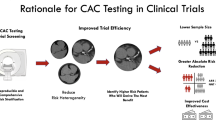
Abstract
Purpose of Review
The risk of incident atherosclerotic cardiovascular disease (ASCVD) in primary prevention is typically lower than in secondary prevention. However, there is a spectrum of risk among individuals undergoing primary prevention with the risk in some individuals approaching those of secondary prevention. We review the clinical conditions wherein the risk in primary prevention is similar to that observed in secondary prevention.
Recent Findings
Among individuals without established ASCVD, coronary artery calcium (CAC) scores ≥ 300 AU are associated with ASCVD event rates similar to secondary prevention populations. CAC score ≥ 1,000 AU are associated with an ASCVD risk seen in very high-risk secondary prevention populations. Interpretation of these observations must however consider differences in the risk reduction strategies.
Summary
Current guidelines dichotomize ASCVD prevention into primary and secondary prevention, but certain primary prevention patients have an ASCVD risk equivalent to that of secondary prevention populations. Identifying higher risk primary prevention populations will allow for better risk mitigation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary prevention in cardiovascular health refers to efforts taken to prevent the first occurrence of atherosclerotic cardiovascular disease (ASCVD) while in secondary prevention the goal is to prevent recurrence of ASCVD events in patients with established ASCVD [1]. According to the 2018 American Heart Association (AHA)/American College of Cardiology (ACC)/multi-society cholesterol guidelines, clinical ASCVD includes a history of myocardial infarction (MI), stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral artery disease (PAD) including aortic aneurysm, all of atherosclerotic origin [2]. Current ACC/AHA and European Society of Cardiology (ESC) guidelines recommend prevention strategies based on the assignment of individuals to primary or secondary prevention, a distinction whose goal is to help better identify higher risk individuals and thereby guide intensity of therapy [2, 3].
Cardiovascular risk assessment in primary prevention is generally performed with risk calculators, such as the ACC/AHA pooled cohort equation (PCE), the Framingham Risk Score, or the European Systematic COronary Risk Evaluation-2 (SCORE-2) model [1, 4, 5]. The PCE uses risk factors including age, sex, blood pressure, total cholesterol, high density lipoprotein (HDL) cholesterol, smoking history, presence of diabetes, and presence of hypertension to calculate the 10-year ASCVD risk in adults ages 40–79 years of age. In addition to these traditional cardiovascular risk factors, the AHA/ACC cholesterol guidelines introduced risk-enhancing factors as a supplement to the PCE to acknowledge that other factors not considered in PCE may affect one’s risk and hence further help improve an individual's risk assessment Example of risk enhancing factors include chronic inflammatory conditions, chronic kidney disease (CKD), an ankle brachial index (ABI) < 0.9 and elevated lipoprotein(a). More recently the AHA published the Predicting Risk of CVD EVENTS (PREVENT) equation as a contemporary sex-specific, race-free model for predicting risk of total cardiovascular disease (CVD), including ASCVD and heart failure, in adults 30–79 years of age [6]. These models are vital tools for individual cardiovascular risk assessment as individuals at highest risk for ASCVD will derive the greatest absolute benefit of preventive strategies such as lipid-lowering therapy and anti-hypertensive medications.
On a population level, primary prevention patients are supposed to be a lower risk population compared to secondary prevention. However, both primary and secondary prevention categories encompass a wide spectrum of individual risk [7]. For instance, certain individuals with elevated coronary artery calcium (CAC) scores, typically considered for primary prevention, exhibit risk levels comparable to those with previous MIs (MI) (i.e. secondary prevention) [8]. Likewise, among patients in the secondary prevention group who effectively manage their risk factors, the likelihood of recurrent cardiovascular events can be equivalent or even lower than some primary prevention individuals [9]. This review highlights important distinctions between primary and secondary prevention populations and describes scenarios in which primary prevention encroaches on secondary prevention.
ASCVD Risk in the Secondary Prevention Population
Randomized control trials such as ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab) and FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) provide a contemporary framework for cardiovascular risk among secondary prevention patients [10, 11]. Both studies included secondary prevention patients with low density lipoprotein cholesterol (LDL-c) ≥ 70 mg/dL or non-HDL-c ≥ 100 mg/dL despite maximum tolerated statin therapy. However, the FOURIER trial included adults 40–85 years of age with a history of MI, nonhemorrhagic stroke, or symptomatic peripheral artery disease (PAD); whereas the ODYSSEY OUTCOMES trial enrolled adults ≥ 40 years old with an acute coronary syndrome (MI or unstable angina) 1–12 months before randomization. Primary endpoints for both studies were a composite of major adverse cardiovascular events (MACE) including cardiovascular death, MI, stroke, unstable angina, or coronary revascularization. Among the placebo treated patients, the primary endpoint occurred 11.3% after median follow up of 2.2 years in the FOURIER trial and 11.1% after a median duration of 2.8 years in the ODYSSEY OUTCOMES trial.
These trials demonstrated further ASCVD risk reduction with the addition of Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors to maximum tolerated statin therapy. Following these and other trials, an update to the ACC/AHA cholesterol guidelines was released in 2018 with specific recommendations for considering the use of non-statin medications in patients with LDL-c ≥ 70 mg/dL or non-HDL-c ≥ 100 mg/dL despite maximum tolerated statin therapy (2). This guideline classified patients with established ASCVD as high-risk and very high-risk, with very high-risk defined by the presence of multiple major ASCVD events or 1 major ASCVD event in addition to multiple high-risk conditions (age ≥ 65, heterozygous familial hypercholesterolemia (FH), prior revascularization, hypertension, diabetes mellitus, chronic kidney disease, current smoking, persistently elevated LDL-c despite maximally tolerated statin therapy and ezetimibe, and, congestive heart failure).
Studies have stratified ASCVD event rates among secondary prevention patients based on these high-risk features. In the ODYSSEY outcomes trial, the MACE rate per 1,000 person-years was 20.4 for high-risk patients and 54.8 for very high-risk patients [12]. In very high-risk patients, those with multiple ASCVD events had a MACE rate of 80.1 compared to 40.2 for those with one event plus high-risk features. A Market Scan database study found MACE rates of 17.0 and 53.1 per 1,000 person-years for high-risk and very high-risk adults, respectively, with rates of 89.8 and 43.1 for those with ≥ 2 events vs. one event plus high-risk conditions [13]. The FOURIER trial showed significantly higher risk among patients with recent MI, residual multivessel coronary disease, or multiple prior MI, with greater absolute risk reduction and lower numbers needed to treat with Evolocumab [14].
These studies demonstrate the variability in risk among secondary prevention patients. To this end, 10-year risk models have been developed, such as the SMART (Secondary Manifestations of ARTerial disease) score to quantify the risk of recurrent vascular events in this population [15]. In the SMART study, patients with various forms of vascular disease (CAD, cerebrovascular disease, PAD) had a median 10-year risk of recurrent MACE of 17%, but there was substantial variability in estimated 10-year risk, ranging from < 10% in 18% of patients and > 30% in 22% of patients (9). Compared to adults with a lower 10-year risk (< 10%), those with a higher 10-year risk (≥ 30%) were older, had a higher prevalence of polyvascular disease, and exhibited more modifiable risk factors that did not meet guideline-recommended targets, including systolic blood pressure, LDL cholesterol levels, smoking status, and physical activity. The authors noted that if all modifiable risk factors were maintained at guideline-recommended levels, half of the patients would have a 10-year risk of less than 10%. In 2022, the SMART score was updated to the SMART2 algorithm, which was recalibrated and externally validated for estimation of 10-year risk residual risk among adults aged 40–80 years with established ASCVD [16].
More recently, a study by Mok et al. developed a universal risk prediction tool in the assessment of both primary and secondary prevention patients [17]. Using data from the Atherosclerosis Risk In Communities (ARIC) study, the model incorporated traditional risk factors (age, diabetes, smoking, hypertension) with cardiac biomarkers (high sensitivity C-reactive protein, high sensitivity troponin T, and N-terminal pro-B type brain natriuretic peptide) resulting in excellent discrimination and calibration irrespective of ASCVD status. Importantly, the same set of predictors were associated with ASCVD events in both primary and secondary prevention populations. Also of note, the 5-year observed risk in the highest quintile of predicted risk in primary prevention patients was higher than the lowest two quintiles of secondary prevention patients. This highlights the overlap in risk among primary prevention patients with poorly controlled risk factors and secondary prevention patients with well controlled risk factors.
ASCVD Risk in the Primary Prevention Population, High Risk Conditions, and ASCVD “Risk Equivalents”
ASCVD Risk Based on Estimated 10-Year Risk Prediction
The ACC/AHA PCE remains the recommended risk calculator for individuals without ASCVD and has also been incorporated into the 2017 ACC/ AHA Hypertension guidelines. The PCE has several limitations: it is only applicable to white and black US adults aged 40–79 years and could over-or underestimate risk in some populations [18,19,20]. For example, it has been reported to underestimate risk in patients living with human immunodeficiency virus infection, rheumatologic/ inflammatory disorders, and low socioeconomic status [21,22,23]. As part of the updated 2018 cholesterol guidelines and the 2019 primary prevention guidelines, a systematic review of studies validating the PCE concluded that it is well calibrated near decision thresholds in large U.S. general populations [24]. Therefore, despite its limitations, the PCE remains an effective tool for ASCVD risk estimation and is an excellent starting point for discussion of ASCVD prevention with patients (1).
Adults with high ASCVD risk based on the PCE (10-year risk > 20%) have been shown to have event rates similar to some lower risk secondary prevention patients. In the Multiethnic Study of Atherosclerosis (MESA) cohort, adults with a 10-year ASCVD risk ≥ 20% (mean risk 32.4%) had an ASCVD event rate of 22.8 per 1,000 person years, which is similar to event rates observed in secondary prevention patients (typically > 20 events per 1,000 person years) [25, 26]. Compared to those with a 10-year ASCVD risk < 20%, adults with estimated risk ≥ 20% were older and had a higher prevalence of hypertension, diabetes, and were more likely to be taking lipid lowering therapy. Similarly, in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study, adults ages 45–79 years without diabetes mellitus who were not taking a statin (LDL-c between 70–189 mg/dL) had an ASCVD incidence rate of 22.2 per 1,000 person-years when PCE 10-year predicted risk was ≥ 20% in the setting of “established risk factors,” which included smoking, hypertension, total cholesterol ≥ 200 mg/dL, or HDL-c < 50 mg/dL for women (< 40 mg/dL for men) [27].
While direct comparisons cannot be drawn between these findings and incidence rates reported in secondary prevention populations, these results suggest that a 10-year risk ≥ 20% measured by the PCE could be associated with a risk of incident ASCVD that is seen in lower risk secondary prevention patients (as opposed to very-high risk patients).
Coronary Artery Calcium
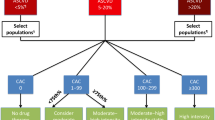
Coronary artery calcium (CAC) is a highly specific marker of coronary atherosclerosis. First described in the early 1990s by Agatston et al., a CAC scoring system was developed using the number, areas, and peak Hounsfield numbers during non-contrast, electrocardiographically gated computed tomography (PMID 2407762). Several cohort studies have demonstrated that CAC scoring is an excellent diagnostic modality for detection of subclinical coronary atherosclerosis and risk stratification in asymptomatic primary prevention patients [28, 29]. Current guidelines advocate for CAC scoring in primary prevention patients with intermediate ASCVD risk to guide decision making regarding initiation of statin therapy, which should be considered if the CAC score exceeds 100 Agatston units (AU) or greater than the 75th percentile of CAC score distribution for age and gender (1).
Over the last 10 years, several studies have attempted to identify CAC score thresholds at which the risk of ASCVD events in asymptomatic primary prevention patients equates to the ASCVD risk of secondary prevention patients. In a study assessing the allocation of statin therapy based on CAC scoring in the MESA cohort, a CAC score ≥ 100 AU was associated with an event rate similar to that seen in secondary prevention patients (26.5 per 1,000 person years) in adults not taking statins (26). A subsequent analysis from the MESA study found that a CAC score of 1,000 or higher corresponds to an annualized 3-point MACE rate (nonfatal MI, nonfatal stroke, and fatal CVD) of 3.4 per 100 person-years which is similar to that observed in a high-risk, stable, treated secondary prevention population (3.3), and surpassing rates seen in lower-risk subgroups from the FOURIER trial [30]. In this study, adults with a CAC score ≥ 1,000 AU were older (mean age 71 years); however, only 30% were on statin therapy and hence how the event rates would compare with secondary prevention populations who will have better preventive treatment is not known. In another analysis, authors evaluated 4,949 adults enrolled in the CONFIRM registry [31] and compared MACE among those with CAC scores exceeding 300 AU to those with established ASCVD. Individuals with CAC scores surpassing 300 AU exhibited MACE rates of MACE (~ 20%), which was comparable to those with established ASCVD (23%), translating to event rates of 73.9 and 77.8 per 1,000 person-years, respectively.
Several studies from the CAC consortium have also examined CAC thresholds that equate to secondary prevention level risk in asymptomatic primary prevention patients. In a cohort of adults with intermediate 10-year ASCVD risk, a CAC score of 781 AU corresponded to an annualized ASCVD mortality rate observed in the placebo arm of the FOURIER trial (0.766 per 100 person-years) (8). Among those with diabetes, CAC scores between 300–375 AU were associated with ASCVD mortality risk equivalent to secondary prevention. A recent study by Razavi et al. identified severe left main coronary artery calcium (≥ 25% burden or a vessel-specific score of ≥ 300) and diabetes as factors that were associated with very-high risk ASCVD mortality rates (fivefold higher crude ASCVD mortality rate) in primary prevention patients with CAC score ≥ 1,000 AU [32].
Findings from studies examining CAC thresholds that equate to secondary prevention level risk are summarized in Table 1. Taken together, these data suggest that a CAC scores ≥ 300 AU in primary prevention patients, especially in the setting of diabetes, are associated with an equivalent risk of MACE as those treated for established ASCVD. Additionally, a CAC score ≥ 1,000 AU may represent a uniquely high-risk primary prevention population with an ASCVD event rate similar to very high-risk secondary prevention patients. However, one important caveat that should be remembered is that the above analyses are all based on observational, epidemiological data. It is likely that individuals with established ASCVD had more intensive risk factor management when compared to those with elevated CAC, and hence if similar treatment strategies were pursued the incidence of MACE may be different. Hence randomized controlled trial data as always will be helpful and welcome.
With increasing evidence demonstrating that primary prevention patients with very high CAC scores have a similar risk profile for ASCVD events to that of patients with established disease, many experts are advocating for consideration of nonstatin therapies such as PCSK9 inhibitors in this population (7). The VESALIUS-CV (Effect of EVolocumab on Major Cardiovascular Events in PatientS at High CArdiovascuLar Risk WithoUt Prior Myocardial Infarction or Stroke; NCT03872401) is an ongoing randomized trial that will assess the effectiveness of PCSK9 inhibitors in this population.
Diabetes
Among the risk factors in the PCE, diabetes mellitus (DM) is often cited as the most impactful cardiovascular (CV) risk factor after age. Diabetes was previously considered an ASCVD “risk equivalent,” based on earlier studies from the Framingham cohort and other studies demonstrating higher ASCVD risk in individuals with DM compared to their non-diabetic counterparts [33, 34]. However, more recent studies challenge the idea of diabetes as a cardiovascular risk equivalent. A meta-analysis of 13 studies with 45,108 participants found that those with DM but no history of MI had a 43% lower likelihood of experiencing an MI compared to those without DM who had a previous MI (summary odds ratio 0.56, 95% CI 0.53–0.60) [35]. Similarly, a large cohort study of 1,586,061 adults aged 30–90 years showed a much lower CHD risk among patients with DM without CHD compared to those with CHD but no DM [36]. Another study on coronary artery calcium (CAC) found that individuals with DM and no detectable CAC had a survival rate similar to those without DM and no CAC (98.8% and 99.4%, respectively, p = 0.5) [37].
These observations demonstrate that DM, by itself, is not necessarily a cardiovascular risk equivalent. However, as described elsewhere in this review, the co-existence of DM with other cardiovascular risk factors and risk markers (i.e. CAC, elevated Lp(a)) can have multiplicative effects on ASCVD risk in both primary and secondary prevention settings [38].
Chronic Kidney Disease (CKD)
Individuals with CKD have a higher risk of ASCVD events and higher mortality rates compared to the general population [39]. Multiple studies have shown that reduced estimated glomerular filtration rate (eGFR) and proteinuria above 300 mg/day are independently linked to an increased risk of cardiovascular events, even in low-risk populations [40,41,42,43,44].
While CKD was once classified as a CAD risk equivalent, recent research suggests that the risk of MI in CKD patients, though high, is not equivalent to those with a prior MI [45]. For example, Tonelli et al. reported the highest unadjusted rate of MI in individuals with a prior MI (18.5 per 1000 person-years, 95% CI 17.4–19.8), followed by those with both diabetes and CKD but no prior MI [46]. The MI rate in patients with diabetes but without prior MI was lower than in those with CKD (5.4 vs 6.9 per 1000 person-years; p < 0.0001). After adjusting for socioeconomic status and comorbidities, individuals with CKD (eGFR < 60 mL/min per 1.73 m2) had a lower rate of first MI than those with diabetes, suggesting age as a contributing factor. Statin use varied significantly among populations in Tonelli's study, posing a limitation for comparing outcomes. Despite CKD's strict definition (eGFR below 45 mL/min per 1.73 m2 with severe proteinuria), the MI rate was not significantly higher than in those with a prior MI (12.4 vs 8.5 per 1000 person-years). Consequently, current guidelines no longer equate CKD's CAD risk to that of a prior MI, reflecting the evolving understanding of CKD's impact on cardiovascular risk.
Primary Severe Hypercholesterolemia/Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is a common monogenic disorder affecting almost 1 in 200 people worldwide [47]. Inherited defects in LDL-c metabolism result in markedly elevated levels of circulating LDL-c, accelerating the progression of atherosclerosis.
In an analysis from 6 US cohorts, patients with FH phenotype (LDL-c ≥ 190 mg/dl) were found to have an increased risk for ASCVD compared to controls (LDL-c < 130 mg/dl) [48]. The ASCVD event rates in those with FH over the age of 60 years are comparable to ASCVD event rates seen in secondary prevention populations (22.9 and 27.2 per 1,000 person years in men ages 60–70 years and 70–79 years, respectively).
The ASCVD risk in FH patients can be modified by genetic testing for known pathogenic variants, namely LDL-c receptor, APOB (apolipoprotein B), and PCSK9 [49]. In one study, compared to patients with LDL-c ≤ 130 mg/dL and no FH mutation, patients with LDL-c cholesterol ≥ 190 mg/dl and no FH mutation had a sixfold higher risk for CAD, whereas those with both LDL-c cholesterol ≥ 190 mg/dl and an FH mutation demonstrated a 22-fold increased risk (OR 22.3; 95% CI: 10.7—53.2) [50]. This suggests that genetic testing can have a role in personalized and tailored risk assessment in FH patients, allowing identification of a subset of FH patients whose ASCVD risk may be comparable to secondary prevention patients.
CAC scoring in FH patients can also help identify higher-risk FH individuals. In a prospective study of 206 adults with heterozygous FH, elevated CAC scores were associated with a similar MACE event rate described in secondary prevention populations [51]. Those with CAC score 1–100 or > 100 had 26.4 and 44.1 events per 1,000 person-years, respectively, after a median follow up of 3.7 years. Of note, this cohort was relatively young (mean age 45 ± 14 years) and the majority were on statin therapy.
Lipoprotein (a)
Lp(a) (Lipoprotein [a]) is a plasma lipoprotein comprised of an LDL particle covalently bound to apolipoprotein (a) [apoA]. Elevated Lp(a) is considered a risk-enhancing feature in the 2018 ACC/AHA Cholesterol guidelines (2).
Currently, there is no Lp(a) threshold that equates to secondary prevention level ASCVD risk. However, in the presence of other risk factors such as DM or elevated CAC score, adults with elevated Lp(a) can have an ASCVD risk near that of secondary prevention populations. In a pooled analysis of 5 US cohorts, patients with DM and Lp(a) levels in the < 50th, 50th- < 75th, 75th—< 90th, and ≥ 90th percentiles had 20.2, 26, 26.7, and 32.1 ASCVD events per 1,000 person years, respectively [52]. Furthermore, an analysis from the MESA and Dallas Heart Study showed that adults with an Lp(a) ≥ 50 mg/dL and a CAC score ≥ 100 had a > 20% cumulative ASCVD incidence over 10 years, which approaches the rate of events in secondary prevention populations [53].
Conclusion
Over the past decade, there has been a proliferation of pharmacological choices aimed at reducing the risk of ASCVD, encompassing innovative lipid-lowering drugs, medications for hypertension, antithrombotic agents, and treatments for diabetes. However, alongside this expanded array of preventive measures for cardiovascular health come increased expenses, polypharmacy with the potential for drug interactions, and potential adverse effects. Consequently, the crucial task in contemporary clinical practice is to identify those individuals who will derive the greatest risk reduction from more aggressive preventive treatments.
Current guidelines endorse different risk reduction strategies for primary and secondary prevention, which is based on previous observations indicating that a history of ASCVD events is associated with greater risk of future ASCVD events than any combination of traditional CVD risk factors in individuals without a documented history of ASCVD. However, substantial evidence over the last few years now suggests that risk assessment is much more nuanced with substantial overlap in risk among certain primary and secondary prevention populations. The diversity of risk within each category suggests that this binary system may be antiquated and oversimplified, which calls for a more sophisticated and individualized approach to risk assessment. Future guidelines should strongly consider shifting the concept of primary and secondary prevention to a continuous risk spectrum so that treatment strategies are guided instead of defined by the presence of documented ASCVD.
Data Availability
No datasets were generated or analysed during the current study.
References
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–209.
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337.
Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94(1):20–4.
Mulas O, Abruzzese E, Luciano L, Iurlo A, Attolico I, Castagnetti F, et al. The new Systematic Coronary Risk Evaluation (SCORE2 and SCORE2-OP) estimates the risk of arterial occlusive events in chronic myeloid leukemia patients treated with nilotinib or ponatinib. Ann Hematol. 2024;103(2):427–36.
Khan SS, Matsushita K, Sang Y, Ballew SH, Grams ME, Surapaneni A, et al. Development and Validation of the American Heart Association’s PREVENT Equations. Circulation. 2024;149(6):430–49.
Saba PS, Al Kindi S, Nasir K. Redefining Cardiovascular Risk Assessment as a Spectrum: From Binary to Continuous. J Am Coll Cardiol. 2024;83(5):574–6.
Dzaye O, Razavi AC, Michos ED, Mortensen MB, Dardari ZA, Nasir K, et al. Coronary artery calcium scores indicating secondary prevention level risk: Findings from the CAC consortium and FOURIER trial. Atherosclerosis. 2022;347:70–6.
Kaasenbrood L, Boekholdt SM, van der Graaf Y, Ray KK, Peters RJ, Kastelein JJ, et al. Distribution of Estimated 10-Year Risk of Recurrent Vascular Events and Residual Risk in a Secondary Prevention Population. Circulation. 2016;134(19):1419–29.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379(22):2097–107.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713–22.
Roe MT, Li QH, Bhatt DL, Bittner VA, Diaz R, Goodman SG, et al. Risk Categorization Using New American College of Cardiology/American Heart Association Guidelines for Cholesterol Management and Its Relation to Alirocumab Treatment Following Acute Coronary Syndromes. Circulation. 2019;140(19):1578–89.
Colantonio LD, Shannon ED, Orroth KK, Zaha R, Jackson EA, Rosenson RS, et al. Ischemic Event Rates in Very-High-Risk Adults. J Am Coll Cardiol. 2019;74(20):2496–507.
Sabatine MS, De Ferrari GM, Giugliano RP, Huber K, Lewis BS, Ferreira J, et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: Analysis From FOURIER. Circulation. 2018;138(8):756–66.
Dorresteijn JA, Visseren FL, Wassink AM, Gondrie MJ, Steyerberg EW, Ridker PM, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99(12):866–72.
Hageman SHJ, McKay AJ, Ueda P, Gunn LH, Jernberg T, Hagstrom E, et al. Estimation of recurrent atherosclerotic cardiovascular event risk in patients with established cardiovascular disease: the updated SMART2 algorithm. Eur Heart J. 2022;43(18):1715–27.
Mok Y, Dardari Z, Sang Y, Hu X, Bancks MP, Mathews L, et al. Universal Risk Prediction for Individuals With and Without Atherosclerotic Cardiovascular Disease. J Am Coll Cardiol. 2024;83(5):562–73.
Medina-Inojosa JR, Somers VK, Garcia M, Thomas RJ, Allison T, Chaudry R, et al. Performance of the ACC/AHA Pooled Cohort Cardiovascular Risk Equations in Clinical Practice. J Am Coll Cardiol. 2023;82(15):1499–508.
Rana JS, Tabada GH, Solomon MD, Lo JC, Jaffe MG, Sung SH, et al. Accuracy of the Atherosclerotic Cardiovascular Risk Equation in a Large Contemporary. Multiethnic Population J Am Coll Cardiol. 2016;67(18):2118–30.
Damen JA, Pajouheshnia R, Heus P, Moons KGM, Reitsma JB, Scholten R, et al. Performance of the Framingham risk models and pooled cohort equations for predicting 10-year risk of cardiovascular disease: a systematic review and meta-analysis. BMC Med. 2019;17(1):109.
Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, et al. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017;2(2):155–62.
Crowson CS, Gabriel SE, Semb AG, van Riel P, Karpouzas G, Dessein PH, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56(7):1102–10.
Colantonio LD, Richman JS, Carson AP, Lloyd-Jones DM, Howard G, Deng L, et al. Performance of the Atherosclerotic Cardiovascular Disease Pooled Cohort Risk Equations by Social Deprivation Status. J Am Heart Assoc. 2017;6(3).
Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr, Sperling LS, Virani SS, et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019;73(24):3153–67.
Whelton SP, Marshall CH, Cainzos-Achirica M, Dzaye O, Blumenthal RS, Nasir K, et al. Pooled Cohort Equations and the competing risk of cardiovascular disease versus cancer: Multi-Ethnic study of atherosclerosis. Am J Prev Cardiol. 2021;7:100212.
Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129(1):77–86.
Kong N, Sakhuja S, Colantonio LD, Levitan EB, Lloyd-Jones DM, Cushman M, et al. Atherosclerotic cardiovascular disease events among adults with high predicted risk without established risk factors. Am J Prev Cardiol. 2024;17:100612.
Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–8.
McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643–53.
Peng AW, Dardari ZA, Blumenthal RS, Dzaye O, Obisesan OH, Iftekhar Uddin SM, et al. Very High Coronary Artery Calcium (>/=1000) and Association With Cardiovascular Disease Events, Non-Cardiovascular Disease Outcomes, and Mortality: Results From MESA. Circulation. 2021;143(16):1571–83.
Budoff MJ, Kinninger A, Gransar H, Achenbach S, Al-Mallah M, Bax JJ, et al. When Does a Calcium Score Equate to Secondary Prevention?: Insights From the Multinational CONFIRM Registry. JACC Cardiovasc Imaging. 2023;16(9):1181–9.
Razavi AC, Shaw LJ, Berman DS, Budoff MJ, Wong ND, Vaccarino V, et al. Left Main Coronary Artery Calcium and Diabetes Confer Very-High-Risk Equivalence in Coronary Artery Calcium >1,000. JACC Cardiovasc Imaging. 2024.
Kannel WB, McGee DL. Diabetes and cardiovascular disease The Framingham study. JAMA. 1979;241(19):2035–8.
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34.
Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26(2):142–8.
Rana JS, Liu JY, Moffet HH, Jaffe MG, Sidney S, Karter AJ. Ethnic Differences in Risk of Coronary Heart Disease in a Large Contemporary Population. Am J Prev Med. 2016;50(5):637–41.
Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43(9):1663–9.
Swamy S, Noor SM, Mathew RO. Cardiovascular Disease in Diabetes and Chronic Kidney Disease. J Clin Med. 2023;12(22):6984.
Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81.
Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41(1):47–55.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305.
McCullough PA, Jurkovitz CT, Pergola PE, McGill JB, Brown WW, Collins AJ, et al. Independent components of chronic kidney disease as a cardiovascular risk state: results from the Kidney Early Evaluation Program (KEEP). Arch Intern Med. 2007;167(11):1122–9.
Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154(1):12–21.
Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14(11):2919–25.
Deo R, Fyr CL, Fried LF, Newman AB, Harris TB, Angleman S, et al. Kidney dysfunction and fatal cardiovascular disease–an association independent of atherosclerotic events: results from the Health, Aging, and Body Composition (Health ABC) study. Am Heart J. 2008;155(1):62–8.
Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–14.
Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478–90.
Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-Term Risk of Atherosclerotic Cardiovascular Disease in US Adults With the Familial Hypercholesterolemia Phenotype. Circulation. 2016;134(1):9–19.
Sturm AC, Knowles JW, Gidding SS, Ahmad ZS, Ahmed CD, Ballantyne CM, et al. Clinical Genetic Testing for Familial Hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2018;72(6):662–80.
Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol. 2016;67(22):2578–89.
Miname MH, Bittencourt MS, Moraes SR, Alves RIM, Silva PRS, Jannes CE, et al. Coronary Artery Calcium and Cardiovascular Events in Patients With Familial Hypercholesterolemia Receiving Standard Lipid-Lowering Therapy. JACC Cardiovasc Imaging. 2019;12(9):1797–804.
Wong ND, Fan W, Hu X, Ballantyne C, Hoodgeveen RC, Tsai MY, et al. Lipoprotein(a) and Long-Term Cardiovascular Risk in a Multi-Ethnic Pooled Prospective Cohort. J Am Coll Cardiol. 2024;83(16):1511–25.
Mehta A, Vasquez N, Ayers CR, Patel J, Hooda A, Khera A, et al. Independent Association of Lipoprotein(a) and Coronary Artery Calcification With Atherosclerotic Cardiovascular Risk. J Am Coll Cardiol. 2022;79(8):757–68.
Funding
The authors did not receive any funding or support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
M.D. wrote and edited the abstract and main manuscript text. A.K., J.R., and O.O. wrote and edited sections within the manuscript. J.R. prepared Table 1. V.N. edited the abstract and main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
No human or animal subjects were used in this study.
Conflicts of Interest
The authors declare that they have no financial conflicts of interest related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deshotels, M.R., Kotta, P.A., Rico Mesa, J.S. et al. When Does Primary Prevention Encroach on Secondary Prevention?. Curr Atheroscler Rep 26, 511–519 (2024). https://doi.org/10.1007/s11883-024-01227-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-024-01227-1