Abstract
Purpose of Review
The purpose of this review is to study the epidemiology, presentation, pathogenesis and management of ketamine cystitis and its complications.
Recent Findings
Recent data suggest that the toxicity of ketamine can be due to direct cytotoxicity, independent of a NMDA receptor-mediated mechanism. Bladder barrier dysfunction, neurogenic inflammation with abnormal neurotransmission and immunological mechanisms have also been proposed, based on data from animal models and in vitro studies. Effects on the bladder are dependent on the dose and duration of ketamine use. Clinical studies have showed that the model of outreach clinics with involvement of local urology services holds most promise, with a tiered approach to management based on stage of the disease.
Summary
Chronic ketamine use is associated with significant lower urinary tract symptoms including frequency, urgency, haematuria and bladder pain. This may progress to irreversible bladder dysfunction with poor capacity and compliance and upper tract dysfunction may also occur. The key to successful treatment is ketamine cessation. The role of medications is not well established but there may be benefit from drugs used for IC/BPS and bladder Botox. Patients with end stage disease will require surgery, but there is a high complication rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine is an N-methyl-d-aspartate (NMDA) antagonist, which was first synthesised in 1964 and widely adopted for use in human anaesthesia, due to its profound analgesia, amnesia and excellent safety profile [1]. However, patients reported a variety of unpleasant symptoms including agitation and vivid dreams, when recovering from anaesthetic, which led to ketamine being withdrawn from mainstream anaesthetic use. The very psychedelic effects, which limited its use in anaesthetics, have made it attractive to recreational drug users. Since ketamine first appeared on the ‘rave’ scene in the 1990s, its use amongst recreational drug abusers has grown significantly [2]. The 2017 global drugs survey found a major rise in the use of psychedelics, with 26% of UK respondents admitted using ketamine in the past 12 months compared to 20% in 2014 [3]. In Hong Kong, ketamine has been the most common abused substance in teenagers since 2005 [4].
Ketamine is obtained in powder form and administered through snorting or inhaling. Recreational users report varying sensations of weightlessness, colourful visions, hallucinations and euphoria, with some users experiencing a ‘near death experience’ of travelling through a dark tunnel into a light at high speed with buzzing, whistling sounds [5]. Tolerance rapidly develops, forcing users to seek increasingly larger doses to experience the same effects [1]. Ketamine is metabolised by the hepatic microsomal enzymes to norketamine, which is then hydroxylated to dehydronorketamine and then conjugated with glucuronate before being excreted in the urine [6]. In 2007, two case series from Hong Kong and Canada described severe lower urinary symptoms (LUTS), haematuria and bladder pain in young patients who had used ketamine recreationally [7, 8]. Since then, published evidence has grown, which have confirmed the emergence of ‘ketamine cystitis’ (KC)—a condition characterised by small contracted inflamed bladder with frequent upper urinary tract involvement, which develops in 20–30% patients abusing ketamine [9,10,11,12,13,14,15,16,17,18,19, 20•, 21••, 22•]. Although there is a growing need for urology services to manage these patients, there are no evidence-based recommendations for investigations or treatment of KC, due to the lack of large prospective clinical studies. Here, we review the existing literature, regarding the urological complications of chronic ketamine use, our current understanding of its pathophysiology and treatment.
Epidemiology
Ketamine is a controlled drug in many countries. In the UK, it became a class C drug in 2006. In Taiwan, it has been a schedule III drug since 2002 [23••]. The epidemiology of KC was studied in a survey of 3806 participants from the dance, music and clubbing scene in the UK [24]; 1947 (51%) reported ‘ever use’ of ketamine, whilst 1258 (34%) had used ketamine in the past year. The mean age was 23.5 (SD 5.8) years and 70.3% were male; 27% of participants reported at least one urinary symptom; 31% reported using 0.125 g or less during a typical session (low user), 35% reported using 0.25 or 0.5 g (medium) and 34% 1 g (high) or more. With regard to frequency, users were divided into low (1–4 days/month, 70% of subjects), medium (5–8 days/month, 16%) and high (≥ 9 days/month, equating to more than twice weekly, 13.4%). Muetzelfeldt found that 20% of frequent (> 4 times per week) users, 6.7% of infrequent (two times per month to four times per week) users and 13.3% of ex-users reported ‘cystitis or bladder problems’ [25]. Thus, infrequent users may have mild ‘cystitis’ type symptoms, but frequent usage with larger doses may result in irreversible damage with significant symptoms.
In a different population to the UK study, Tam et al. reported that of the 318 patients that self-registered for assessment of their urinary symptoms related to ketamine use, 55% were female. Mean age was 24.4 (SD 3.1) years and patients had used ketamine for a mean (SD) period of 81 (36) months. The mean (SD) ketamine use per week was 18.5 (15.8) g [21••]. Ketamine users tend to be polydrug users, with most common associated drugs being methamphetamine, cocaine and cannabis. Most users also admitted to consuming alcohol at the same time [17, 19, 21••, 24]. Despite the polydrug use, the symptoms from chronic ketamine use appear to be due to ketamine itself rather than an adulterant [7, 18]. Urinary symptoms suggestive of KC have also been reported after use of ketamine for chronic pain [26,27,28]. The ketamine doses were in the range of 50 mg QDS to 200 mg five times a day, which is less than the amount typically used by recreational ketamine users, but duration of ketamine use was in months, implying chronic use. In all except one case (where symptoms persisted despite stopping ketamine, ketamine use duration was 3 years), symptoms improved/resolved following stopping/lowering dose of ketamine. Ketamine also shows promise as a novel treatment for depression. However, there have been no reports of KC when ketamine has been used intermittently in short spells in subanaesthetic doses for this indication (typically 0.5 mg/kg iv repeated every few days for a finite number of sessions) [29]. Therefore, whilst there is clearly a relationship between dose, frequency and duration of ketamine use to the emergence of symptoms, there may be unidentified factors, such as individual susceptibility that determine the development of KC.
Presentation
Patients with KC present with dysuria, bladder pain and severe urinary frequency with urgency. Some patients also have gross haematuria. The critical amount and duration of ketamine exposure that results in symptoms is not known. Tsai et al. found that patients developed symptoms sometimes as early as 1 month after starting the drug but severe symptoms was obvious at 1 year [15]. A study of community dwelling adolescents using ketamine found that usage of three or more times a week for at least 2 years produced measurable bothersome symptoms [17]. In addition to bladder dysfunction, patients can also present with unilateral or bilateral hydronephrosis with or without renal impairment [9, 10, 12, 15,16,17, 19, 20, 21••, 22, 30, 31, 32•]. Up to 7% patients in some case series presented with impaired renal function due to hydronephrosis [9, 12, 19, 21••]. These patients may require upper tract decompression or even dialysis to stabilise their renal function [9, 12, 19, 32•]. Wu suggested stratifying patients with KC into three clinical stages, according to the severity of disease, dose and duration of ketamine abuse. Patients were grouped into stage I (inflammatory stage), stage II (initial bladder fibrosis stage) and stage III (end stage fibrosis, contracture stage). Patients had been using ketamine at < 0.5 g/week for < 2 years (stage I), 0.5–2 g/week for 2-4 years (stage II) and > 2 g/week for > 4 years(stage III disease) [22•]. They found that advanced stages did not always manifest as increasing LUTS severity, indicating that symptoms may not reflect disease progression. However, patients were more likely to have upper tract involvement with worsening renal function in stage III.
Pathophysiology
The underlying pathogenesis of destruction of urinary tract from chronic ketamine use is not clear. Several mechanisms have been proposed based on data from animal models and in vitro studies using human bladder tissue.
Direct Cytotoxicity
High concentrations of ketamine and its metabolites in urine might cause direct toxic effects on the bladder cells [9]. Near total loss of urothelium was seen in a cystectomy specimen of a patient with KC, but the urachal epithelium which was not exposed to urine remained healthy, implicating direct contact with ketamine metabolites was necessary for effect [33]. The rationale for direct cytotoxicity is based on the histopathological findings of ulcerated bladder urothelium and interstitial fibrosis [23]. Wai et al. suggested that hydroquinone, which is one possible metabolite of ketamine, could directly fragment DNA and chromosome in cells [34]. Baker et al. showed that the effect of ketamine on bladder tissue was not mediated by NMDA receptor antagonism, but direct cytotoxicity by activating the intrinsic apoptotic pathway [35].
Bladder Barrier Dysfunction
E-cadherin is a cell adhesion molecule and loss of E-cadherin expression is associated with bladder inflammatory diseases with bladder barrier dysfunction, such as interstitial cystitis/bladder pain syndrome (IC/BPS) [36]. Lee et al. found decreased E-cadherin and increased apoptosis in KC and IC/BPS bladders, but these were more severe in KC bladder tissues [30]. Gu found urinary nitric oxide and antiproliferative factor levels were increased in ketamine-treated rats within the first 30 h after administration [37]. The investigators concluded that ketamine, or its urinary metabolites, disrupted the proliferation of bladder epithelial cells, resulting in defective bladder epithelial barrier. However, it is possible, that the converse is true, i.e., bladder barrier dysfunction could be the result of bladder inflammation.
Neurogenic Inflammation and Abnormal Neurotransmission
Nerve hyperplasia is a unique finding in histopathological examination of bladder tissue from KC. This is likely to account for the extreme pain experienced by KC patients. Urothelial damage was a notable feature of all ketamine cystitis specimens and where urothelium remained, increased nerve growth factor (NGFR) expression was observed. This would indicate that the development of pain in ketamine cystitis is mediated through a specific neurogenic mechanism that may also implicate the urothelium [38]. Meng et al. reported enhanced noncholinergic contractions and P2X1 receptor expression in the ketamine bladder indicating that dysregulation of purinergic neurotransmission may underlie detrusor overactivity in cases of KC [39].
Immunological Reaction
Bladder mucosa of KC patients is infiltrated by mast cells and eosinophils, suggesting an immunological basis to the pathogenesis [7, 23, 40]. In a study of IgE, patients with KC had higher serum IgE than patients with IC/BPS, acute bacterial cystitis or controls. Serum IgE and the severity of eosinophil infiltration were associated with bladder pain severity and small maximal bladder capacity. KC patients who stopped ketamine lowered their serum IgE levels whilst the levels remained high in patients who did not stop ketamine. Thus, IgE-mediated inflammation appears to play a role, although further immunohistochemical staining for IgE in bladder tissue is necessary to prove this that hypersensitivity contributes to the pathogenesis [41]. It is likely that KC as a pathological entity is distinct from what has been described as classical eosinophilic cystitis [7].
Nitric Oxide Synthase (NOS) and the Cyclooxygenase (COX) Pathway
iNOS is the enzyme responsible for inflammation. This is not expressed in resting cells and is induced by cytokines. Overexpression of iNOS has been found in rat bladders with KC [37, 42]. Ketamine could stimulate neurotransmitters to induce the NOS-COX pathway, which could play a role in the pathogenesis of KC.
It is likely that a combination of the above mechanisms leads to the key pathophysiological change in KC which is a severe bladder inflammation. Chronic use causes gradual replacement of elastic connective tissue and muscle fibres with fibrosis in the bladder, as shown in animal models, which could be epithelial to mesenchymal transformation mediated by transforming growth factor β [43, 44]. A small contracted bladder, with varying degrees of inflammation, lead to the typical symptoms of KC.
Pathogenesis of Upper Tract Damage
Although the bladder is the primary site affected in KC, with toxic effects possibly accentuated by the longer contact time, the upper tracts can be involved either concurrently or separately, through the same or several other mechanisms.
-
Development of a small shrunken poorly compliant bladder, with or without vesicoureteric reflux, results in impaired drainage from the kidney [9, 15, 19, 32•].
-
Ureteric wall can also be directly involved in an inflammatory process similar to that in the bladder, as evidenced by wall thickness and enhancement of ureteric wall on CT Urogram suggesting transmural inflammation [16, 20•]. This could then lead to development of ureteric strictures or ureterovesical junction obstruction [9, 14, 16, 20•, 32•]
-
A third mechanism is the development of acute papillary necrosis, which could be due to irreversible damage to the medullary papillary interstitial cells by ketamine [9].
-
A single case has been reported, of obstruction of both pelvi-calyceal systems with gelatinous debris which was confirmed to be ketamine metabolites and cannabinoids [12].
Ketamine-induced kidney damage has also been shown in animal models. Yeung showed mononuclear cell infiltration into murine ureter and kidney including glomeruli and the blood vessels, suggesting that a chronic interstitial inflammatory process is induced by ketamine [45]. Wai found hydropic degeneration of the kidney tubules in mice after 6 weeks of treatment with ketamine. Long-term ketamine administration (28 weeks) led to atresia of glomeruli in the kidney and proteinuria. The damages in both liver and kidney of these mice were more severe when the animals were treated with both ketamine and alcohol [46].
Investigations
The purpose of investigations in ketamine cystitis is to exclude other pathology, confirm the diagnosis and identify complications like upper tract damage. The findings vary and change as the disease progresses. Full blood count and renal and liver function tests (LFT) are mandatory at initial assessment and follow up. Abnormal LFTs with a cholestatic picture has been reported [8, 12, 47]. A urine culture is advisable, as although the urine is generally sterile, a few patients present with sterile pyuria, and/or a concomitant urinary tract infection which is almost always secondary [9, 19, 21••]. Urine cytology is sometimes requested and is almost always negative [19].
A voiding diary is a simple non-invasive technique to assess voided volume and frequency with increased frequency and voided volumes being typically under 200 ml [9, 20•, 48]. Pelvic pain, urgency and frequency questionnaire (PUF) score has been validated and used in evaluating patients with KC [9, 17]. The PUF total score ranges from 0 to 35, with scores ≥ 15 considered significant [9, 21••]. The mean PUF total score in Chu’s series was 25 (SD 7.5) [9]. Similarly, Yee et al. found a significantly higher mean (± SD) PUF total scores in active (23.3 ± 6.7) compared to ex-users (19.8 ± 7.7) P < 0.0005. In comparison, normal subjects with a negative history of ketamine abuse have a mean (SD) PUF total score: 2.1 ± 2.4 [48••].
A severe reduction in the bladder capacity is the most common finding at urodynamics, typically < 150 ml [9, 15]. Other findings include poor compliance with vesicoureteric reflux on voiding cystometrogram (VCMG), detrusor overactivity at very low bladder volumes (as low as 14 ml) and urgency incontinence and ineffective bladder emptying [9, 15, 21••, 49]. Often, patients refuse to have urodynamics, as they are unable to tolerate the pain [15, 19, 22•].
Upper tract imaging is best done with an ultrasound in the first instance. Reduced bladder wall volume and bladder wall thickening has been reported by almost all researchers. Some patients were unable to fill their bladder due to the extreme discomfort. Hydronephrosis is found in 8–51% of cases [9, 16, 17, 19, 20, 21••]. Bladder wall calcification has also been reported [21••]. The difference in incidence of upper tract damage could possibly be due to the disease being picked up at different stages in the natural history of the disease. In those with hydronephrosis, CT urography is thought to be the investigation of choice [16, 20•, 50]. Diffuse bladder wall thickening with enhancement, perivesical inflammation and small bladder volume were the commonest findings. There was also involvement of upper tracts with hydronephrosis, ureteral wall thickening and enhancement. Huang also found vesicovaginal fistula in 15% of patients [20•]. Interestingly in their study, they did not find a correlation between upper tract involvement and the severity of cystitis.
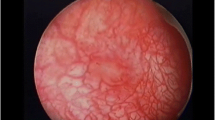
Cystoscopy is a key investigation to exclude other pathology, best done under a general anaesthetic, as it is unlikely to be tolerated with the patient awake. Also, one has to be careful, as significant bleeding and bladder perforation have been reported [19, 51]. Although patients with mild LUTS in the very early stages of the disease have a normal bladder appearance, typical cystoscopic findings in severely symptomatic patients include low bladder capacity (in most series < 300 mls), contracted nondistensible bladder, varying degrees of inflammation and neovascularisation, fragile mucosa with easy tearing on distension and ulceration [7, 9, 19, 32•]. After hydrodistension, there may be glomerulations, diffuse bleeding from the bladder or persistent bleeding from the ulcer [40]. Where performed, ureteroscopic findings show mucosal swelling and oedema, which is distinctly different from patients with IC/BPS who rarely have ureter involvement [41]. Bladder biopsies reveal ulceration and chronic inflammatory changes similar to those seen in IC/BPS and are listed in Table 1. Similarly, ureteric tissue biopsies have showed inflammatory infiltrates through the ureteric wall with periureteric fibrosis [55].
The author and several others have reported high attendance default rates for appointments of upto 59% [18, 19, 48]. In a unique study of users experience of KC, users reported that fear and embarrassment adversely affected medical help seeking, which was often inadequate, with limited assessments, incorrect diagnosis, inappropriate advice and inadequate support [56]. Participants also felt that they were judged, ignored and treated with contempt by most health professionals. This demonstrated a need for a more compassionate and integrated multi-agency approach. Tam et al. reported on a dedicated one stop clinic set up for assessment and management of ketamine users in Hong Kong. Appointments to this clinic were made through a hotline without the need for medical referrals or prior assessment. All patients were evaluated at the first visit by a standard protocol which included the PUF questionnaire, uroflowmetry, urinary tract ultrasound and full history and physical examination by a urologist. Social workers were encouraged to attend. After a diagnosis of ketamine cystitis was made, patients were counselled about their condition and first line treatment was prescribed. All study patients were successfully evaluated at the first visit using this approach [21••].
Management
Behavioural and Medical Treatment
The goal of treatment is to relieve symptoms and prevent deterioration of renal function. Cessation of ketamine intake is the only effective treatment but the effect varies depending on the stage of disease and duration of ketamine use. Patients with near normal bladder capacities who stop ketamine may return to normal bladder function [9, 19]. In the largest UK study, 51% reported improvement in urinary symptoms after cessation of ketamine use [24]. Tam found that status of ex-abuser (stopped ketamine for ≥ 4 weeks before first visit) was the only protective factor against severe symptoms as judged by a PUF score ≥ 28, voided volume ≤ 35 ml or bladder capacity ≤ 60 ml [21••]. In some patients, however, symptoms might continue despite abstinence and in some cases worsen over time [10, 19, 21••, 24, 25]. Cheung reported that 90% of female ex-ketamine users continued to have active urinary symptoms; this was highest in those who had used ketamine for 2 years or more [57]. LUTS in ketamine users is due to both the ongoing inflammatory process and fibrosis that develops subsequently. Cessation may partly resolve the cystitis, but contraction of the bladder wall is irreversible, which may explain the continuation of symptoms in these patients.
Compliance with drug cessation can be difficult, especially with high users with bladder pain, who take ketamine to relieve the pain, but end up in a vicious cycle of pain and more ketamine. According to one report, 79% of ketamine users were dependent on the drug and 54% reported withdrawal symptoms when ketamine was stopped; 26% had a lifetime history of a psychiatric diagnosis apart from substance use. Drug expectancy assessment showed that although ketamine users realised the negative effects of illicit drugs, they continued to use the same drugs or try different combinations in expectation of positive effects [58]. Key elements to aid cessation are avoidance of ketamine using social scene, access to general psychological social support and availability of alternative analgesia [59]. In Bristol, UK, the chronic pain specialists have developed a regime which includes buprenorphine patches with cocodamol and amitriptyline at night. This offered adequate pain control, allowing users to avoid ketamine [18]. The Club Drug clinic in London is another initiative, which specialises in club drugs including ketamine and have a team of specialist addiction doctors and psychologists, nurses, counsellors and peer mentors. There are established pathways for referral to specialties, including urologists [60].
Anti-cholinergic agents have been used to treat overactive bladder symptoms of KC. However, the overall response rate is poor [9, 15, 19]. This is not entirely unexpected as the urgency of KC might result from non-cholinergic activation, as has been shown by Meng et al. [39]. Moreover, symptoms result from an inflamed contracted bladder where an anti-cholinergic is unlikely to be beneficial. NSAIDS and steroids have also been used, but the response rate is poor [15, 19].
Based on defective glycosaminoglycan (GAG) layer model of KC, treatment with GAG substitution therapy like Elmiron and intravesical hyaluronic acid and chondroitin sulphate has been tried with some benefit [7, 15, 61]. However, the numbers and follow-up are small, and the benefits could have been due to cessation of ketamine. In their prospective series, Yee et al. found that in 8 of 17 patients who completed treatment with intravesical sodium hyaluronate, there was a significant improvement in voided volume for all patients after treatment, and 5 of them could step down their oral medication usage [48].
Intravesical Botulinum Toxin Injections
Botulinum toxin significantly improves pain and frequency in patients with IC/BPS. Based on a similar pathophysiological model, Zeng et al. treated 36 patients with 200 units of Botulinum toxin A combined with bladder hydrodistension and 1 month post-treatment; all patients had marked relief of symptoms [62]. The follow-up is short and these results have not been reproduced in other series [32•]. One reason could be the loss of bladder wall elasticity. Similarly, bladder hydrodistension has produced variable results [15, 19, 22•].
In one retrospective study of stage-based treatment, whilst behavioural treatment, pharmacotherapy and bladder hydrodistension were suitable for patients at stages I–II, surgery was recommended for patients with stage III disease, who had upper tract damage, the longest history and highest dosage of ketamine use. The authors reported significant improvement in voiding parameters and PUF scores after treatment in all patients in the three stages [22•]. A similar four-tiered approach was applied prospectively to patients in Yee’s series, namely anti-inflammatory or anti-cholinergic drugs, opioid analgesics or pregabalin, intravesical hyaluronic acid and hydrodistention and augmentation enterocystoplasty (AE); 69.7% of patients who had first line treatment and 67.7% of patients who had second line treatment symptomatic reported improvement [48].
Reconstructive Surgery
Urinary tract reconstruction is required in severely symptomatic patients who fail initial treatment or to prevent further damage to upper tracts in those with hydronephrosis due to end stage contracted bladders with poor compliance, vesicoureteric reflux or ureteric strictures. Wu proposed surgery when functional bladder capacity is < 100 ml [22•]. Options for surgical reconstruction include augmentation enterocystoplasty (AE) with or without supratrigonal cystectomy and ureteric reimplantation, total cystectomy with orthotopic neobladder, heterotopic neobladder with Mitrofanoff or urinary diversion with or without a cystectomy [9, 19, 31, 32, 63, 64].
Chung et al. reported improved pain scores, bladder capacity and patient perception of bladder condition (PPBC) in 14 patients after AE with or without ureteric reimplantation. Hydronephrosis resolved in all nine cases, where this was present preoperatively and VUR disappeared in five of eight cases [31]. Jhang reported that 28/53 patients that had partial cystectomy with AE had good outcome [64]. However, others have reported poor results [9, 63]. Ng performed AE in four cases but had a poor outcome. Three of four patients experienced further deterioration in renal function due to development of ureteric strictures and sepsis [63]. The factors contributing to this were resumption of ketamine abuse after surgery (in all four cases). It was further postulated that the metabolites excreted in the urine could be reabsorbed by the ileum, resulting in further prolonged exposure to ketamine. They concluded that augmentation may be harmful and suggested that a simpler ileal conduit urinary diversion may be a better option. Chu performed AE in one patient but he had progressive upper damage after operation due to upper ureteric stricture, as he continued to abuse ketamine [9].
Sihra et al. reported their experience of major reconstructive surgery in a tertiary centre in the UK and found an increased rate of complications with 71% having one or more post-operative complication and 30% further subsequent complications [32•]. The higher rate of complications was thought to be related to more extensive inflammatory disease, extending up the ureters retroperitoneally, which required more extensive reconstruction. However, 8/14 (57%) patients did do well, with resolution of pain and remained with normal renal function. They all had surgery for severe LUTS and pain from small contracted bladder. Patients who did not do well had renal impairment or ureteric strictures preoperatively. Although the numbers are small to make any generalisations, there seems to be a trend for better outcomes in a subset of patients, whose upper tracts have not yet been damaged.
Continuation of ketamine following surgery is of major concern, as are issues like compliance with self-catheterisation and follow-up, which can significantly compromise results after surgery. The effect of ketamine on intestinal segments is unknown. Major surgery should therefore only be performed after very careful patient selection, in a multidisciplinary setting.
Conclusions
Ketamine cystitis is now an established health problem worldwide, which poses particular problems in management, not least due to characteristics of the patient population. These patients are less likely to seek help by themselves; hence, there is a need for liaison service or outreach one stop clinics to remove barriers to seeking medical help. This would enable a thorough baseline assessment and patient counselling about harmful effects of chronic ketamine abuse and the central message about ketamine cessation. Treatment plans should be individualised, depending on the stage of the disease. Patients referred to urological services should be managed in a multidisciplinary setting, with pain and substance misuse specialists, as any urological treatment without ketamine cessation is doomed to failure. Having a dedicated care worker to coordinate appointments and to provide ongoing support would be invaluable. Although our understanding of this challenging condition is increasing, more research is required to fully understand the pathogenesis of ketamine cystitis and implications for treatment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Morgan CJ, Curran HV. Independent scientific committee on drugs. Ketamine use: a review. Addiction. 2012;107(1):27–38.
Dalgarno PJ, Shewan D. Illicit use of ketamine in Scotland. J Psychoactive Drugs. 1996;28(2):191–9.
Global drug survey [Online]. 2017 [accessed 2018 January2]; Available from URL: https://www.globaldrugsurvey.com/gds2017-launch/results-released/
Yiu-Cheung C. Acute and chronic toxicity pattern in ketamine abusers in Hong Kong. J Med Toxicol. 2012 Sep;8(3):267–70.
Meng E, Wu ST, Cha TL, Sun GH, Yu DS, Chang SY. A murderer of young bladders: ketamine-associated cystitis. Urol Sci. 2013;24:113–6.
Moore KA, Sklerov J, Levine B et al. Urine concentrations of ketamine and norketamine following illegal consumption. J Anal Toxicol 200;25(7):583–588.
Shahani R, Streutker C, Dickson B, Stewart RJ. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology. 2007;69:810–2.
Chu PS, Kwok SC, Lam KM, et al. ‘Street ketamine’-associated bladder dysfunction: a report of ten cases. Hong Kong Med J. 2007;13(4):311–3.
Chu PS, Ma WK, Wong SC, et al. The destruction of the lower urinary tract by ketamine abuse: a new syndrome? BJU Int. 2008;102:1616–22.
Cottrell AM, Gillatt DA. Ketamine-associated urinary tract pathology: the tip of the iceberg for urologists? Br J Med Surg Urol. 2008;1:136–8.
Colebunders B, Van Erps P. Cystitis due to the use of ketamine as a recreational drug: a case report. J Med Case Rep. 2008;2:219.
Selby NM, Anderson J, Bungay P, Chesterton LJ, Kolhe NV. Obstructive nephropathy and kidney injury associated with ketamine abuse. NDT Plus. 2008;1(5):310–2.
Shahzad S, Antil S, Ferrie B, Ganta S. UP-3.073: dystrophic calcification of urinary bladder associated with ketamine use. Urology. 2009;74:S317.
Chiew YW, Yang CS. Disabling frequent urination in a young adult. Ketamine-associated ulcerative cystitis. Kidney Int. 2009;76(1):123–4.
Tsai TH, Cha TL, Lin CM, Tsao CW, Tang SH, Chuang FP, et al. Ketamine-associated bladder dysfunction. Int J Urol. 2009;16:826–9.
Mason K, Cottrell AM, Corrigan AG, Gillatt DA, Mitchelmore AE. Ketamine-associated lower urinary tract destruction: a new radiological challenge. Clin Radiol. 2010;65:795–800.
Mak SK, Chan MT, Bower WF, et al. Lower urinary tract changes in young adults using ketamine. J Urol. 2011;186(2):610–4.
Wood D, Cottrell A, Baker SC, Southgate J, Harris M, Fulford S, et al. Recreational ketamine: from pleasure to pain. BJU Int. 2011;107:1881–4.
Misra S, Chetwood A, Coker C, Thomas P. Ketamine cystitis: practical considerations in management. Scand J Urol. 2014;48(5):482–8.
Huang LK, Wang JH, Shen SH, et al. Evaluation of the extent of ketamine-induced uropathy: the role of CT urography. Postgrad Med J. 2014;90(1062):185–90. In depth discussion of role of imaging in ketamine cystitis.
Tam YH, Ng CF, Pang KK, et al. One-stop clinic for ketamine-associated uropathy: report on service delivery model, patients' characteristics and non-invasive investigations at baseline by a cross-sectional study in a prospective cohort of 318 teenagers and young adults. BJU Int. 2014;114(5):754–60. Report on service delivery using a one stop clinic for assessment of ketamine cystitis.
Wu P, Wang Q, Huang Z, et al. Clinical staging of ketamine-associated urinary dysfunction: a strategy for assessment and treatment. World J Urol. 2016;34(9):1329–36. Report on treatment based on stage.
Jhang JF, Hsu YH, Kuo HC. Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. Int J Urol. 2015;22(9):816–25. Review article focussing on pathophysiology.
Winstock AR, Mitcheson L, Gillatt DA, Cottrell AM. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int. 2012;110:1762–6.
Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJA, Curran HV. Journey through the K-hole: phenomenological aspects of ketamine use. Drug Alcohol Depend. 2008;95(3):219–29.
Gregoire MC, MacLellan DL, Finley GA. A paediatric case of ketamine-associated cystitis. Urology. 2008;71:1232–3.
Storr TM, Quibell R. Can ketamine prescribed for pain cause damage to the urinary tract? Palliat Med. 2009;23(7):670–2.
Shahzad K, Svec A, Al-Koussayer O, et al. Analgesic ketamine use leading to cystectomy: a case report. Br J Med Surg Urol. 2012;5:188–91.
Andrade C. Ketamine for depression, 1: clinical summary of issues related to efficacy, adverse effects, and mechanism of action. J Clin Psychiatry. 2017;78(4):e415–9.
Lee CL, Jiang YH, Kuo HC. Increased apoptosis and suburothelial inflammation in patients with ketamine-related cystitis: a comparison with non-ulcerative interstitial cystitis and controls. BJU Int. 2013;112(8):1156–62.
Chung SD, Wang CC, Kuo HC. Augmentation enterocystoplasty is effective in relieving refractory ketamine-related bladder pain. Neurourol Urodyn. 2014;33(8):1207–11.
Sihra N, Ockrim J, Wood D. The effects of recreational ketamine cystitis on urinary tract reconstruction—a surgical challenge. BJU Int. 2017 Dec 12; https://doi.org/10.1111/bju.14094. One of the larger series describing reconstructive surgery for ketamine cystitis
Kidger E, Stahlschmidt J, Garthwaite M, Fulford S, Southgate J, Baker SC. A rare urachal cyst in a case of ketamine-induced cystitis provides mechanistic insights. Urology. 2016;90:223.e1–7.
Wai MS, Luan P, Jiang Y, Chan WM, Tsui TY, Tang HC, et al. Long term ketamine and ketamine plus alcohol toxicity—what can we learn from animal models? Mini Rev Med Chem. 2013;13(2):273–9.
Baker SC, Shabir S, Georgopoulos NT, Southgate J. Ketamine-induced apoptosis in normal human urothelial cells: a direct, N-methyl-d-aspartate receptor-independent pathway characterized by mitochondrial stress. Am J Pathol. 2016;186(5):1267–77.
Shie JH, Kuo HC. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int. 2011;108(2 Pt 2):E136–41.
Gu D, Huang J, Yin Y, Shan Z, Zheng S, Wu P. Long-term ketamine abuse induces cystitis in rats by impairing the bladder epithelial barrier. Mol Biol Rep. 2014;41(11):7313–22.
Baker SC, Stahlschmidt J, Oxley J, Hinley J, Eardley I, Marsh F, et al. Nerve hyperplasia: a unique feature of ketamine cystitis. Acta Neuropathol Commun. 2013;1:64.
Meng E, Chang HY, Chang SY, Sun GH, Yu DS, Cha TL. Involvement of purinergic neurotransmission in ketamine induced bladder dysfunction. J Urol. 2011;186(3):1134–41.
Wei YB, Yang JR, Yin Z, Guo Q, Liang BL, Zhou KQ. Genitourinary toxicity of ketamine. Hong Kong Med J. 2013;19(4):341–8.
Jhang JF, Hsu YH, Jiang YH, Kuo HC. Elevated serum IgE may be associated with development of ketamine cystitis. J Urol. 2014;192(4):1249–56.
Chuang SM, Liu KM, Li YL, Jang MY, Lee HH, Wu WJ, et al. Dual involvements of cyclooxygenase and nitric oxide synthase expressions in ketamine-induced ulcerative cystitis in rat bladder. Neurourol Urodyn. 2013;32(8):1137–43.
Tan S, Chan WM, Wai MS, et al. Ketamine effects on the urogenital system—changes in the urinary bladder and sperm motility. Microsc Res Tech. 2011;74(12):1192–8.
Wang J, Chen Y, Gu D, Zhang G, Chen J, Zhao J, et al. Ketamine-induced bladder fibrosis involves epithelial-to-mesenchymal transition mediated by transforming growth factor-β1. Am J Physiol Renal Physiol. 2017;313(4):F961–72.
Yeung LY, Rudd JA, Lam WP, Mak YT, Yew DT. Mice are prone to kidney pathology after prolonged ketamine addiction. Toxicol Lett. 2009;191(2–3):275–8.
Wai MS, Chan WM, Zhang AQ, Wu Y, Yew DT. Long-term ketamine and ketamine plus alcohol treatments produced damages in liver and kidney. Hum Exp Toxicol. 2012;31(9):877–86.
Lo RS, Krishnamoorthy R, Freeman JG, Austin AS. Cholestasis and biliary dilatation associated with chronic ketamine abuse: a case series. Singap Med J. 2011;52(3):e52–5.
Yee CH, Lai PT, Lee WM, et al. Clinical outcome of a prospective case series of patients with ketamine cystitis who underwent standardized treatment protocol. Urology. 2015;86(2):236–43. Outcome of treatment from the largest prospective series of ketamine cystitis.
Lai Y, Wu S, Ni L, Chen Z, Li X, Yang S, et al. Ketamine-associated urinary tract dysfunction: an underrecognized clinical entity. Urol Int. 2012;89(1):93–6.
Middela S, Pearce I. Ketamine-induced vesicopathy: a literature review. Int J Clin Pract. 2011;65(1):27–30.
Raja H, Srirangam SJ, Pillai MK. Ketamine induced bladder dysfunction BJUI [Online] 2011 [accessed 2018 January 3]; Available from URL: http://www.bjui.org/ContentFullItem.aspx?id=629
Oxley JD, Cottrell AM, Adams S, Gillatt D. Ketamine cystitis as a mimic of carcinoma in situ. Histopathology. 2009;55:705–8.
Ho CC, Pezhman H, Praveen S, Goh EH, Lee BC, Zulkifli MZ, et al. Ketamine-associated ulcerative cystitis: a case report and literature review. Malays J Med Sci. 2010;17(2):61–5.
Jalil R, Gupta S. Illicit ketamine and its bladder consequences: is it irreversible? BMJ Case Rep. 2012;2012:bcr2012007244.
Hopcroft SA, Cottrell AM, Mason K, Abrams P, Oxley JD. Ureteric intestinal metaplasia in association with chronic recreational ketamine abuse. J Clin Pathol. 2011;64(6):551–2.
Logan K, Gill P, Shaw C, et al. Users experience of ketamine bladder syndrome (KBS). Neurourol Urodyn. 2015;34(S3):47–8.
Cheung RYK, Chan SSC, Lee JHS, Pang AW, Choy KW, Chung TK. Urinary symptoms and impaired quality of life in female ketamine users: persistence after cessation of use. Hong Kong Med J. 2011;17:267–73.
Chen R, Lee AM, Chan R et al. A study on the cognitive impairment and other harmful effects from ecstasy and ketamine abuse. Narcotics Division, Security Bureau. The Government of the Hong Kong Special Administrative Region. Narcotics Division 2004.
Forster JA, Harrison SC. Ketamine uropathy: rising to the challenges of a new condition. BJU Int. 2012;109(9):1277–8.
Club drug clinic [Online]. 2018 [accessed 2018 January2 ]; Available from URL: http://clubdrugclinic.cnwl.nhs.uk/
Smart C, Kabir M, Pati J. Treatment of ketamine-associated cystitis with chondroitin sulphate. Br J Nurs. 2013;22(18):S4. S6, S8-9
Zeng J, Lai H, Zheng D, Zhong L, Huang Z, Wang S, et al. Effective treatment of ketamine-associated cystitis with botulinum toxin type a injection combined with bladder hydrodistention. J Int Med Res. 2017;45(2):792–7.
Ng CF, Chiu PK, Li ML, et al. Clinical outcomes of augmentation cystoplasty in patients suffering from ketamine-related bladder contractures. Int Urol Nephrol. 2013;45(5):1245–51.
Jhang JF, Birder LA, Chancellor MB, Kuo HC. Patient characteristics for different therapeutic strategies in the management ketamine cystitis. Neurourol Urodyn. 2017;36(3):687–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards.
Additional information
This article is part of the Topical Collection on Overactive Bladder and Lower Urinary Tract Symptoms
Rights and permissions
About this article
Cite this article
Misra, S. Ketamine-Associated Bladder Dysfunction—a Review of the Literature. Curr Bladder Dysfunct Rep 13, 145–152 (2018). https://doi.org/10.1007/s11884-018-0476-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11884-018-0476-1