral valve and pulmonary vein flow velocities and flow volume across the mitral valve. Am Heart J. 1993;125(4):1054–66.
Paelinck BP et al. Feasibility of tissue magnetic resonance imaging: a pilot study in comparison with tissue Doppler imaging and invasive measurement. J Am Coll Cardiol. 2005;45(7):1109–16.
Ambale-Venkatesh B et al. Diastolic function assessed from tagged MRI predicts heart failure and atrial fibrillation over an 8-year follow-up period: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging. 2014;15(4):442–9.
Ellims AH et al. Diffuse myocardial fibrosis evaluated by post-contrast t1 mapping correlates with left ventricular stiffness. J Am Coll Cardiol. 2014;63(11):1112–8.
• Rommel KP et al. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2016;67(15):1815–25. This study provides evidence that CMR-derived T1 mapping, a technique to quantify diffuse myocardial fibrosis, can independently predict invasively measured LV stiffness in patients with HFpEF.
Su MY et al. CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. J Am Coll Cardiol Img. 2014;7(10):991–7.
Collins JD. Global and regional functional assessment of ischemic heart disease with cardiac MR imaging. Radiol Clin N Am. 2015;53(2):369–95.
•• Lang RM et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. This document provide a comprehensive guidelines for the echocardiographic chamber quantification as recommended by the ASE/EACVI.
Cerqueira MD et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42.
Raff GL et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3(2):122–36.
Hundley WG et al. Society for cardiovascular magnetic resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson. 2009;11:5.
Heimdal A et al. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998;11(11):1013–9.
Leitman M et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17(10):1021–9.
Stefani L et al. Two-dimensional tracking and TDI are consistent methods for evaluating myocardial longitudinal peak strain in left and right ventricle basal segments in athletes. Cardiovasc Ultrasound. 2007;5:7.
Lamash Y et al. Strain analysis from 4-D cardiac CT image data. IEEE Trans Biomed Eng. 2015;62(2):511–21.
Rizvi A et al. Analysis of ventricular function by CT. J Cardiovasc Comput Tomogr. 2015;9(1):1–12.
Cerqueira MD, Harp GD, Ritchie JL. Quantitative gated blood pool tomographic assessment of regional ejection fraction: definition of normal limits. J Am Coll Cardiol. 1992;20(4):934–41.
Zeb I et al. Computerized left ventricular regional ejection fraction analysis for detection of ischemic coronary artery disease with multidetector CT angiography. Int J Cardiovasc Imaging. 2013;29(3):685–92.
Pourmorteza A et al. Regional cardiac function assessment in 4D CT: comparison between SQUEEZ and ejection fraction. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4966–9.
• Pourmorteza A et al. A new method for cardiac computed tomography regional function assessment: stretch quantifier for endocardial engraved zones (SQUEEZ). Circ Cardiovasc Imaging. 2012;5(2):243–50. This study provides evidence that the regional myocardial function obtained by CT SQUEEZ, assessed among 162 segments in the 9 hearts, has good correlation with the tagged MRI as a reference standard for noninvasive regional myocardial function.
Pourmorteza A. et al. Correlation of CT-based regional cardiac function (SQUEEZ) with myocardial strain calculated from tagged MRI: an experimental study. Int J Cardiovasc Imaging. 2015.
Bansal M, Sengupta PP. Longitudinal and circumferential strain in patients with regional LV dysfunction. Curr Cardiol Rep. 2013;15(3):339.
Schuster A et al. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson. 2011;13:58.
Hor KN et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. J Am Coll Cardiol Img. 2010;3(2):144–51.
Kuetting D et al. Comparison of magnetic resonance feature tracking with harmonic phase imaging analysis (CSPAMM) for assessment of global and regional diastolic function. Eur J Radiol. 2015;84(1):100–7.
Jeung MY et al. Myocardial tagging with MR imaging: overview of normal and pathologic findings. Radiographics. 2012;32(5):1381–98.
Jung B et al. Investigating myocardial motion by MRI using tissue phase mapping. Eur J Cardiothorac Surg. 2006;29 Suppl 1:S150–7.
Kim D, Kellman P. Improved cine displacement-encoded MRI using balanced steady-state free precession and time-adaptive sensitivity encoding parallel imaging at 3 T. NMR Biomed. 2007;20(6):591–601.
Simpson RM, Keegan J, Firmin DN. MR assessment of regional myocardial mechanics. J Magn Reson Imaging. 2013;37(3):576–99.
Osman NF et al. Imaging longitudinal cardiac strain on short-axis images using strain-encoded MRI. Magn Reson Med. 2001;46(2):324–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Menhel Kinno, Prashant Nagpal, Stephen Horgan, and Alfonso H. Waller declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Echocardiography
Rights and permissions
About this article
Cite this article
Kinno, M., Nagpal, P., Horgan, S. et al. Comparison of Echocardiography, Cardiac Magnetic Resonance, and Computed Tomographic Imaging for the Evaluation of Left Ventricular Myocardial Function: Part 2 (Diastolic and Regional Assessment). Curr Cardiol Rep 19, 6 (2017). https://doi.org/10.1007/s11886-017-0816-3
Published:
DOI: https://doi.org/10.1007/s11886-017-0816-3