Abstract
Purpose of Review
In this review, we explore the intriguing and evolving connections between bacterial extracellular membrane nanovesicles (BEMNs) and atherosclerosis development, highlighting the evidence on molecular mechanisms by which BEMNs can promote the athero-inflammatory process that is central to the progression of atherosclerosis.
Recent Findings
Atherosclerosis is a chronic inflammatory disease primarily driven by metabolic and lifestyle factors; however, some studies have suggested that bacterial infections may contribute to the development of both atherogenesis and inflammation in atherosclerotic lesions. In particular, the participation of BEMNs in atherosclerosis pathogenesis has attracted special attention.
Summary
We provide some general insights into how the immune system responds to potential threats such as BEMNs during the development of atherosclerosis. A comprehensive understanding of contribution of BEMNs to atherosclerosis pathogenesis may lead to the development of targeted interventions for the prevention and treatment of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of low-density lipoprotein (LDL) cholesterol, fibrous elements, and calcification in the walls of large and medium-sized arteries. It is the leading cause of many cardiovascular diseases (CVDs), including coronary heart disease, myocardial infarction, and stroke, which pose a huge burden to health and socioeconomic development [1]. The pathogenesis of atherosclerosis is a complex and multifaceted process that involves various cellular and molecular events related to endothelial dysfunction and subendothelial lipid build-up, resulting in chronic inflammation in the arterial intima [2]. Atherosclerosis is primarily driven by metabolic and lifestyle factors, like high levels of blood cholesterol, high blood pressure, smoking, and type 2 diabetes mellitus; however, there is some evidence suggesting that bacteria may contribute to the atherogenesis in atherosclerotic lesions. A direct link between atherosclerosis and atherosclerotic CVD (ASCVD) and infection has been established [3, 4••]. The presence of specific Helicobacter pylori (H. pylori) DNA in atherosclerotic lesions has been demonstrated, providing biological evidence that this infection is a contributing factor to atherosclerosis pathophysiology [5]. Moreover, the contribution of bacterial extracellular membrane nanovesicles (BEMNs) to atherosclerosis pathogenesis attracted special attention. Highly invasive BEMNs are able to penetrate through the vascular endothelial cell (VEC) membrane, inducing inflammation and the development of atherosclerosis [6]. Notably, BEMNs can trigger an inflammatory response independently of maternal bacteria [7] and are able to up-regulate the expression of various cytokines and adhesion molecules, initiating a cascade of inflammatory reactions in the vascular wall [8]. In this review, we explore the intriguing and evolving connections between BEMNs and atherosclerosis development, highlighting the evidence on molecular mechanisms by which BEMNs can promote the athero-inflammatory process that is central to the progression of atherosclerosis. A comprehensive understanding of BEMNs` contribution to atherosclerosis pathogenesis may lead to the development of targeted interventions for the prevention and treatment of the disease.
Involvement of BEMNs in Atherosclerosis
BEMNs are nanosized (20–300 nm in diameter) spherical structures enclosed by a bilayer membrane formed from the mother cell envelope. They are secreted by almost all types of eukaryotic and prokaryotic cells, including both Gram-negative and Gram-positive bacteria [9]. They are produced during all stages of natural bacterial growth both in vivo and in vitro and the ubiquitous production of BEMNs by bacteria is nowadays recognized as a novel bacterial secretion system. Unlike bacterial cells, these membrane-bound nanostructures are incapable of independent replication [10]. BEMNs secreted by bacteria are exceedingly numerous with a cell-to-vesicle ratio of approximately 1:2000 [11]. Due to their small dimensions, BEMNs can carry and deliver a variety of compounds to host tissues in concentrated and protected forms. These compounds include outer membrane proteins, lipopolysaccharides (LPS), phospholipids, peptidoglycan, periplasmic, cytoplasmic and membrane-bound proteins, periplasmic components, nucleic acids (DNA, RNA), pathogen-associated molecular patterns (PAMPs), toxins, ion metabolites, and signaling molecules [12•, 13, 14]. The evidence indicating that BEMNs exert proteolytic damage to VEC membrane to overcome biological barriers and reach the sites not accessible to whole bacteria was reviewed [15]. A recent study has shown that BEMNs can also serve as vehicles for the delivery of virulence factors [9]. They deliver bacterial virulence factors deep into host tissues, promoting and dysregulating the immune response. Pathogenic BEMNs change host cell functions and trigger inflammatory reactions [16]. Therefore, BEMNs are perfect causative agents for a number of infectious and inflammatory diseases, including periodontitis, endocarditis, peptic ulcer disease, cystic fibrosis, gastric cancer, and atherosclerosis among others [15]. Several specific contributions of BEMNs to atherosclerosis are presented in the next subsections. It is important to note that research on BEMNs` contribution to atherosclerosis is ongoing, and the specific contributions may vary depending on the bacterial species and individual host factors.
BEMNs Derived from Helicobacter Pylori
H. pylori bacterium colonizes the gastric mucosa of over 50% of the population around the world [17]. Its association with atherosclerosis represents one of the current paradigms of the disease pathogenesis [4••, 18]. Two most studied virulence factors of H. pylori such as cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) have been shown to influence the disease state [18, 19]. In particular, CagA-positive H. pylori infection contributes to the atherosclerosis progression by promoting atherogenesis and macrophage-derived foam cell formation via the release of CagA-containing BEMNs [18]. The mechanism of CagA action is associated with the inhibition the transcription of cholesterol removal transporters by suppressing the expression of transcription factors such as peroxisome proliferator-activated receptor gamma (PPAR-γ) and liver X receptor alpha (LXR-α). In this way, CagA-associated BEMNs enhance foam cell formation. Another study suggested that H. pylori-derived BEMNs containing CagA can accelerate atherosclerotic lesion formation through endothelium injury. It was demonstrated that these BEMNs activated reactive oxygen species (ROS)/nuclear factor κB (NF-κB) signaling pathway in human VECs, as well as promoted the expressions of proinflammatory cytokines such as interleukin-6 (IL)-6 and tumor necrosis factor-alpha (TNF-α) and, thus, impaired vascular endothelial function [6].
BEMNs Derived from Escherichia Coli
Escherichia coli(E. coli) BEMNs are able to stimulate the inflammatory response in the vascular wall and, thereby, may contribute to ASCVD development. It was shown that E. coli BEMNs enhanced the expression of adhesion molecules and pro-inflammatory cytokines and promoted the leukocyte binding on VECs in vitro [20]. Notably, E. coli BEMNs were much more effective in inducing both intercellular adhesion molecule-1 (ICAM-1) expression and leukocyte adhesion in comparison with free LPS and the Cytolysin A protein, a bacterial virulence factor. Besides, an in vivo study showed that BEMNs from E. coli can strongly upregulate functional cell adhesion molecules through the release of IL-8/C-X-C motif chemokine ligand 1 (CXCL1) from endothelial cells involving NF-κB- and toll-like receptors 4 (TLR4)-dependent mechanisms [21].
BEMNs Derived from Porphyromonas Gingivalis
Pathogenic Porphyromonas gingivalis (P. gingivalis) is not only a dominating cause of periodontitis but it was also found to be implicated in the development other diseases, including atherosclerosis [22]. BEMNs derived from periodontal P. gingivalis have been associated with atherosclerosis [23]. In fact, both P. gingivalis and its derived BEMNs are able to upregulate the expression of chemoattractant proteins, including CXCL1, CXCL2, and CXCL8, and endothelial-leukocyte adhesion molecule such as E-selectin; however, BEMNs are more potent activators of the innate inflammatory response related to atherogenesis [23]. BEMNs of P. gingivalis released from areas of periodontitis into the circulation can deliver virulence factors to the arterial intima and induce or promote foam cell formation in macrophages, and contribute to the development of atherosclerotic plaque [24]. Moreover, noticeable effects of P. gingivalis BEMNs on macrophage inflammatory phenotype, mitochondrial function, inflammasome activation, and pyroptotic cell death have been reported [25]. They stimulated macrophages to release more of TNF-α, IL-1β, IL-18, IL-12p70, IL-6, IL-10, interferon beta (IFN-β), and nitric oxide (NO) compared to maternal bacteria, which released much less of these mediators. P. gingivalis BEMNs induced a metabolism change from oxidative phosphorylation (OXPHOS) to glycolysis in macrophages, decreased mitochondrial oxygen intake, and increased ROS production. Stimulated with P. gingivalis BEMNs, macrophages activate caspase-1, produce a large amounts of lactate dehydrogenase, and initiate a pyroptotic cell death. These effects may have potent implications for their roles in atherosclerosis. These BEMNs are able to stimulate VSMCs differentiation and calcification [26]. Calcification of VSMCs is linked to the atherosclerotic plaque rupture [27].
Furthermore, BEMNs derived from P. gingivalis can permeate into coronary vessels, providing the link between periodontitis and CVD [28]. A study reported that P. gingivalis BEMNs can mediate increased endothelial permeability via a gingipain-dependent mechanism that involves proteolytic cleavage of endothelial adhesion molecule PECAM-1, responsible for maintaining vascular integrity [29]. Gingipains are major virulence factors produced by P. gingivalis. This mechanism significantly drives the risk of CVD. Gingipains` activity can also initiate disturbance of the complement system. They break down the C3, C4, and C5 complement components, as well as exert proteolytic destruction of the CD46 complement regulator, reducing bacterial elimination and increasing inflammation [30]. In particular, complement C5a has been found in atherosclerotic lesions, which acts as a proatherogenic moiety, promoting apoptosis of VECs and VSMCs and inducing the production of the metalloproteases MMP1 and MMP9 in human macrophages [31]. These metalloproteases play a major role in plaque destabilization and rupture. Gingipains, proteases, and LPS from P. gingivalis, which provoke the accumulation of C5a and antimicrobial peptides in atherosclerotic lesions, may putatively contribute to the progression of atherosclerosis [22].
Taken together, these data suggest a robust role that BEMNs play in the pathogenesis of atherosclerosis.
Molecular Attributes and Mechanisms Involved in the Innate Immune Response to BEMNs during the Development of Atherosclerosis
According to the current understanding, the innate immune system serves as the first barrier against microbial pathogens and executes its protection by non-specific immune defense and surveillance by innate immune cells. Innate immunity is essentially characterized by a variety of germline-encoded receptors, i.e., pattern recognition receptors (PRRs) with high affinity to tightly conserved motifs in pathogens, which typically recognize PAMPs, including BEMNs. During atherogenesis, BEMNs admittedly furnish inflammatory stimuli, creating a pro-inflammatory milieu and stimulating immune responses at sites of atherosclerotic lesion formation. The scientific basis of inflammation in atherogenesis, demonstrating that inflammatory processes promote the initiation of atheroma in vascular walls, was discussed in the review of Libby, 2012 [32]. It was found that PRRs are involved in direct host-bacterial interactions through BEMNs [33]. A wide variety of PRRs, including TLRs, G-protein-coupled receptors (GPCRs), and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are expressed on the surface of innate immune system cells, such as macrophages, monocytes, neutrophils, dendritic cells (DCs), natural killer (NK) cells, and mast cells. These cells play key roles in the early stages of atherosclerosis development [34]. The innate immune system exerts its antimicrobial defense function through other entities, including inflammasomes, nuclear factor κappa B (NF-κB), cytokines, chemokines, and mitochondria, which are also activated during the development of atherosclerosis in response to BEMNs. The schematic representation of the innate immune response to BEMNs in atherosclerotic lesions is shown in Fig. 1.
Innate immune system signaling triggered by BEMNs during atherosclerosis developmens. Abbreviations: BEMNs, bacterial extracellular membrane nanovesicles; GPCRs, G-protein-coupled receptors; MyD88, myeloid differentiation factor 88; MtDys, mitochondrial dysfunction; NF-kB, nuclear factor kappa B; NLR, nucleotide-binding oligomerization domain (NOD)-like receptors; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; PRRs, pattern recognition receptors; TLR, toll-like receptors
Toll-like Receptors
TLRs are the major component of the innate immune system, which plays a very important role in the development of the immune response [35]. These receptors are essentially characterized by an extracellular leucine-rich repeat domain, which mediates the recognition of PAMPs, a transmembrane domain in conjunction with its cytosolic or intracellular Toll/IL-1R-like domains necessary for downstream signaling pathways [35]. In accordance to their localization, TLRs are largely divided into two subfamilies, cell surface TLRs and intracellular TLRs. Cell surface TLRs recognize microbial membrane components, such as lipids, lipoproteins, and proteins. For example, TLR2, TLR1, and TLR6 recognize an array of PAMPs including lipoproteins, peptidoglycans, lipotechoic acids, zymosan, mannan, and tGPI-mucin [36]. Intracellular TLRs recognize nucleic acids derived from invading bacterial and viral pathogens [37]. TLR activation is accomplished via the myeloid differentiation factor 88 (MyD88) and the Toll/IL-1 receptor domain-related adaptor protein that induces interferon (TRIF) [38]. Experimental studies have established an important role of MyD88 and TRIF for TLR signaling, linking innate immunity and atherogenesis [39]. The effect of a TRIF deficiency on atherosclerosis in LDL receptor knockout mice showed that MyD88 participates in atherogenesis. Moreover, MyD88 deficiency leads to decreases in plaque size, LDL content, expression of proinflammatory genes, cytokines, and chemokines such as IL-12 and monocyte chemoattractant protein-1 [39]. Besides, MyD88 is involved in signaling of the IL-1 family of receptors and thus implicates TLRs in atherosclerosis. The IL-1 receptor family is the main regulator of both atherogenesis and inflammation, particularly through the activity of proatherogenic IL-1β and IL-18 [40]. In addition, MyD88 and TRIF regulate the stimulation of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) and IL-1β in response to TLR ligands. A study demonstrated that the innate immune system is activated via TLR2/4/MyD88/TRIF/mitogen-activated protein kinases (MAPK)/NF-κB and NLRP3/caspase-1 activation pathways [41].
G-Protein-Coupled Receptors
GPCRs are another diverse family of cell-surface receptors of the innate immune system, which deals with microbial intruders and regulates inflammation and immunity. In particular, GPCRs are responsible for the immune responses of macrophages toward extracellular pathogens [42]. Monocyte accumulation in the atherosclerotic plaque can be promoted by the functional activity CX3CR1, also known as the fractalkine receptor of the GPCR family [43]. Decreased atherosclerosis in CX3CR1 deficient mice confirms the pivotal role of this receptor during atherogenesis [44]. In atherogenesis, monocytes recruited to the site of atherosclerotic lesion formation turn into macrophages, accumulate lipids, and display morphological features of foam cells, which establish the foundation for plaque formation. The central role of macrophages in the initiation of atherosclerotic inflammation has been discussed [45]. Macrophages are professional phagocytes aimed at removing not only small particles and apoptotic cells, but also pathogens from the atherosclerotic tissue via phagocytosis. Moreover, the expression of C3a complement system receptors, members of the rhodopsin-like GPCR family, is significantly increased during inflammation, as demonstrated in human atherosclerotic coronary plaques [46]. The notion of complement as being mainly a host-defense system against microbial pathogens has been expanded markedly in the last decades [47]. An animal study showed that inhibition of C3a diminishes atherosclerosis, in particular, the C3a/C3a receptor axis mediates negative regulation of proinflammatory responses and macrophage polarization toward the anti-inflammatory phenotype [48].
Nucleotide-Binding Oligomerization Domain (NOD)-like Receptors
One class of PRRs, NLRs, are intracellular cytoplasmic sensors of PAMPs, including BEMN-containing PAMPs [49]. The most well-studied NLRs are NOD1 and NOD2, which can detect bacterial peptidoglycans and BEMNs [50]. In particular, NOD2 mediates bacterial clearance in the cytoplasm of myeloid cells and regulates innate immune responses via the detection of peptidoglycan derivatives diaminopimelic acid (DAP) through the recognition of bacterial cell wall fragment, the muramyl dipeptide (MDP) ligand, which is commonly distributed among both Gram positive and Gram negative bacteria [51]. It was demonstrated that NOD2-deficient mice are prone to bacterial infection, including Staphylococcus aureus [52], Chlamydophila pneumonia [53], H. pylori [54], and Bacillus anthracis [55]. This susceptibility is attributable to reduced proinflammatory cytokine production and leukocyte recruitment [55]. MDP of NOD2 was found in the atherosclerotic plaque [56]. Therefore, NOD2 may play some role as an immune regulator in atherosclerosis. Atherosclerosis development can be enhanced by the presence of P. gingivalis infection, and this enhancement is pronounced in ApoE and NOD2 double-deficient mice to a greater extent [57]. When MDP is used to activate NOD2, levels of serum inflammatory cytokines are decreased [57]. The results of these studies suggest that NOD2 exhibits anti-inflammatory and anti-atherogenic effects. Additionally, several, members of the NOD-receptor family, such as NLRP1, NLRP3, and NLRC4 among others, have been reported to operate via inflammasomes, which couple up microbial and endogenous danger signals to caspase-1 activation [58]. The caspase-1 pathway is a major inflammatory pathway. For example, NOD receptors have been described to synergize with NLRP3 sensors that detect a broad range of microbial motifs to activate the NLRP3 inflammasomes [59].
Inflammasomes
Inflammasomes are assembled by PRRs following the recognition of pathogenic microorganisms and BEMNs in the cytosol of host cells, and they activate inflammatory caspases to produce proinflammatory cytokines and generate pyroptosis, an inflammatory form of cell death [60]. The most studied inflammasome, the NLRP3 inflammasome, is the critical component of the innate immune system that governs the activation of the inflammatory caspase-1 pathway and the production of proinflammatory cytokines such as IL-1β and IL-18 in response to microbial infection [61]. A positive correlation of NLRP3 level with the severity of coronary atherosclerosis in patients with acute coronary disease was demonstrated [62]. In atherosclerosis, mature IL-1β induces an inflammatory response in ECs and promotes the accumulation of inflammatory cells in local vascular intima, which often occurs at the initiation of atherosclerosis [63]. Moreover, an experimental study showed that IL-1β plays a crucial role in the progression of bacteria-associated atherosclerosis and IL-1β was regarded as a proatherogenic cytokine [64]. Produced mainly by monocytes/macrophages, IL-18 is critical for the secretion of interferon gamma (INF-γ) and cytokines; it also enhances the cytolytic activity of NK cells, providing an important link between the innate and adaptive immune responses [65]. Several studies using transfected cell lines and NALP3 knockout mice showed that bacterial products, such as peptidoglycans, MDP, and bacterial toxins, can activate the NALP3 inflammasome [66, 67]. Additionally, BEMNs containing Gram-negative bacterial cell wall component LPS can activate caspase-11 with the aid of guanylate binding proteins that are the regulators of BEMN-mediated inflammation [68].
A two-signal model for the NLRP3 inflammasome activation was described [61]. The priming signal (signal 1) is delivered by microbial components or endogenous cytokines, leading to the activation of the NF-κB pathway and consequently to the upregulation of the NLRP3 inflammasome and pro-interleukin-1β (pro-IL-1β). Then, the NLRP3 inflammasome endures posttranslational modifications, which enable its activation. The activating signal (signal 2) is represented by various stimuli including extracellular adenosine triphosphate (ATP), pore-forming toxins, RNA viruses, and particulate matter. Signal 2 is able to directly activate inflammasome assembly. In this way, NLRP3 regulates the pathogen (including BEMNs)-induced inflammation and fundamentally impacts the progression of bacteria-enhanced atherosclerosis [69].
Nuclear Factor κB
Transcription factor NF-κB plays a crucial role in regulating the expression of genes involved in inflammation, immune responses, and cell survival [70]. Activation of NF-κB is a key component of the body's defense mechanisms against microbial infections. Microbial components, such as bacterial endotoxins such as LPS can activate NF-κB through the TLR signaling pathway [71]. At the early stages of atherosclerosis, the activation of NF-κB by microbial components can amplify the inflammatory response within the arterial walls, promoting the development and progression of atherosclerosis [70]. The activation of NF-κB by microbial components can have several effects on the vascular wall: (i) Inflammation: NF-κB activation leads to the production of pro-inflammatory cytokines such as TNF-α and IL-1. These cytokines promote inflammation within the arterial walls, contributing to the progression of atherosclerosis. (ii) Recruitment of immune cells: NF-κB activation also results in the expression of adhesion molecules on the surface of endothelial cells lining the arteries. These molecules help recruit immune cells such as monocytes to the site of injury in the arterial wall. (iii) Foam cell formation: Activated monocytes can infiltrate the arterial wall and differentiate into macrophages. These macrophages can engulf oxidized LDL (oxLDL) and become foam cells, which are a hallmark of atherosclerotic plaques. (iv) Smooth muscle cell proliferation: NF-κB activation can stimulate the proliferation of vascular smooth muscle cells (VSMCs), contributing to the thickening of the arterial wall and the formation of atherosclerotic plaques.
Cytokines
Microbial components such as LPS from Gram-negative bacteria and other PAMPs can activate the innate immune system through monocytes and macrophages and trigger the release of various cytokines during atherosclerosis [72]. Cytokines are a subset of low-molecular weight proteins necessary for cell signaling. Involved in the immune cell activation, these proteins can further exacerbate inflammation and contribute to atherosclerosis [73]. Generally, cytokines can be classified as pro- or anti-atherogenic. Classical atherogenic cytokines include interferon gamma (IFN-γ) and TNF-α. IFN-γ promotes inflammatory response in macrophages, NK cells, and VSMCs and increases oxLDL accumulation and foam cell formation, as reviewed by Leon and Zuckerman, 2005 [74]. TNF-α promotes the expression of adhesion molecules and chemokines on VECs, which are critical in recruiting immune cells to the sites of inflammation within arterial walls [75]. Moreover, several mechanisms were described that support the proatherogenic effects of TNF-α on the endothelium, including its role in oxidative stress, decreasing NO bioavailability, and increasing vascular permeability to circulating blood components and cells [76]. Atherosclerosis progression is associated with the local increase in TNF-α expression in atherosclerotic lesions [77]. Besides, IL-1α and IL-1β are pro-inflammatory cytokines, produced by monocytes and macrophages. Majority of innate immune cells express either IL-1 family cytokines, their receptors, or both, and hence almost all innate immune cells are influenced by IL-1 signaling. The IL-1 family cytokines include a number of proinflammatory cytokines (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ) and one anti-inflammatory cytokine (IL-37) [78]. They are profoundly involved in regulation of both, the innate and the adaptive immune responses. Some experiments in murine models proven proatherogenic properties of IL-1α and IL-1β that are involved into the upregulation of adhesion molecules expression by endothelial cells along with macrophage activation [79]. Together, IL-1RA possesses endogenous anti-inflammatory properties as a potent inhibitor of IL-1 signaling pathways [80]. In atherosclerosis, the production of IL-1β is determined by the activation of the NLRP3 inflammasome, as described above. Also, IL-1β can affect the proliferation and migration of VSMCs [81]. Cells known to express IL-18 include macrophages, VECs, and VSMCs [82]. IL-18 aggravates atherosclerosis by enhancement of an inflammatory response through an IFN-γ–related mechanism [82, 83]. In addition, IL-6, an early and key regulator of inflammation, has been long-identified in human atherosclerotic lesions [84]. It is synthesized by many cells of the arterial wall including macrophages, VSMCs, and VECs. The effects of IL-6 in atherosclerosis have been extensively reviewed [85]. In particular, IL-6, can promote LDL uptake in macrophages and stimulate VECs to secret adhesion molecules.
Chemokines
Microbial components can be recognized by TLR4 on immune cells, including macrophages and dendritic cells. This recognition is part of the innate immune system's response to pathogens. When microbial components activate TLR4, immune cells at the site of infection or injury produce chemokines. Chemokines are a group of small signaling proteins that play a crucial role in immune cell trafficking. Chemokines, which trigger directed chemotaxis of immune cells to the site of infection or tissue damage, include four subfamilies: CXC, CC, CX3C, and XC. When E. coli-derived BEMNs bind to TLR4, it initiates a signaling cascade leading to the activation of NF-κB, recruiting neutrophils via the release of IL-8/CXCL1 from endothelial cells [21]. In the context of atherosclerosis, this means that immune cells like monocytes are attracted to the arterial wall, promoting the inflammatory response. Animal studies demonstrated that CXCL1 drives the development of inflammation early in atherosclerosis. CXCL1 can recruit monocytes and neutrophils to the atherosclerotic plaque via its receptor CXCR2 [86]. CXCL1 supports the development of atherosclerosis by controlling the migration, diffusion, and differentiation of macrophages. Macrophage CXCR2 expression in atherosclerotic lesions is central in the progression of early atherosclerotic lesions, such as fatty streaks [87]. Distinct monocyte subsets also exploit CX3CR1 and CCR5 receptors to accumulate in atherosclerotic lesions [43]. Thus, chemokines and their receptors are the key factors in monocyte recruitment, which are able independently promote atherogenesis.
Mitochondria
The role of mitochondria as an innate immune signaling platform triggered by microbial components in atherosclerosis is an interesting and evolving area of research. The release of microbial components could potentially engage mitochondrial DAMP-mediated immune responses. When cells are stressed or damaged, mitochondria can become dysfunctional, leading to the release of mitochondrial DNA (mtDNA) and mitochondrial reactive oxygen species (mtROS) [88]. MtDNA, can activate PRRs of the innate immune system, and inflammasomes. The actions of PRRs in the development of pathogen-associated atherosclerosis were described in previous subsections. MtDNA has been suggested to act as a mitochondrial danger signal. Such a signal triggers the activation of the NLRP3 inflammasome [89]. Mitochondrial ROS can induce the assembly and activation of the NLRP3 inflammasome [90]. The exposure to toxins delivered by BEMNs is a significant cause of mitochondrial disruption. These nanovesicles from Neisseria gonorrhoeae, E. coli, and Pseudomonas aeruginosa induce mitochondrial apoptosis and activation of the NLRP3 inflammasome [91]. This study showed that BEMN’s toxins trigger inhibition of host protein synthesis, caspase-11-mediated pyroptosis, and activation and release of IL-1β. In atherosclerosis, the endothelial lining of blood vessels can become damaged, allowing the infiltration of lipids and immune cells. These immune cells, when exposed to mitochondrial DAMPs, can become activated, further promoting inflammation within the atherosclerotic plaque. The deterioration of mitochondria leads to a high ROS production, oxidative stress, chronic inflammation, and atherosclerosis progression [92]. Thus, mitochondria can serve as innate immune signaling platforms in atherosclerosis through the release of mtDNA, which can activate innate immune responses in response to microbial components, carried by BEMNs.
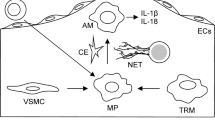
Atherosclerosis and Anti-Microbial Responses of Immune Cells(Fig. 2)
Cellular aspects of infection-enhanced atherosclerosis. Abbreviations: BEMNs, bacterial extracellular membrane nanovesicles; B Cell, B lymphocyte; CD4 + T cell, CD4 T (helper) lymphocyte; CD8 + T cell, CD8 T (cytotoxic) lymphocyte; IFN-γ, interferon gamma, IL, interleukin; M-CSF, macrophage colony-stimulating factor; TNF-α, tumor necrosis factor alpha; VECs, vascular endothelial cells
The vascular endothelium is a heterogeneous monolayer shaped by VECs, which lay the luminal side of all blood vessels, representing the first line of defense for molecules, cells, and pathogens circulating in the bloodstream [93]. It was found that BEMNs contribute the atherosclerotic plaque formation via endothelial damage [6]. At atherosclerotic lesion formation sites, damaged VECs release chemokines that trigger the inflammatory cascade and attract immune cells, such as monocytes. Monocytes are blood-derived mononuclear phagocytic cells that migrate around the body and can deliver rapid innate immune effector responses in response to microbial pathogen infections. Chemokines and their ligands as chemo-attractants that promote monocyte recruitment into arterial walls are explained above. Monocytes, followed by their penetration to the vascular wall, differentiate into macrophages or DCs according to the environmental signals [94]. Their study showed that monocytes are required to be stimulated by granulocyte–macrophage colony-stimulating factor (GM-CSF) together with IL-4, in order to differentiate into DCs. Besides, both IFN-γ and macrophage colony-stimulating factor (M-CSF) switch monocyte differentiation to CD64 + macrophages. Macrophages and DCs play different roles in the immune response. While DCs induce specific immune responses, macrophages and specifically IFN-γ–activated macrophages have powerful antibacterial and antitumoral activities [94]. As a part of the innate immune system, monocytes also influence adaptive immune responses [94].
Furthermore, DCs are the antigen presenting cells (APCs); their properties have been reviewed in detail [95]. Their main function is to deliver pathogen signals to naïve T cells. Besides, DCs have phagocytic activity as immature cells and a great ability to generate cytokines as mature cells. During infection, DCs recognize PAMPs via TLRs and transmit the signal via MyD88. After they associate with a presentable antigen, become activated, and interact with T and B cells to initiate the adaptive immune response. Therefore, DCs operate as intermediates between the innate and adaptive immune responses [96]. The role of vascular DCs in atherosclerosis was vigorously discussed [97].
Macrophages are the most significant cell type of innate immunity and the initial stages of atherosclerosis. In fact, they are multifunctional in atherogenesis [98]. First, macrophages are equipped with an extensive repertoire of PRRs that make them highly efficient at phagocytosis and the production of cytokines and growth factors. Second, macrophages can also function as APCs and orchestrate the immune response. Third, macrophages engulf LDL-cholesterol followed by foam cell formation and the development of atherosclerotic plaque. In the course of atherosclerosis progression, macrophages interact with VECs, VSMCs, T cells, and DCs [99]. Traditionally, activated macrophages have been divided into two groups: M1 and M2 macrophages. M1 are classically activated macrophages by T helper 1 (Th1) cytokines such as TNF-α and IFN-γ and/or by other stimuli such as microbial LPS, participating in the elimination of pathogens, activating the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system, and, in turn, generating ROS [98, 100]. Aerobic glycolysis is also induced in M1 macrophages upon activation, which enhances their proinflammatory activity [101]. M1 macrophages are pro-inflammatory macrophages, which have been found both in human and mouse atherosclerotic plaques, and their production of the pro-inflammatory mediators is believed to maintain local inflammation and the degradation of extracellular matrix components, leading to atherosclerosis progression [102]. Pro-inflammatory M1 macrophages are usually activated through TLR4 or NF-κB pathways [94]. These are the mechanisms that M1 macrophages use for bacterial killing. The alternatively activated M2 macrophages are induced by Th2 cytokines, such as IL-4, IL-10, IL-13, or IL-1β [103]. They play a preventative role in the progression of the disease since they reduce inflammation and OXPHOS. M2 macrophages show increased ATP production by means of electron transport chain OXPHOS. Also, M2 macrophages have lower levels of glycolysis, higher levels of fatty acid oxidation, and increased arginine metabolism. These M2 mechanisms reduce inflammation, increase lipid degradation, inhibit foam cell formation, and therefore likely slow the progression of atherosclerosis [104].
Promoting pathogen elimination from the host, mast cells interact with various cell types, including T-cells [105], macrophages [106], neutrophils [107], DCs [108], SMCs [109], and ECs [110]. These cells mount innate immune responses and play critical roles in the initiation of atherosclerosis. Mast cells are able to sense pathogens through the expression of TLRs and specific Fc receptors. The stimulation of rodent mast cells by LPS via TLR4 induced cytokine production without their degranulation [111]. Also, mast cells can be activated through TLRs with subsequent degranulation. The stimulation through TLR2 by peptidoglycan lead to both degranulation and secretion of cytokines [111]. Owing to the upregulation of FcγRI and FcϵRI receptors, mast cells can bind both IgG and IgE immunoglobulins and become sensitized to host antigens [112]. It was shown that stimulation of TLRs and Fc receptors can have synergistic effects on production of inflammatory cytokines by mast cells, augmenting cytokine transcription via their joint action resulting in the increase in activity of MAPKs [113]. In the arterial intima, at the atherogenesis sites, mast cells are activated to degranulate, and in this way induced to release a large quantity of preformed inflammatory mediators, such as cytokines, chemokines, histamine, proteases, prostaglandins, and leukotrienes [114]. Mast cells are effector cells that initiate inflammatory responses and have a proatherogenic effect during atherogenesis [114].
T cells are the first cells among others recruited within the atheroma [115]. They are important participants in innate and adaptive immune defense mechanisms safeguarding against pathogens such as viruses and bacteria [116]. Atherosclerotic lesions encompass helper (CD4+) and cytotoxic (CD8+) T cells that respond to peptides from bacterial pathogens [117]. Pathogens such as human immunodeficiency virus and cytomegalovirus were accepted as candidate antigens relevant in atherosclerosis [118]. Such interactions result in the secretion of a large amount of cytokines [119]. CD8+ T cells can control infections, by responding to the following cytokines: IL-2, IL-12, IL-15, and IL-18 that promote CD8+ T cell responses to pathogens in an innate fashion and production of IFN-γ [120].
As a part of adaptive immune response, naïve CD8+ T cells primarily interact through the T cell receptor (TCR) with a specific antigen presented via the major histocompatibility complex class I (MHC I) or human leukocyte antigen on the surface of APCs such as dendritic cells. Activated CD8+ T cells undergo differentiation into effector T cells and clonal expansion. The activation and expansion of T cells is closely regulated to ensure an effective response to infection and preventing immunopathology [121]. Abundant evidence indicates that cytotoxic T cells play central roles in the pathology of atherosclerosis through cytotoxic mechanisms contributing to atheroma development and progression [122]. Atherosclerosis-associated viral antigens are able to activate, reactivate, and differentiate CD8+ T cells to elaborate antigen-specific cytotoxic T cell-mediated responses [123].
In order to develop into effector populations that fight microbial infections, naive CD4+ T cells require to recognize peptide antigens presented by MHC class II molecules on activated APCs. PRR-mediated signaling activates APCs to enhance their expression of MHC class II molecules, co-stimulatory molecules such as CD80 and CD86, and pro-inflammatory cytokines, such as type I interferon, TNF-α, IL-1, IL-6, and IL-12 [124]. Another activation pathway is carried out by means of B7 proteins, which are recognized by co-receptor such as CD28. The expression of B7 proteins on the surface of APCs is provoked by pathogens [125]. Activated CD4+ T cells undergo differentiation into Th subsets. They are generated under the distinct influence of cytokine environment: Th1 (IFN-γ), Th2 (IL-4/IL-5/IL-13), Th17 (IL-17/IL-22), follicular (Tfh) (IL-21), and regulatory Treg (IL-10, TGF-β, IL-35) [126]. Helper function of CD4+ T cells is to promote antibody production by B cells during pathogen exposure [124]. It is important to note that the subset of CD4+ Th1 cells is pro-atherogenic. They promote atherosclerosis development by secretion of pro-inflammatory cytokines, such as IFN-γ, TNF-α, IL-2, IL-3, which sustains chronic inflammation and foam cells formation [127]. Recent review summarized the evidence on the molecular mechanisms by which Th1 cells contribute to atherosclerosis [128]. On the contrary, Treg cells display anti-atherogenic activities by inhibiting ongoing macrophage and T-cell-mediated proinflammatory responses [129], while the role of Th2 cells remains under discussion [117].
During the development of the atherosclerotic lesion, B cells can play both atheroprotective and pro-atherogenic roles by executing their main effector functions, such as antibody production, secretion of cytokines, and antigen presentation/interaction with T cells, as discussed in [130]. These functions depend on the B cell subset and their activation state. Naïve B cells become activated by detecting antigens via B cell receptors (BCRs), resulting in their differentiation into antibody-secreting plasma cells. This process is supported by Tfh cells and results in the release of various classes of immunoglobulins. Apparently, TLRs are also involved in B cell activation and differentiation. The involvement of both BCRs and TLRs leads to quicker induction of B cells. In atherosclerosis, dual TLR and BCR signaling allows B cells to be a part of the innate and adaptive immune responses [130, 131].
Perspective Therapeutic Concepts of Infection-Enhanced Atherosclerosis
Bacteria are known to aggravate atherosclerosis. In this regard, anti-atherosclerotic therapy can be aimed at the immune response caused by pathogenic BEMNs and the elimination of vascular inflammation. For this, several approaches can be suggested, which have been identified in pre-clinical settings, but the efficacy, side effects, and optimal dosage of potential drugs need to be verified in clinical trials.
The interruption of PRR signaling may be a reliable way to alleviate ASCVD. The suppression of TLR signaling using inhibitors can be an important target. For example, MyD88 inhibitors may be advantageous in this regard [132]. Because of the pivotal role of NF-κB dysregulation in atherosclerosis, this signaling pathway can become a highly potential therapeutic target for its treatment [133]. Also, the NLRP3-related pathway is a promising pharmacological target to attenuate ASCVD, reviewed in [134]. Several studies have emphasized potential pharmacological inhibitors in different sites of the NLRP3’s complex signaling cascade, including 1) inhibition of upstream signaling, 2) impediment of the NLRP3 assembly, and 3) neutralization of the inflammatory cytokines that are released when the inflammasome is activated [135,136,137]. The notable role of the complement system in BEMNs-associated atherosclerosis development represents its potential for ASCVD treatment. It was shown that pexelizumab, a humanized monoclonal antibody against C5, significantly reduced rates of acute myocardial infarction [138]. A great deal of evidence suggests that targeting cytokines can be a part of atherosclerosis treatment [139]. For example, an early study using murine models of atherosclerosis proposed that blocking the activity of IL-1 and TNF-α and -β should be considered as a therapeutic option for the disease [140]. Numerous options for targeting chemokines have been investigated experimentally. An in vivo study using a hypercholesterolemic mouse model reported that blocking a chemokine pathway with the CC chemokine antagonist Met-RANTES can diminish the progression of atherosclerosis [141]. As an antagonist for the chemokine receptors CCR5 and CXCR3, TAK-779, can reduce the size of the atherosclerotic lesion and the content of Th1 cells in plaques [142]. Other potential anti-chemokine drugs, which showed anti-atherosclerotic effects, were also identified. Some examples of these drugs are listed in Table 1.
As for the cellular aspect of vascular inflammation, inhibition of macrophage activation can be an effective concept for ASCVD treatment. Animal studies suggested that the therapeutic strategies of switching macrophage phenotypes can aid in plaque regression [149]. In this study, the signal transducer and activator of transcription 3/6 (STAT3/6) altered macrophage M1 phenotype to M2 resulting in atherosclerotic plaque stabilization. Moreover, an independent group of researchers proposed that cholesterol-lowering drugs such as statins and proprotein convertase subtillisin/kexin type 9 (PCSK9) inhibitors are able to reduce atherosclerosis [150]. This might not only be because of the efficacy of these drugs in lipid-lowering but also, they might be playing a role in switching the macrophage phenotype and promoting plaque regression. This concept needs further exploration with advanced technologies under treated conditions. In addition, therapeutic suppression of Th1 cell activity can be a promising strategy to decrease atherosclerosis. This could be achieved by inhibiting the differentiation of naive T cells into Th1 cells or by inhibiting the effector functions of Th1 cells. Blocking the activity of IL-12 or IL-18 can limit the differentiation of Th1 cells [151].
Conclusion Remarks
The investigation into the role of BEMNs in atherosclerosis has shed light on a potential and previously overlooked trigger in its complex pathogenesis. Through our comprehensive analysis of the available literature, we have summarized compelling evidence supporting the involvement of these nanovesicles in the atherosclerotic process. The studies presented in this review underscore the intricate interplay between bacterial components such as BEMNs and the formation of atherosclerotic lesions. Several promising therapeutic concepts for infection-enhanced atherosclerosis have been suggested that can be tested in human clinical trials.
Future Perspectives
While the link between microbial components and atherosclerosis is not fully established, it is an interesting avenue for further exploration in understanding the disease's complex pathophysiology. In this regard, it is worth exploring whether pathogenic microbes that enter a cell are capable of activating the inflammasome not via PAMPs, but through modified cellular metabolism and mitochondrial activity. There is an example that indicates this possibility. An in vivo study revealed that the NLRP3 inflammasome mediates innate immunity to the influenza A virus by identifying its RNA [152]. This was established to be a result of the influenza virus M2 protein action, a proton-selective ion channel, recognized to efficiently reduce the mitochondrial membrane potential [153]. Mitochondrial membrane potential is crucial for mitochondrial antiviral signaling (MAVS)-mediated antiviral signaling [154] and thus the physiological function of mitochondria is implicated in innate antiviral immunity. High levels of mtROS provoke oligomerization of MAVS with subsequent activation of NF-κB to regulate host defence and inflammation [155].
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cardiovascular diseases (CVDs) World Health Organization 2021; WHO: Geneva, Switzerland. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 03 Nov 2023.
Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. https://doi.org/10.1083/jcb.201412052.
Ford PJ, Yamazaki K, Seymour GJ. Cardiovascular and oral disease interactions: what is the evidence? Prim Dent Care. 2007;14:59–66. https://doi.org/10.1308/135576107780556806.
Lusta KA, Poznyak AV, Sukhorukov VN, Eremin II, Nadelyaeva II, Orekhov AN. Hypotheses on Atherogenesis Triggering: Does the Infectious Nature of Atherosclerosis Development Have a Substruction? Cells. 2023;12:707. https://doi.org/10.3390/cells12050707. (This paper examined current hypotheses for the triggering of atherogenesis, with special focus on the contribution of bacterial and viral infections to the pathogenesis of atherosclerosis and cardiovascular disease. The reviewed studies emphasized the intricate relationship between bacterial components such as BEMNs and the formation of atherosclerotic lesions.).
Qiang L, Hu J, Tian M, Li Y, Ren C, Deng Y, Jiang Y. Extracellular vesicles from helicobacter pylori-infected cells and helicobacter pylori outer membrane vesicles in atherosclerosis. Helicobacter. 2022;27:e12877. https://doi.org/10.1111/hel.12877.
Wang N, Zhou F, Chen C, Luo H, Guo J, Wang W, Yang J, Li L. Role of Outer Membrane Vesicles From Helicobacter pylori in Atherosclerosis. Front Cell Dev Biol. 2021;9:673993. https://doi.org/10.3389/fcell.2021.673993.
Yu Y-J, Wang X-H, Fan G-C. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol Sin. 2018;39:514–33. https://doi.org/10.1038/aps.2017.82.
Ho M-H, Guo Z-M, Chunga J, Goodwin JS, Xie H. Characterization of Innate Immune Responses of Human Endothelial Cells Induced by Porphyromonas gingivalis and Their Derived Outer Membrane Vesicles. Front Cell Infect Microbiol. 2016;6:139. https://doi.org/10.3389/fcimb.2016.00139.
Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. https://doi.org/10.1016/j.semcdb.2015.02.006.
Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–87. https://doi.org/10.1038/nri3837.
Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Chen Y-Y, Singleton W, Gause KT, Yan Y, Caruso F, Reynolds EC. Differential Responses of Pattern Recognition Receptors to Outer Membrane Vesicles of Three Periodontal Pathogens. PLoS ONE. 2016;11:e0151967. https://doi.org/10.1371/journal.pone.0151967.
Tarashi S, Zamani MS, Omrani MD, Fateh A, Moshiri A, Saedisomeolia A, Siadat SD, Kubow S. Commensal and Pathogenic Bacterial-Derived Extracellular Vesicles in Host-Bacterial and Interbacterial Dialogues: Two Sides of the Same Coin. J Immunol Res. 2022;2022:8092170. https://doi.org/10.1155/2022/8092170. (Both the protective and harmful roles of commensal and pathogenic BEMNs in host-bacterial and interbacterial interactions have been explored. The ability of BEMNs to pass through epithelial, endothelial, and blood-brain barriers highlights the potential advantages of these delivery molecules in the targeted therapy of various diseases, atherosclerosis including.).
Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–19. https://doi.org/10.1038/nrmicro3525.
Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology. 2014;160:2109–21. https://doi.org/10.1099/mic.0.079400-0.
Lusta KA, Poznyak AV, Litvinova L, Poggio P, Orekhov AN, Melnichenko AA. Involvement of Bacterial Extracellular Membrane Nanovesicles in Infectious Diseases and Their Application in Medicine. Pharmaceutics. 2022;14:2597. https://doi.org/10.3390/pharmaceutics14122597.
Peng Y, Yin S, Wang M. Extracellular vesicles of bacteria as potential targets for immune interventions. Hum Vaccin Immunother. 2020;17:897–903 https://doi.org/10.1080/21645515.2020.1799667.
Matysiak-Budnik T, Mégraud F. Epidemiology of Helicobacter pylori infection with special reference to professional risk. J Physiol Pharmacol. 1997;48(Suppl 4):3–17.
Yang S, Xia Y-P, Luo X-Y, Chen S-L, Li B-W, Ye Z-M, Chen S-C, Mao L, Jin H-J, Li Y-N, Hu B. Exosomal CagA derived from Helicobacter pylori-infected gastric epithelial cells induces macrophage foam cell formation and promotes atherosclerosis. J Mol Cell Cardiol. 2019;135:40–51. https://doi.org/10.1016/j.yjmcc.2019.07.011.
Tobin NP, Henehan GT, Murphy RP, Atherton JC, Guinan AF, Kerrigan SW, Cox D, Cahill PA, Cummins PM. Helicobacter pylori-induced inhibition of vascular endothelial cell functions: a role for VacA-dependent nitric oxide reduction. Am J Physiol Heart Circ Physiol. 2008;295:H1403–13. https://doi.org/10.1152/ajpheart.00240.2008.
Kim JH, Yoon YJ, Lee J, Choi E-J, Yi N, Park K-S, Park J, Lötvall J, Kim Y-K, Gho YS. Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo. PLoS ONE. 2013;8:e59276. https://doi.org/10.1371/journal.pone.0059276.
Lee J, Yoon YJ, Kim JH, Dinh NTH, Go G, Tae S, Park K-S, Park HT, Lee C, Roh T-Y, Di Vizio D, Gho YS. Outer Membrane Vesicles Derived From Escherichia coli Regulate Neutrophil Migration by Induction of Endothelial IL-8. Front Microbiol. 2018;9:2268. https://doi.org/10.3389/fmicb.2018.02268.
Hussain M, Stover CM, Dupont AP. Gingivalis in Periodontal disease and atherosclerosis – scenes of action for antimicrobial peptides and complement. Front Immunol. 2015;6. https://doi.org/10.3389/fimmu.2015.00045.
Ho M-H, Guo Z-M, Chunga J, Goodwin JS, Xie H. Characterization of innate immune responses of human endothelial cells induced by porphyromonas gingivalis and their derived outer membrane vesicles. Front Cell Infect Microbiol. 2016; 6. https://doi.org/10.3389/fcimb.2016.00139.
Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb Pathog. 2003;35:259–67. https://doi.org/10.1016/j.micpath.2003.07.002.
Fleetwood AJ, Lee MKS, Singleton W, Achuthan A, Lee M-C, O’Brien-Simpson NM, Cook AD, Murphy AJ, Dashper SG, Reynolds EC, Hamilton JA. Metabolic Remodeling, Inflammasome Activation, and Pyroptosis in Macrophages Stimulated by Porphyromonas gingivalis and Its Outer Membrane Vesicles. Front Cell Infect Microbiol. 2017;7:351. https://doi.org/10.3389/fcimb.2017.00351.
Yang WW, Guo B, Jia WY, Jia Y. Porphyromonas gingivalis-derived outer membrane vesicles promote calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Open Bio. 2016;6:1310–9. https://doi.org/10.1002/2211-5463.12151.
Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590–600. https://doi.org/10.1093/cvr/cvy010.
Zhang Z, Liu D, Liu S, Zhang S, Pan Y. The Role of Porphyromonas gingivalis Outer Membrane Vesicles in Periodontal Disease and Related Systemic Diseases. Front Cell Infect Microbiol. 2021;10:585917. https://doi.org/10.3389/fcimb.2020.585917.
Farrugia C, Stafford GP, Murdoch C. Porphyromonas gingivalis Outer Membrane Vesicles Increase Vascular Permeability. J Dent Res. 2020;99:1494–501. https://doi.org/10.1177/0022034520943187.
Aleksijević LH, Aleksijević M, Škrlec I, Šram M, Šram M, Talapko J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens. 2022;11:1173. https://doi.org/10.3390/pathogens11101173.
Speidl WS, Kasd SP, Hutter R, Katsaros KM, Kaun C, Bauriedel G, Maurer G, Huber K, Badimon JJ, Wojta J. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J. 2011;25:35–44. https://doi.org/10.1096/fj.10-156083.
Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51. https://doi.org/10.1161/ATVBAHA.108.179705.
Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, Siadat SD. Microbiota-Derived Extracellular Vesicles as New Systemic Regulators. Front Microbiol. 2017; 8.
Chávez-Sánchez L, Espinosa-Luna JE, Chávez-Rueda K, Legorreta-Haquet MV, Montoya-Díaz E, Blanco-Favela F. Innate immune system cells in atherosclerosis. Arch Med Res. 2014;45:1–14. https://doi.org/10.1016/j.arcmed.2013.11.007.
Vijay K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int Immunopharmacol. 2018;59:391–412. https://doi.org/10.1016/j.intimp.2018.03.002.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. https://doi.org/10.1038/ni.1863.
Blasius AL, Beutler B. Intracellular Toll-like Receptors. Immunity. 2010;3:305–15. https://doi.org/10.1016/j.immuni.2010.03.012.
Kawasaki T, Kawai T. Toll-Like Receptor Signaling Pathways. Front Immunol. 2014;5:461. https://doi.org/10.3389/fimmu.2014.00461.
Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res. 2009;50:S340–5. https://doi.org/10.1194/jlr.R800056-JLR200.
González L, Rivera K, Andia ME, Martínez Rodriguez G. The IL-1 Family and Its Role in Atherosclerosis. Int J Mol Sci. 2022;24:17. https://doi.org/10.3390/ijms24010017.
Palová-Jelínková L, Dáňová K, Drašarová H, Dvořák M, Funda DP, Fundová P, Kotrbová-Kozak A, Černá M, Kamanová J, Martin SF, Freudenberg M, Tučková L. Pepsin Digest of Wheat Gliadin Fraction Increases Production of IL-1β via TLR4/MyD88/TRIF/MAPK/NF-κB Signaling Pathway and an NLRP3 Inflammasome Activation. Plos One 2013; 8:e62426 https://doi.org/10.1371/journal.pone.0062426.
Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, Saijo K, Glass CK, Hume DA, Kellie S, Sweet MJ. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;4:5. https://doi.org/10.1186/1745-7580-4-5.
Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. https://doi.org/10.1172/JCI28549.
Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1–/– mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–40. https://doi.org/10.1172/JCI15555.
Chowdhury AS, Tamanna S, Kar K. Role of macrophages in atherosclerosis. Asian J Med Biol Res. 2020;6:366–74. https://doi.org/10.3329/ajmbr.v6i3.49784.
Oksjoki R, Laine P, Helske S, Vehmaan-Kreula P, Mäyränpää MI, Gasque P, Kovanen PT, Pentikäinen MO. Receptors for the anaphylatoxins C3a and C5a are expressed in human atherosclerotic coronary plaques. Atherosclerosis. 2007;195:90–9. https://doi.org/10.1016/j.atherosclerosis.2006.12.016.
Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97. https://doi.org/10.1038/ni.1923.
Wei L-L, Ma N, Wu K-Y, Wang J-X, Diao T-Y, Zhao S-J, Bai L, Liu E, Li Z-F, Zhou W, Chen D, Li K. Protective Role of C3aR (C3a Anaphylatoxin Receptor) Against Atherosclerosis in Atherosclerosis-Prone Mice. Arterioscler Thromb Vasc Biol. 2020;40:2070–83. https://doi.org/10.1161/ATVBAHA.120.314150.
Krishnaswamy JK, Chu T, Eisenbarth SC. Beyond pattern recognition: NOD-like receptors in dendritic cells. Trends Immunol. 2013;34:224–33. https://doi.org/10.1016/j.it.2012.12.003.
Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. https://doi.org/10.1038/nri3565.
Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection *. J Biol Chem. 2003;278:8869–72. https://doi.org/10.1074/jbc.C200651200.
Deshmukh HS, Hamburger JB, Ahn SH, McCafferty DG, Yang SR, Fowler VG. Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun. 2009;77:1376–82. https://doi.org/10.1128/IAI.00940-08.
Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, Doherty TM, Underhill D, Crother TR, Arditi M. Correction: The NOD/RIP2 Pathway Is Essential for Host Defenses Against Chlamydophila pneumoniae Lung Infection. PLOS Pathog. 2009; 5. https://doi.org/10.1371/journal.ppat.1000379.
Rosenstiel P, Hellmig S, Hampe J, Ott S, Till A, Fischbach W, Sahly H, Lucius R, Fölsch UR, Philpott D, Schreiber S. Influence of polymorphisms in the NOD1/CARD4 and NOD2/CARD15 genes on the clinical outcome of Helicobacter pylori infection. Cell Microbiol. 2006;8:1188–98. https://doi.org/10.1111/j.1462-5822.2006.00701.x.
Loving CL, Osorio M, Kim Y-G, Nuñez G, Hughes MA, Merkel TJ. Nod1/Nod2-Mediated Recognition Plays a Critical Role in Induction of Adaptive Immunity to Anthrax after Aerosol Exposure. Infect Immun. 2009;77:4529–37. https://doi.org/10.1128/IAI.00563-09.
Laman JD, Schoneveld AH, Moll FL, van Meurs M, Pasterkamp G. Significance of peptidoglycan, a proinflammatory bacterial antigen in atherosclerotic arteries and its association with vulnerable plaques. Am J Cardiol. 2002;90:119–23. https://doi.org/10.1016/S0002-9149(02)02432-3.
Yuan H, Zelkha S, Burkatovskaya M, Gupte R, Leeman SE, Amar S. Pivotal role of NOD2 in inflammatory processes affecting atherosclerosis and periodontal bone loss. Proc Natl Acad Sci. 2013;110:E5059–68. https://doi.org/10.1073/pnas.1320862110.
Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The Inflammasome: A Caspase-1 Activation Platform Regulating Immune Responses and Disease Pathogenesis. Nat Immunol. 2009;10:241. https://doi.org/10.1038/ni.1703.
Zhong Y, Kinio A, Saleh M. Functions of NOD-like receptors in human diseases. Front Immunol. 2013;4. https://doi.org/10.3389/fimmu.2013.00333.
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20. https://doi.org/10.1038/nri.2016.58.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20:3328. https://doi.org/10.3390/ijms20133328.
Afrasyab A, Qu P, Zhao Y, Peng K, Wang H, Lou D, Niu N, Yuan D. Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart Vessels. 2016;31:1218–29. https://doi.org/10.1007/s00380-015-0723-8.
Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA. Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985;121:394–403.
Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 Receptor Signaling Mediates Atherosclerosis Associated With Bacterial Exposure and/or a High-Fat Diet in a Murine Apolipoprotein E Heterozygote Model. Circulation. 2004;110:1678–85. https://doi.org/10.1161/01.CIR.0000142085.39015.31.
Badimon L. Interleukin-18: a potent pro-inflammatory cytokine in atherosclerosis: EXPERT’S PERSPECTIVE. Cardiovasc Res. 2012;96:172–5. https://doi.org/10.1093/cvr/cvs226.
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. https://doi.org/10.1038/nature04515.
Martinon F, Agostini L, Meylan E, Tschopp J. Identification of Bacterial Muramyl Dipeptide as Activator of the NALP3/Cryopyrin Inflammasome. Curr Biol. 2004;14:1929–34. https://doi.org/10.1016/j.cub.2004.10.027.
Finethy R, Luoma S, Orench-Rivera N, Feeley EM, Haldar AK, Yamamoto M, Kanneganti T, Kuehn MJ, Coers J. Inflammasome Activation by Bacterial Outer Membrane Vesicles Requires Guanylate Binding Proteins. mBio. 2017; 8. https://doi.org/10.1128/mbio.01188-17.
Karasawa T, Takahashi M. Role of NLRP3 Inflammasomes in Atherosclerosis. J Atheroscler Thromb. 2017;24:443–51. https://doi.org/10.5551/jat.RV17001.
Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:1–9. https://doi.org/10.1038/sigtrans.2017.23.
Wan J, Shan Y, Fan Y, Fan C, Chen S, Sun J, Zhu L, Qin L, Yu M, Lin Z. NF-κB inhibition attenuates LPS-induced TLR4 activation in monocyte cells. Mol Med Rep. 2016;14:4505–10. https://doi.org/10.3892/mmr.2016.5825.
Gorabi AM, Kiaie N, Khosrojerdi A, Jamialahmadi T, Al-Rasadi K, Johnston TP, Sahebkar A. Implications for the role of lipopolysaccharide in the development of atherosclerosis. Trends Cardiovasc Med. 2022;32:525–33. https://doi.org/10.1016/j.tcm.2021.08.015.
Ma J, Luo J, Sun Y, Zhao Z. Cytokines associated with immune response in atherosclerosis. Am J Transl Res. 2022;14:6424–44.
Leon MLA, Zuckerman SH. Gamma interferon: a central mediator in atherosclerosis. Inflamm res. 2005;54:395–411. https://doi.org/10.1007/s00011-005-1377-2.
Lee AS, Kim JS, Lee YJ, Kang DG, Lee HS. Anti-TNF-α Activity of Portulaca oleracea in Vascular Endothelial Cells. Int J Mol Sci. 2012;13:5628–44. https://doi.org/10.3390/ijms13055628.
Urschel K, Cicha I. TNF-α in the cardiovascular system: from physiology to therapy. Int J Interferon Cytokine Mediator Res. 2015;7:9–25. https://doi.org/10.2147/IJICMR.S64894.
Fatkhullina AR, Peshkova IO, Koltsova EK. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry (Mosc). 2016;81:1358–70. https://doi.org/10.1134/S0006297916110134.
Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. https://doi.org/10.1111/imr.12621.
Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of Interleukin-1β Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. https://doi.org/10.1161/01.ATV.0000064374.15232.C3.
Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res. 2018;122:1722–40. https://doi.org/10.1161/CIRCRESAHA.118.311362.
Eun SY, Ko YS, Park SW, Chang KC, Kim HJ. IL-1β enhances vascular smooth muscle cell proliferation and migration via P2Y2 receptor-mediated RAGE expression and HMGB1 release. Vascul Pharmacol. 2015;72:108–17. https://doi.org/10.1016/j.vph.2015.04.013.
Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schönbeck U. Expression of Interleukin (IL)-18 and Functional IL-18 Receptor on Human Vascular Endothelial Cells, Smooth Muscle Cells, and Macrophages : Implications for Atherogenesis. J Exp Med. 2002;195:245–57. https://doi.org/10.1084/jem.20011022.
Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 Enhances Atherosclerosis in Apolipoprotein E−/− Mice Through Release of Interferon-γ. Circ Res. 2002;90:e34–8. https://doi.org/10.1161/hh0202.105292.
Kishikawa H, Shimokama T, Watanabe T. Localization of T lymphocytes and macrophages expressing IL-1, IL-2 receptor, IL-6 and TNF in human aortic intima. Role of cell-mediated immunity in human atherogenesis. Vichows Archiv A Pathol Anat. 1993;423:433–42. https://doi.org/10.1007/BF01606532.
Reiss AB, Siegart NM, De Leon J. Interleukin-6 in atherosclerosis: atherogenic or atheroprotective? Clin Lipidol. 2017;12:14–23. https://doi.org/10.1080/17584299.2017.1319787.
Soehnlein O, Drechsler M, Döring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gómez A, Mandl M, Vijayan S, Projahn D, Garlichs CD, Koenen RR, Hristov M, Lutgens E, Zernecke A, Weber C. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–81. https://doi.org/10.1002/emmm.201201717.
Boisvert WA, Rose DM, Johnson KA, Fuentes ME, Lira SA, Curtiss LK, Terkeltaub RA. Up-Regulated Expression of the CXCR2 Ligand KC/GRO-α in Atherosclerotic Lesions Plays a Central Role in Macrophage Accumulation and Lesion Progression. Am J Pathol. 2006;168:1385–95. https://doi.org/10.2353/ajpath.2006.040748.
Ciccarelli G, Conte S, Cimmino G, Maiorano P, Morrione A, Giordano A. Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int J Mol Sci. 2023;24:1086. https://doi.org/10.3390/ijms24021086.
Nakahira K, Haspel JA, Rathinam VAK, Lee S-J, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AMK. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30. https://doi.org/10.1038/ni.1980.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. https://doi.org/10.1038/nature09663.
Deo P, Chow SH, Han M-L, Speir M, Huang C, Schittenhelm RB, Dhital S, Emery J, Li J, Kile BT, Vince JE, Lawlor KE, Naderer T. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat Microbiol. 2020;5:1418–27. https://doi.org/10.1038/s41564-020-0773-2.
Salnikova D, Orekhova V, Grechko A, Starodubova A, Bezsonov E, Popkova T, Orekhov A. Mitochondrial Dysfunction in Vascular Wall Cells and Its Role in Atherosclerosis. Int J Mol Sci. 2021;22:8990. https://doi.org/10.3390/ijms22168990.
Yuan SY, Rigor RR. Regulation of endothelial barrier function. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54117/.
Delneste Y, Charbonnier P, Herbault N, Magistrelli G, Caron G, Bonnefoy J-Y, Jeannin P. Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–50. https://doi.org/10.1182/blood-2002-04-1164.
Mellman I, Steinman RM. Dendritic Cells: Specialized and Regulated Antigen Processing Machines. Cell. 2001;106:255–8. https://doi.org/10.1016/S0092-8674(01)00449-4.
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. https://doi.org/10.1126/science.1178331.
Alberts-Grill N, Denning TL, Rezvan A, Jo H. The role of the vascular dendritic cell network in atherosclerosis. Am J Physiol Cell Physiol. 2013;305:C1–21. https://doi.org/10.1152/ajpcell.00017.2013.
Mehu M, Narasimhulu CA, Singla DK. Inflammatory Cells in Atherosclerosis. Antioxidants (Basel). 2022;11:233. https://doi.org/10.3390/antiox11020233.
Takahashi K, Takeya M, Sakashita N. Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc. 2002;35:179–203. https://doi.org/10.1007/s007950200023.
Zhang S, Kim CC, Batra S, McKerrow JH, Loke P. Delineation of Diverse Macrophage Activation Programs in Response to Intracellular Parasites and Cytokines. PLoS Negl Trop Dis. 2010;4:e648. https://doi.org/10.1371/journal.pntd.0000648.
Galván-Peña S, O’Neill LAJ. Metabolic reprograming in macrophage polarization. Front Immunol 2014;5. https://doi.org/10.3389/fimmu.2014.00420.
Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) Deletion Polarizes Macrophages Toward an Inflammatory Phenotype and Increases Atherosclerosis. Circ Res. 2012;110:416–27. https://doi.org/10.1161/CIRCRESAHA.111.253377.
Wu J, He S, Song Z, Chen S, Lin X, Sun H, Zhou P, Peng Q, Du S, Zheng S, Liu X. Macrophage polarization states in atherosclerosis. Front Immunol, 2023;14, 1185587. https://doi.org/10.3389/fimmu.2023.1185587.
Eshghjoo S, Kim DM, Jayaraman A, Sun Y, Alaniz RC. Macrophage Polarization in Atherosclerosis. Genes (Basel). 2022;13:756. https://doi.org/10.3390/genes13050756.
Orinska Z, Bulanova E, Budagian V, Metz M, Maurer M, Bulfone-Paus S. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood. 2005;106:978–87. https://doi.org/10.1182/blood-2004-07-2656.
Ketavarapu JM, Rodriguez AR, Yu J-J, Cong Y, Murthy AK, Forsthuber TG, Guentzel MN, Klose KE, Berton MT, Arulanandam BP. Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc Natl Acad Sci U S A. 2008;105:9313–8. https://doi.org/10.1073/pnas.0707636105.
Wezel A, Lagraauw HM, van der Velden D, de Jager SCA, Quax PHA, Kuiper J, Bot I. Mast cells mediate neutrophil recruitment during atherosclerotic plaque progression. Atherosclerosis. 2015;241:289–96. https://doi.org/10.1016/j.atherosclerosis.2015.05.028.
Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, Staats HF, Abraham SN. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–42. https://doi.org/10.1016/j.chom.2009.09.004.
Margulis A, Nocka KH, Brennan AM, Deng B, Fleming M, Goldman SJ, Kasaian MT. Mast Cell-Dependent Contraction of Human Airway Smooth Muscle Cell-Containing Collagen Gels: Influence of Cytokines, Matrix Metalloproteases, and Serine Proteases. J Immunol. 2009;183:1739–50. https://doi.org/10.4049/jimmunol.0803951.
Sendo T, Sumimura T, Itoh Y, Goromaru T, Aki K, Yano T, Oike M, Ito Y, Mori S, Nishibori M, Oishi R. Involvement of proteinase-activated receptor-2 in mast cell tryptase-induced barrier dysfunction in bovine aortic endothelial cells. Cell Signal. 2003;15:773–81. https://doi.org/10.1016/s0898-6568(03)00014-7.
Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–9. https://doi.org/10.1172/JCI14704.
Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–52. https://doi.org/10.1038/nri2782.
Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcϵR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–8. https://doi.org/10.1182/blood-2005-06-2271.
Kovanen PT and Bot I. Mast cells in atherosclerotic cardiovascular disease – Activators and actions. Eur J Phar. 2017;816:37–46. https://doi.org/10.1016/j.ejphar.2017.10.013.
Fernandez DM, Rahman AH, Fernandez N, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill C, Wang Z, Remark R, Li JR, Pina C, Faries C, Awad AJ, Moss N, Bjorkegren JLM, Kim-Schulze S, Gnjatic S, Ma’ayan A, Mocco J, Faries P, Merad M, Giannarelli C. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019; 25:1576–1588 https://doi.org/10.1038/s41591-019-0590-4.
Zhang N, Bevan MJ. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity. 2011;35:161–8. https://doi.org/10.1016/j.immuni.2011.07.010.
Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17:387–401. https://doi.org/10.1038/s41569-020-0352-5.
Pothineni NVK, Subramany S, Kuriakose K, Shirazi LF, Romeo F, Shah PK, Mehta JL. Infections, atherosclerosis, and coronary heart disease. Eur Heart J. 2017;38:3195–201. https://doi.org/10.1093/eurheartj/ehx362.
Ammirati E, Moroni F, Magnoni M, Camici PG. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin Exp Immunol. 2015;179:173–87. https://doi.org/10.1111/cei.12477.
Berg RE, Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18:338–43. https://doi.org/10.1016/j.coi.2006.03.010.
Mayer A, Zhang Y, Perelson AS, Wingreen NS. Regulation of T cell expansion by antigen presentation dynamics. Proc Natl Acad Sci. 2019;116:5914–9. https://doi.org/10.1073/pnas.1812800116.
Kyaw T, Peter K, Li Y, Tipping P, Toh B-H, Bobik A. Cytotoxic lymphocytes and atherosclerosis: significance, mechanisms and therapeutic challenges. Br J Pharmacol. 2017;174:3956–72. https://doi.org/10.1111/bph.13845.
Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol. 2016;16:367–77. https://doi.org/10.1038/nri.2016.38.
Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–48. https://doi.org/10.1038/nri3152.
Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3-23. https://doi.org/10.1016/j.jaci.2009.12.980.
Zhu X, Zhu J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int J Mol Sci. 2020;21:8011. https://doi.org/10.3390/ijms21218011.
Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell–APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–26. https://doi.org/10.1172/JCI61758.
Chen J, Xiang X, Nie L, Guo X, Zhang F, Wen C, Xia Y, Mao L. The emerging role of Th1 cells in atherosclerosis and its implications for therapy. Front Immunol. 2023;13:1079668. https://doi.org/10.3389/fimmu.2022.1079668.
Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, Sansbury BE, Corr EM, van Solingen C, Koelwyn GJ, Shanley LC, Beckett L, Peled D, Lafaille JJ, Spite M, Loke P, Fisher EA, Moore KJ. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ Res. 2020;127:335–53. https://doi.org/10.1161/CIRCRESAHA.119.316461.
Ma SD, Mussbacher M, Galkina EV. Functional Role of B Cells in Atherosclerosis. Cells. 2021;10:270. https://doi.org/10.3390/cells10020270.
Rawlings DJ, Schwartz MA, Jackson SW, Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol. 2012;12:282–94. https://doi.org/10.1038/nri3190.
Saikh KU. MyD88 and beyond: a perspective on MyD88-targeted therapeutic approach for modulation of host immunity. Immunol Res. 2021;69:117–28. https://doi.org/10.1007/s12026-021-09188-2.
Zhou Y, Cui C, Ma X, Luo W, Zheng SG, Qiu W. Nuclear Factor κB (NF-κB)-mediated inflammation in multiple sclerosis. Front Immunol. 2020;11:391. https://doi.org/10.3389/fimmu.2020.00391.
Poznyak AV, Melnichenko AA, Wetzker R, Gerasimova EV, Orekhov AN. NLPR3 Inflammasomes and Their Significance for Atherosclerosis. Biomedicines. 2020;8:205. https://doi.org/10.3390/biomedicines8070205.
Hu Z, Murakami T, Suzuki K, Tamura H, Kuwahara-Arai K, Iba T, Nagaoka I. Antimicrobial Cathelicidin Peptide LL-37 Inhibits the LPS/ATP-Induced Pyroptosis of Macrophages by Dual Mechanism. PLoS ONE. 2014;9:e85765. https://doi.org/10.1371/journal.pone.0085765.
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, Xin C, Zhu D, Li Y, Yan W, Xiong L, Gao E, Wang H, Tao L. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109:415. https://doi.org/10.1007/s00395-014-0415-z.
Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015;21:263–9. https://doi.org/10.1038/nm.3804.
Granger CB, Mahaffey KW, Weaver WD, Theroux P, Hochman JS, Filloon TG, Rollins S, Todaro TG, Nicolau JC, Ruzyllo W, Armstrong PW. Pexelizumab, an Anti-C5 Complement Antibody, as Adjunctive Therapy to Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction. Circulation. 2003;108:1184–90. https://doi.org/10.1161/01.CIR.0000087447.12918.85.
Poznyak AV, Bharadwaj D, Prasad G, Grechko AV, Sazonova MA, Orekhov AN. Anti-Inflammatory Therapy for Atherosclerosis: Focusing on Cytokines. Int J Mol Sci. 2021;22:7061. https://doi.org/10.3390/ijms22137061.
Elhage R, Maret A, Pieraggi M-T, Thiers JC, Arnal JF, Bayard F. Differential Effects of Interleukin-1 Receptor Antagonist and Tumor Necrosis Factor Binding Protein on Fatty-Streak Formation in Apolipoprotein E-Deficient Mice. Circulation. 1998;97:242–4. https://doi.org/10.1161/01.CIR.97.3.242.
Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AEI, Mach F. Antagonism of RANTES Receptors Reduces Atherosclerotic Plaque Formation in Mice. Circ Res. 2004;94:253–61. https://doi.org/10.1161/01.RES.0000109793.17591.4E.
van Wanrooij EJA, Happé H, Hauer AD, de Vos P, Imanishi T, Fujiwara H, van Berkel TJC, Kuiper J. HIV Entry Inhibitor TAK-779 Attenuates Atherogenesis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2005;25:2642–7. https://doi.org/10.1161/01.ATV.0000192018.90021.c0.
Braunersreuther V, Steffens S, Arnaud C, Pelli G, Burger F, Proudfoot A, Mach F. A Novel RANTES Antagonist Prevents Progression of Established Atherosclerotic Lesions in Mice. Arterioscler Thromb Vasc Biol. 2008;28:1090–6. https://doi.org/10.1161/ATVBAHA.108.165423.
Copin J-C, da Silva RF, Fraga-Silva RA, Capettini L, Quintao S, Lenglet S bastien, Pelli G, Galan K, Burger F, Braunersreuther V, Schaller K, Deruaz M, Proudfoot AE, Dallegri F, Stergiopulos N, Santos RA, Gasche Y, Mach F, Montecucco F, Fabrizio.Treatment with Evasin-3 Reduces Atherosclerotic Vulnerability for Ischemic Stroke, but Not Brain Injury in Mice. J Cereb Blood Flow Metab. 2013; 33:490–498 https://doi.org/10.1038/jcbfm.2012.198.
Müller I, Schönberger T, Schneider M, Borst O, Ziegler M, Seizer P, Leder C, Müller K, Lang M, Appenzeller F, Lunov O, Büchele B, Fahrleitner M, Olbrich M, Langer H, Geisler T, Lang F, Chatterjee M, de Boer JF, Tietge UJF, Bernhagen J, Simmet T, Gawaz M. Gremlin-1 Is an Inhibitor of Macrophage Migration Inhibitory Factor and Attenuates Atherosclerotic Plaque Growth in ApoE−/− Mice *. J Biol Chem. 2013;288:31635–45. https://doi.org/10.1074/jbc.M113.477745.
Cipriani S, Francisci D, Mencarelli A, Renga B, Schiaroli E, D’Amore C, Baldelli F, Fiorucci S. Efficacy of the CCR5 Antagonist Maraviroc in Reducing Early, Ritonavir-Induced Atherogenesis and Advanced Plaque Progression in Mice. Circulation. 2013;127:2114–24. https://doi.org/10.1161/CIRCULATIONAHA.113.001278.
Yao L, Heuser-Baker J, Herlea-Pana O, Iida R, Wang Q, Zou M-H, Barlic-Dicen J. Bone Marrow Endothelial Progenitors Augment Atherosclerotic Plaque Regression in a Mouse Model of Plasma Lipid Lowering. Stem Cells. 2012;30:2720–31. https://doi.org/10.1002/stem.1256.
Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, Kramp BK, Piccinini AM, Paludan SR, Kowalska MA, Kungl AJ, Hackeng TM, Mayo KH, Weber C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15:97–103. https://doi.org/10.1038/nm.1898.
Gong M, Zhuo X, Ma A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med Sci Monit Basic Res. 2017;23:240–9. https://doi.org/10.12659/msmbr.904014.
Guo S, Xia X, Gu H, Zhang D. Proprotein Convertase Subtilisin/Kexin-Type 9 and Lipid Metabolism. In: Jiang X-C, editor. Lipid Transfer in Lipoprotein Metabolism and Cardiovascular Disease. Singapore: Springer; 2020. p. 137–56.
Grönberg C, Nilsson J, Wigren M. Recent advances on CD4+ T cells in atherosclerosis and its implications for therapy. Eur J Pharmacol. 2017;816:58–66. https://doi.org/10.1016/j.ejphar.2017.04.029.
Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP-Y. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–65. https://doi.org/10.1016/j.immuni.2009.02.005.
Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–10. https://doi.org/10.1038/ni.1861.
Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial Membrane Potential Is Required for MAVS-Mediated Antiviral Signaling. Sci Signaling. 2011; 4:ra7–ra7 https://doi.org/10.1126/scisignal.2001147.
Buskiewicz IA, Montgomery T, Yasewicz EC, Huber SA, Murphy MP, Hartley RC, Kelly R, Crow MK, Perl A, Budd RC, Koenig A. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci Signaling. 2016;9:ra115–ra115. https://doi.org/10.1126/scisignal.aaf1933.
Funding
This work was supported by the Russian Science Foundation (Grant #20–15-00264).
Author information
Authors and Affiliations
Contributions
K.A.L. had the idea for the article and provided the first draft; V.A.K., V.N.S., and V.Y.G. performed the literature search and data analysis; V.A.K. prepared Figs. 1 and 2; V.I.S. edited the text; A.N.O. critically revised the work. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lusta, K.A., Summerhill, V.I., Khotina, V.A. et al. The Role of Bacterial Extracellular Membrane Nanovesicles in Atherosclerosis: Unraveling a Potential Trigger. Curr Atheroscler Rep 26, 289–304 (2024). https://doi.org/10.1007/s11883-024-01206-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-024-01206-6