Abstract
Purpose of Review
Genome-wide association studies have repeatedly linked the metalloproteinase ADAMTS7 to coronary artery disease. Here we aim to highlight recent findings surrounding the human genetics of ADAMTS7, novel mouse models that investigate ADAMTS7 function, and potential substrates of ADAMTS7 cleavage.
Recent Findings
Recent genome-wide association studies in coronary artery disease have replicated the GWAS signal for ADAMTS7 and shown that the signal holds true even across different ethnic groups. However, the direction of effect in humans remains unclear. A recent novel mouse model revealed that the proatherogenicity of ADAMTS7 is derived from its catalytic functions, while at the translational level, vaccinating mice against ADAMTS7 reduced atherosclerosis. Finally, in vitro proteomics approaches have identified extracellular matrix proteins as candidate substrates that may be causal for the proatherogenicity of ADAMTS7.
Summary
ADAMTS7 represents an enticing target for therapeutic intervention. The recent studies highlighted here have replicated prior findings, confirming the genetic link between ADAMTS7 and atherosclerosis, while providing further evidence in mice that ADAMTS7 is a targetable proatherogenic enzyme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in the USA [1]. Despite the immense success of lipid-lowering therapies in treating coronary artery disease (CAD), there remains a great deal of residual risk, underscoring the need for identifying novel lipid-independent therapeutics. Genome-wide association studies (GWAS) are an unbiased approach for identifying regions of the genome that are involved in disease pathogenesis, and CAD GWAS have identified hundreds of genomic loci that significantly associate with CAD in humans [2, 3•, 4, 5]. To date, 279 genomic loci have been associated with CAD by GWAS [2]. As expected, CAD GWAS have identified genes involved in lipid metabolism, such as APOB and LDLR, known regulators of low-density lipoprotein (LDL) cholesterol, a critical risk factor for CAD [6, 7]. However, CAD GWAS have highlighted other biological pathways that contribute to CAD risk independent of lipids. For example, inflammation-related genes such as IL5, IL6R, and CXCL12 have been significantly associated with CAD via GWAS [6]. Inflammation is a critical component of CAD progression [8], and the therapeutic targeting of the pro-inflammatory cytokine interleukin-1β reduces adverse cardiovascular events [9], suggesting that CAD GWAS are identifying biological pathways that are viable therapeutic targets. Vascular remodeling has also been identified by CAD GWAS as a pathway contributing to disease progression [2, 6], and given the above examples, vascular remodeling warrants consideration as a biological pathway for therapeutic intervention.
Single nucleotide polymorphisms (SNPs) in the chromosome 15q25.1 genomic locus have been repeatedly identified as significantly associated with CAD, thus making this a proven, consistent, reproducible CAD GWAS signal [2, 3•, 6, 10,11,12,13]. The signal centers around the gene A disintegrin and metalloproteinase with thrombospondin motifs 7 (ADAMTS7). ADAMTS7 is a member of the ADAMTS family (19 members) of metzincin metalloproteinases, which are secreted proteins that degrade extracellular matrix (ECM) [14, 15]. The ADAMTS proteins have highly homologous metalloprotease domains but vary significantly in their C-terminal structure which confers substrate specificity [16]. These proteins have been previously linked to human disease. ADAMTS13 is the metalloproteinase that cleaves Von Willebrand factor (VWF) [17], and inactivating mutations in ADAMTS13, or its inhibition by auto antibodies, causes the disorder thrombotic thrombocytopenic purpura (TTP), which can cause fatal cardiac events in affected individuals [18, 19]. The first family member described, ADAMTS1, is reported to have both pro and anti-tumorigenic properties. ADAMTS1 can alter vascularization, inhibit angiogenesis, and increase cancer cell proliferation [20], highlighting the diverse roles of ADAMTS proteinases in human disease.
Multiple ADAMTS proteinases have described roles in atherosclerosis. One of the initiating steps of atherosclerosis is the retention of LDL within the intima of the vasculature [21], a process mediated by proteoglycans that bind LDL [22]. The catalytic cleavage of proteoglycans has been well described for ADAMTS1, 4, 5, 8, 9, 15, and 20 [16]; however, this cleavage activity has yielded inconsistent directions of influence on atherosclerosis. Mechanistic studies have shown that ADAMTS5 can cleave the proteoglycans biglycan and versican, both of which have been shown to bind to LDL [23, 24]. Whole-body knockout of ADAMTS5 causes the accumulation of proteoglycans and increased retention of LDL particles in the aorta [25, 26]. Indeed, ADAMTS5 levels are reduced in atherosclerotic lesions in both mice and humans [25, 27]; however, no in vivo atherosclerosis studies have been performed to confirm a role for ADAMTS5 in atherogenesis. In contrast, genetic ablation of Adamts4 on the Apoe-/- background reduced atherosclerosis [28]. While both ADAMTS4 and ADAMTS5 can cleave proteoglycans, the loss of ADAMTS5 is presumed to increase atherosclerosis, whereas the loss of ADAMTS4 reduces atherosclerosis, suggesting higher level substrate specificity not yet completely elucidated. ADAMTS3 is a GWAS hit for both CAD and LDL-C, suggesting it contributes to atherosclerosis via dyslipidemia [2, 10, 29, 30], but there are no studies investigating ADAMTS3 and atherosclerosis. The differing directionality of atherosclerotic disease for ADAMTS4 and ADAMTS5 and the potential role of ADAMTS3 in dyslipidemia reflect the continual need to investigate these proteinases in atherosclerosis.
Our group first identified an association between SNPs near ADAMTS7 and CAD [10], implicating it in CAD pathophysiology. The ADAMTS7 signal has since been replicated multiple times [2, 3•, 4, 6, 11,12,13]. Prior to its identification in CAD GWAS, ADAMTS7 was predominantly studied in the context of osteoarthritis [14], owing to the fact that ADAMTS7 can degrade cartilage, a hallmark of the disease. One of the first studies hinting at a role for ADAMTS7 in atherosclerosis was the in vitro overexpression and knockdown of Adamts7 in rat vascular smooth muscle cells (SMCs) [31]. This study found that ADAMTS7 increases SMC migration, and ADAMTS7 expression could be induced in vitro by cytokines such as TNFa and PDGF-BB, and in vivo via carotid wire injury. After its identification as a CAD locus, follow-up studies have found that Adamts7 whole-body knockout mice had a net reduction in atherosclerosis, implicating ADAMTS7 as a pro-atherogenic metalloproteinase [32]. Importantly, there is no association between the ADAMTS7 genomic locus and any lipid parameters [33, 34], and Adamts7 knockout mice do not exhibit altered cholesterol levels, suggesting that its role in atherosclerosis is lipid-independent [32]. Therefore, targeting ADAMTS7 could be an effective strategy for reducing residual risk outside of lipid lowering.
Causal SNPs at the ADAMTS7 Locus
The first CAD GWAS that implicated ADAMTS7 were performed in patients of European ancestry who had at least 50% occlusion within at least one coronary artery [10]. The lead SNP identified in this study (rs1994016) lies in an intron of ADAMTS7. Given the location of this lead SNP, the locus was assigned to ADAMTS7. A subsequent CAD GWAS performed by the CARDIoGRAM consortium replicated the ADAMTS7 GWAS signal, but instead identified a coding SNP (rs3825807) as the lead SNP [12]. This SNP results in a serine-to-proline substitution (S214P) within the prodomain of ADAMTS7, causing decreased secretion of ADAMTS7 in primary SMCs and presumably reduced function [12, 35]. The identification of a missense variant in ADAMTS7 that reduces ADAMTS7 function and also significantly associates with CAD via GWAS greatly increased the confidence that ADAMTS7 is the causal gene at this locus. This finding also provided directionality in humans, as the loss of function allele is associated with decreased CAD risk, implying that ADAMTS7 is proatherogenic in humans.
Subsequent CAD GWAS have continuously replicated the 15q25.1 GWAS signal [36], yet the location of the lead SNP has changed as the cohort sizes have grown larger. A recent CAD GWAS from the UK Biobank, the CARDIoGRAMplusC4D Consortium, and Biobank Japan studying over 1.3 million individuals localized the lead SNP (rs7173743) at the 15q25.1 locus to a region upstream of the ADAMTS7 gene rather than within the gene (Fig. 1). Non-coding GWAS SNPs are frequently linked to alterations in the expression of nearby genes via eQTL analysis, wherein a specific allele of a SNP is associated with increased or decreased gene expression due to altered enhancer activity, a common functional mechanism of GWAS SNPs. The non-coding location of rs7173743 implies that a similar mechanism is at play in the ADAMTS7 locus. However, in large consortium eQTL data such as the GTEx consortium, the non-coding alleles that associate with increased risk also associate with decreased ADAMTS7 expression in the vasculature [38], implying that ADAMTS7 is antiatherogenic, in stark opposition to the directionality implied by the coding S214P variant.
LocusZoom plot of the 15q25.1 CAD GWAS signal in the subset of people of European ancestry [2]. The GWAS signal centers around ADAMTS7, with the histone acetyltransferase MORF4L1 lying immediately upstream of the gene. Downstream of ADAMTS7 lies a cluster of three nicotine receptors CHRNA3-A5-B4. Linkage disequilibrium is referenced to the lead SNP rs7173743. The coding S214P SNP (rs3825807) is highlighted in yellow. (this figure was created using LocusZoom [37])
The lack of clear directionality in humans is a major shortcoming of the genetics surrounding ADAMTS7. The lead non-coding SNP also has weak linkage to the S214P coding variant, making it difficult to discern which variant, if not both, is driving the genetic signal at this locus. Definitive exome sequencing to identify other loss of function ADAMTS7 variants in humans to support causality and directionality is hampered due to the presence of five highly homologous ADAMTS7 pseudogenes, contaminating variant calling in short-read sequencing studies [39]. Furthermore, as the vast majority of the 15q25.1 GWAS SNPs are non-coding, there remains a pressing need to conduct further functional genomic investigations into non-coding regions near ADAMTS7 to validate any functional effects. Any attempts at therapeutic intervention will necessitate further human genetic data or functional genomic investigations that can confirm a disease directionality for ADAMTS7 in humans.
Candidate Genes in the ADAMTS7 Locus
As stated above, recent GWAS have localized the lead SNPs in the 15q25.1 locus to the region upstream of the ADAMTS7 transcriptional start site. The gene directly upstream of ADAMTS7 is MORF4L1, which codes for the histone acetyltransferase MRG15. The location of the lead SNP (rs7173743) is within the intronic region of one transcript variant of MORF4L1; thus, the GWAS signal is sometimes described as MORF4L1-ADAMTS7. Early studies of MORF4L1 have shown that homozygous knockout of Morf4l1 is embryonically lethal due to reduced cellular proliferation [40]. Subsequent studies of Morf4l1 in the liver revealed its essentiality for the rhythmic regulation of lipid genes [41]. Knockout of MRG15 by CRISPR-Cas9 can reduce blood triglyceride and cholesterol, liver steatosis, and liver expression of essential lipid-related genes such as Srebf1 and Fasn [41]. These in vivo data linking MORF4L1 to lipid genes raise the possibility that MORF4L1 contributes to the GWAS signal at 15q25.1; however, this is unlikely as this locus has not been associated with plasma lipids in large-scale GWAS [34]. To date, there are no investigations of MORF4L1 in rodent models of atherosclerosis.
Immediately, downstream of ADAMTS7 lies a cluster of nicotine receptors, CHRNA3-A5-B4, and data from recent GWAS show that the 15q25.1 signal overlaps these genes. Smoking is a major confounding lifestyle risk factor for CAD, and nicotine receptors are likely candidates to associate with CAD risk. A study examining genetics and smoking across 60,919 CAD patients and 80,243 controls across 29 studies found that smoker status blunts the cardioprotective effect of the 15q25.1 locus [42]. The protective allele of the lead variant in that study (rs7178051) confers a 12% reduction in CAD. With smoking, this protection drops to 5%, equating to a 60% reduction in protection [42]. The protective allele associates with reduced ADAMTS7 expression in human aortic endothelial cells and lymphoblastoid cell lines. Furthermore, cigarette smoke extract induced the expression of ADAMTS7 in human coronary artery SMCs, providing further evidence of the association between smoking and ADAMTS7 and a possible mechanism for the increased CAD associated with smoking [42]. These observations are consistent with mouse data indicating that ADAMTS7 may be pro-atherogenic. Given the data, it is possible that the cluster of nicotine receptors represents a separate GWAS signal for CAD in this locus or are mechanistically linked to the ADAMTS7 signal.
As one of the most reproducible GWAS signals in CAD, there is a possibility that the human genetic signal at 15q25.1 implicates MORF4L1, ADAMTS7, and the nicotine receptors. One noteworthy finding from the most recent GWAS studies is that this GWAS signal replicates in non-Europeans, including those of East Asian, Japanese [43•], and Hispanic descent [3•]. Thus, this locus is likely relevant to cardiovascular health across all ethnicities, underscoring the importance of further studies of the molecular mechanisms underlying this genetic association.
Novel Mouse Models of ADAMTS7
Human genetics provided an impetus to investigate ADAMTS7; subsequent studies of ADAMTS7 have relied heavily on mouse models to further explore both the direction of effect and mechanisms underlying the genetic association [44]. The first study involving ADAMTS7 in the vasculature showed the upregulation of Adamts7 in response to balloon injury in rat arteries [31]. Subsequently, our group showed that whole-body knockout of Adamts7 reduced atherosclerosis [32]. These early rodent studies provided a directionality to the GWAS signal, indicating that ADAMTS7 promotes neointima formation and atherosclerosis.
More recently, two other novel rodent approaches have been reported. The first is a genetic mouse model in which the catalytic activity of ADAMTS7 was rendered inactive [45••]. The second model inactivates ADAMTS7 in vivo through a vaccination-mediated antibody response [46••]. In the first model, the investigators generated a genetically modified mouse with whole-body catalytic inactive ADAMTS7 by substituting a glutamine for a highly conserved glutamic acid at position 373 (E373Q) within the catalytic site, rendering the protein inactive [45••]. Hyperlipidemic mice harboring this inactivated form of ADAMTS7 displayed a reduction in atherosclerosis, confirming that the pro-atherogenicity of ADAMTS7 is conferred by its catalytic function. When compared to the previously described findings in mice with whole-body knockout of Adamts7, the catalytic inactive ADAMTS7 replicated key findings including the reduction of atherosclerosis, blunted SMC migration, and reduced thrombospondin 1 (TSP1) cleavage [32, 45••, 47]. These studies highlight the potential of targeting the catalytic activity of ADAMTS7 in the therapeutic treatment of atherosclerosis.
In the second novel model to investigate ADAMTS7, a vaccine against the ADAMTS7 catalytic domain was used to immunologically inactivate ADAMTS7 [46••]. This peptide vaccine was able to reduce restenosis in mice in response to wire injury, whereas for swine, vaccination against ADAMTS7 reduced stent-associated intimal hyperplasia. Additionally, the vaccination against ADAMTS7 reduced atherosclerosis in mice by both en-face and aortic root measurements in both the Ldlr-/- and Apoe-/- atherosclerotic background. A critical aspect of this study involved the timing of vaccination and initiation of high-fat diet feeding. For the cohort on the Apoe-/- background, the mice were vaccinated before high-fat diet induction and subsequently given boosters throughout high-fat diet feeding. Of the Ldlr-/- cohort, the mice were fed 4 weeks of high-fat diet prior to any vaccination against ADAMTS7. Even with the onset of atherosclerosis, this cohort on the Ldlr-/- background exhibited a significant reduction in plaque burden. As prior literature indicates that ADAMTS7 is induced in early atherogenesis [32], it would be interesting to see if vaccination against ADAMTS7 after longer durations of western diet priming would affect atherosclerosis. This study overall is an important first proof of principle that ADAMTS7 can be therapeutically targeted to reduce atherosclerosis [46••].
It is becoming increasingly clear that the composition of a lesion plays a role in addition to lesion size. Although lesions may be the same size, lesions that are fibrous and high in SMCs are more stable and less likely to cause fatal coronary events [48]. Examination of the aortic root lesions of these mouse models has yielded no changes in lesion composition [32, 45••, 46••]. Regarding macrophage area, both the catalytic inactive and vaccine models reported no differences in macrophage content [45••, 46••]. Additionally, the original whole-body knockout model reported no change in macrophage content in plaques of the brachiocephalic artery, although macrophage content was not reported for the aortic root [32]. All three models showed no change in collagen area within the aortic root as quantified through Masson’s trichrome. Although these two elements of lesion composition remain unchanged, further characterization is warranted for other aspects of lesion composition. There is still a lack of data reporting the necrotic core content, fibrous cap thickness, and SMC content of these lesions. In addition, single cell sequencing approaches (scRNA-seq) could add invaluable insight as to which cell types express ADAMTS7 during disease progression. In summary, Adamts7 knockout reduces atherosclerosis without altering lesion morphology, highlighting that ADAMTS7 may accelerate the formation of atheroma rather than affect lesion progression.
Cleavage Substrates of ADAMTS7
The pro-atherogenicity of ADAMTS7 is derived from its metalloproteinase enzymatic function [45••]. Therefore, the elucidation of specific substrates can not only add mechanistic insights on ADAMTS7 function in atherosclerosis and the vascular contribution to atherosclerotic plaque formation, but also may reveal even more novel therapeutic avenues. Cartilage oligomeric matrix protein (COMP) was the first ADAMTS7 substrate identified using a yeast two-hybrid screen in the context of osteoarthritis [14]. Subsequent proteomics work showed that ADAMTS7 overexpression in SMCs causes a decrease in conditioned media TSP1 levels. ADAMTS7 was shown to cleave TSP1 in an in vitro cleavage assay, suggesting that TSP1 is another substrate for ADAMTS7 cleavage [47]. Since then, a proteomics approach known as terminal amine isotopic labeling of substrates (TAILS) has been used on three different cell types (fibroblast, endothelial cells, and SMCs) to elucidate cleavage targets of ADAMTS7 [49•, 50•]. TAILS is a technique that differentially labels N-termini and is used to specifically quantify cleavage products [51]. Through using this technique, TAILS has generated a rich list of candidate substrates of ADAMTS7.
The first report of TAILS and ADAMTS7 involved co-cultures of human fibroblasts and HEK293Ts transfected with ADAMTS7 cDNA [49•]. This experiment identified differential cleavage in multiple components of the ECM and went the additional step of validating LTBP4 as a true cleavage target of ADAMTS7 using recombinant truncated LTBP4 with purified recombinant ADAMTS7 [49•]. LTBP4 is a member of the ECM that can sequester the ligand TGFB1 and regulate its function [52]. In the context of atherosclerosis, multiple proteins in TGFB1 signaling are CAD GWAS hits, including BMP1, SMAD3, and TGFB1 itself [6]. TGFB1 has known roles in fibrosis and inflammation, two processes important for plaque formation [53]. Within the ECM, tissue inhibitor of metalloproteinases (TIMPs) are endogenous inhibitors of metalloproteinases, including MMPs, ADAMs, and ADAMTSs. After validating that LTBP4 is indeed a target of ADAMTS7, the authors tested four members of the TIMP family to see if they could inhibit ADAMTS7. Of these four TIMPs, TIMP2, 3, and 4 all showed some degree of ADAMTS7 inhibition. TIMP1 had almost no inhibitory activity, while TIMP4 had the highest degree of inhibition, with 60nM of TIMP4 generating a greater than 75% inhibition of 18nM of ADAMTS7 [49•]. This initial proteomics experiment, which provided an ADAMTS7 candidate substrate list, showed that ADAMTS7 can cleave ECM proteins, and implicated ADAMTS7 in the regulation of TGFB signaling.
Subsequent experiments expanded the list of ADAMTS7 candidate substrates by performing TAILS on the secretome of SMCs and endothelial cells [50•]. These TAILS experiments employed an adenovirus to overexpress Adamts7 and identified 91 unique cleavage sites in 48 different proteins. Of these 48 proteins, 16 were found in all datasets reported in this study. Interestingly, ADAMTS7 could cleave fibronectin at 12 different locations, the most unique target sites of any protein identified. Of these 16 proteins identified, EFEMP1 was further validated as a cleavage target of ADAMTS7. ADAMTS3, MMP3, and MMP7 were previously reported to cleave EFEMP1, and ADAMTS7 displayed the same cleavage site preference as these metalloproteinases [45••, 54, 55]. The authors used a novel modification of ADAMTS7 in performing these experiments, where the serine amino acids within the SGSGS site of the mucin domain of ADAMTS7 were replaced with alanines. Without this modification, the hydrophobicity of the mucin site renders ADAMTS7’s purification nearly impossible. These mutations allowed for the recombinant purification of ADAMTS7 for downstream assays, a technique that will be incredibly useful for the study of ADAMTS7. Using endogenous and HA-tagged EFEMP1, the authors validated cleavage of EFEMP1 ADAMTS7 via western blotting. This set of experiments replicated the finding that ADAMTS7 acts on the level of the ECM while both generating additional candidate substrates and validating one specific candidate substrate as a cleavage target of ADAMTS7. Both TAILS studies also identified autocleavage of ADAMTS7. Within the TAILS study of fibroblasts, the authors found that ADAMTS7 auto-cleaves within the spacer domain [49•]. The SMC and endothelial cell TAILS dataset detected autocleavage within the prodomain and mucin domain [50•]. It remains unclear if this autocleavage is critical for normal ADAMTS7 function or, rather, is part of an autoinhibitory negative feedback mechanism.
The described TAILS experiments all clearly show that ADAMTS7 cleaves ECM proteins. Comparison across fibroblasts, SMCs, and endothelial cells identifies only six proteins found in every experiment: BMP6, LTBP1, LTBP3, NID1, FN1, and COL18A1, all of which are ECM proteins. Surprisingly, neither COMP nor TSP1 was strong hits within the TAILS datasets [49•, 50•]. However, the identification of these six substrates and no others may owe to the tissue-specific expression of these six proteins within the three cell types tested. For example, TAGLN was detected as a cleaved protein with the SMCs datasets but not in the other two cell types [50•]. TAGLN is a well-described SMC-associated gene, and it is not surprising that fibroblasts and endothelial cells do not have high expression of TAGLN [56]. TAILS experiments are performed in vitro and cannot capture the entire secretome of the many cell types that make up the vasculature. Although these three cell types cover the vast majority of the secretome of the cells of the vasculature, if, for example, a substrate only produced by macrophages was causal, the reported TAILS experiments would not be able to capture that target substrate. As such, the definitive identification of a causal cleavage target of ADAMTS7 needs to be verified in vivo through an atherosclerosis study.
Conclusion
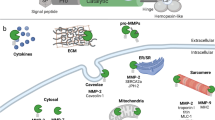
Since its identification by GWAS as a gene of interest for CAD, much work has been done to decipher the role of ADAMTS7 in atherosclerosis. Multiple mouse models have corroborated that ADAMTS7 is pro-atherogenic, and the pro-atherogenicity is derived from its catalytic function [32, 45••]. As ADAMTS7 co-stains with SMCs within human plaques, the causal cell type is highly suggestive of being SMCs [32]. A clear future direction would be the employment of conditional knockout mice to definitively show that SMCs are the causal cell type. At the cellular level, ADAMTS7 can increase the rate of migration of vascular cells. However, the link between this increase in migration and an increase in atherosclerosis is still unresolved (Fig. 2).
Proposed model for the mechanistic effect of ADAMTS7 on the vasculature. ADAMTS7 is induced in response to vascular injury. ADAMTS7 cleaves components of the ECM. This cleavage activity, in turn, leads to an increase in SMC migration through a yet unknown mechanism. It is unknown whether the elevated SMC migration or the cleaved ECM itself is causal for the associated increase in atherosclerosis. (created with BioRender.com)
Furthermore, the vast majority of the SNPS that point towards ADAMTS7 as a gene of interest lie within the non-coding region of the loci. Is the coding variant truly causative, or is there another atherogenic mechanism conferred by the non-coding region? In addition, definitive evidence of ADAMTS7 being proatherogenic in humans is still lacking. More work is needed to show the directionality of disease in humans. The identification of true ADAMTS7 loss of function variants would aid to address this knowledge gap. The current literature also indicates that ADAMTS7 is induced rather than constitutively expressed [32, 45••]. This aspect as to when ADAMTS7 is induced in humans requires further research. In mice, ADAMTS7 is seen in early lesions but not late advanced lesion. As the onset of atherosclerosis can occur in childhood, if the therapeutic window for ADAMTS7 intervention lies within this time, the therapeutic efficacy of ADAMTS7 is greatly diminished.
In summary, human genetic data heavily implicates ADAMTS7 as a gene involved in CAD [10]. Furthermore, basic research has subsequently elucidated ADAMTS7 to be a secreted proatherogenic proteinase [45••]. There remains a need to identify the precise cleavage substrate that leads to the conferred proatherogenicity. Only recently has it been shown in model systems that therapeutic blockade of ADAMTS7 reduce atherosclerosis [46••]. For ADAMTS7-based therapeutics to be translated into the clinic, the directionality of disease conferred by ADAMTS7 in humans needs to be resolved. Nonetheless, this recent publication showing a therapeutic efficacy targeting ADAMTS7 opens the doors to the development of a new class of therapeutics for CAD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023. https://doi.org/10.1161/CIR.0000000000001123.
Aragam KG, Jiang T, Goel A, Kanoni S, Wolford BN, Atri DS, et al. Discovery and systematic characterization of risk variants and genes for coronary artery disease in over a million participants. Nat Genet. 2022;54(12):1803–15. https://doi.org/10.1038/s41588-022-01233-6.
• Tcheandjieu C, Zhu X, Hilliard AT, Clarke SL, Napolioni V, Ma S, et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat Med. 2022;28(8):1679–92. https://doi.org/10.1038/s41591-022-01891-3. This GWAS revealed that ADAMTS7 reached genome wide significance in participants of Hispanic descent.
Coronary Artery Disease Genetics C. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43(4):339–44. https://doi.org/10.1038/ng.782.
Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–30. https://doi.org/10.1038/ng.3396.
Erdmann J, Kessler T, Munoz Venegas L, Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res. 2018;114(9):1241–57. https://doi.org/10.1093/cvr/cvy084.
Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. https://doi.org/10.1016/S0140-6736(13)61752-3.
Libby P, Hansson GK. From focal lipid storage to systemic inflammation: JACC review topic of the week. J Am Coll Cardiol. 2019;74(12):1594–607. https://doi.org/10.1016/j.jacc.2019.07.061.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377(9763):383–92. https://doi.org/10.1016/S0140-6736(10)61996-4.
Lu X, Wang L, Chen S, He L, Yang X, Shi Y, et al. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet. 2012;44(8):890–4. https://doi.org/10.1038/ng.2337.
Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8. https://doi.org/10.1038/ng.784.
van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. 2018;122(3):433–43. https://doi.org/10.1161/CIRCRESAHA.117.312086.
Liu CJ, Kong W, Ilalov K, Yu S, Xu K, Prazak L, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20(7):988–90. https://doi.org/10.1096/fj.05-3877fje.
Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. https://doi.org/10.1042/BJ20040424.
Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 2015;16(1):113. https://doi.org/10.1186/s13059-015-0676-3.
Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, et al. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13). Blood. 2002;100(10):3626–32. https://doi.org/10.1182/blood-2002-05-1397.
Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211–25. https://doi.org/10.1146/annurev-med-061813-013241.
Wiernek SL, Jiang B, Gustafson GM, Dai X. Cardiac implications of thrombotic thrombocytopenic purpura. World J Cardiol. 2018;10(12):254–66. https://doi.org/10.4330/wjc.v10.i12.254.
Tan Ide A, Ricciardelli C, Russell DL. The metalloproteinase ADAMTS1: a comprehensive review of its role in tumorigenic and metastatic pathways. Int J Cancer. 2013;133(10):2263–76. https://doi.org/10.1002/ijc.28127.
Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5(8):927–46.
Camejo G, Hurt-Camejo E, Wiklund O, Bondjers G. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 1998;139(2):205–22. https://doi.org/10.1016/s0021-9150(98)00107-5.
Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15(7):483–94. https://doi.org/10.1038/sj.cr.7290318.
Neufeld EB, Zadrozny LM, Phillips D, Aponte A, Yu ZX, Balaban RS. Decorin and biglycan retain LDL in disease-prone valvular and aortic subendothelial intimal matrix. Atherosclerosis. 2014;233(1):113–21. https://doi.org/10.1016/j.atherosclerosis.2013.12.038.
Didangelos A, Mayr U, Monaco C, Mayr M. Novel role of ADAMTS-5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem. 2012;287(23):19341–5. https://doi.org/10.1074/jbc.C112.350785.
Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–52. https://doi.org/10.1038/nature03417.
Wang Z, Ye D, Ye J, Wang M, Liu J, Jiang H, et al. ADAMTS-5 Decreases in coronary arteries and plasma from patients with coronary artery disease. Dis Markers. 2019;2019:6129748. https://doi.org/10.1155/2019/6129748.
Kumar S, Chen M, Li Y, Wong FH, Thiam CW, Hossain MZ, et al. Loss of ADAMTS4 reduces high fat diet-induced atherosclerosis and enhances plaque stability in ApoE(-/-) mice. Sci Rep. 2016;6:31130. https://doi.org/10.1038/srep31130.
Davis JP, Huyghe JR, Locke AE, Jackson AU, Sim X, Stringham HM, et al. Common, low-frequency, and rare genetic variants associated with lipoprotein subclasses and triglyceride measures in Finnish men from the METSIM study. PLoS Genet. 2017;13(10):e1007079. https://doi.org/10.1371/journal.pgen.1007079.
Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600(7890):675–9. https://doi.org/10.1038/s41586-021-04064-3.
Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104(5):688–98. https://doi.org/10.1161/CIRCRESAHA.108.188425.
Bauer RC, Tohyama J, Cui J, Cheng L, Yang J, Zhang X, et al. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation. 2015;131(13):1202–13. https://doi.org/10.1161/CIRCULATIONAHA.114.012669.
Kathiresan S, Manning AK, Demissie S, D’Agostino RB, Surti A, Guiducci C, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S17. https://doi.org/10.1186/1471-2350-8-S1-S17.
Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, et al. Genetics of blood lipids among ~300,000 multi-ethnic participants of the Million Veteran Program. Nat Genet. 2018;50(11):1514–23. https://doi.org/10.1038/s41588-018-0222-9.
Pu X, Xiao Q, Kiechl S, Chan K, Ng FL, Gor S, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet. 2013;92(3):366–74. https://doi.org/10.1016/j.ajhg.2013.01.012.
Consortium CAD, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33. https://doi.org/10.1038/ng.2480.
Boughton AP, Welch RP, Flickinger M, VandeHaar P, Taliun D, Abecasis GR, et al. LocusZoom.js: interactive and embeddable visualization of genetic association study results. Bioinformatics. 2021;37(18):3017–8. https://doi.org/10.1093/bioinformatics/btab186.
Kim-Hellmuth S, Aguet F, Oliva M, Munoz-Aguirre M, Kasela S, Wucher V, et al. Cell type-specific genetic regulation of gene expression across human tissues. Science. 2020;369(6509). https://doi.org/10.1126/science.aaz8528.
Cheetham SW, Faulkner GJ, Dinger ME. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat Rev Genet. 2020;21(3):191–201. https://doi.org/10.1038/s41576-019-0196-1.
Tominaga K, Kirtane B, Jackson JG, Ikeno Y, Ikeda T, Hawks C, et al. MRG15 regulates embryonic development and cell proliferation. Mol Cell Biol. 2005;25(8):2924–37. https://doi.org/10.1128/MCB.25.8.2924-2937.2005.
Wei Y, Tian C, Zhao Y, Liu X, Liu F, Li S, et al. MRG15 orchestrates rhythmic epigenomic remodelling and controls hepatic lipid metabolism. Nat Metab. 2020;2(5):447–60. https://doi.org/10.1038/s42255-020-0203-z.
Saleheen D, Zhao W, Young R, Nelson CP, Ho W, Ferguson JF, et al. Loss of cardioprotective effects at the aDAMTS7 locus as a result of gene-smoking interactions. Circulation. 2017;135(24):2336–53. https://doi.org/10.1161/CIRCULATIONAHA.116.022069.
• Matsunaga H, Ito K, Akiyama M, Takahashi A, Koyama S, Nomura S, et al. Transethnic meta-analysis of genome-wide association studies identifies three new loci and characterizes population-specific differences for coronary artery disease. Circ Genom Precis Med. 2020;13(3):e002670. https://doi.org/10.1161/CIRCGEN.119.002670. This GWAS revealed that ADAMTS7 reached genome wide significance in participants of Japanese descent.
Pasterkamp G, van der Laan SW, Haitjema S, Foroughi Asl H, Siemelink MA, Bezemer T, et al. Human validation of genes associated with a murine atherosclerotic phenotype. Arterioscler Thromb Vasc Biol. 2016;36(6):1240–6. https://doi.org/10.1161/ATVBAHA.115.306958.
•• Mizoguchi T, MacDonald BT, Bhandary B, Popp NR, Laprise D, Arduini A, et al. Coronary disease association with ADAMTS7 is due to protease activity. Circ Res. 2021;129(4):458–70. https://doi.org/10.1161/CIRCRESAHA.121.319163. This publication revealed that the proatherogenecity of ADAMTS7 observed in mice is due to its catayltic activity.
•• Ma Z, Mao C, Chen X, Yang S, Qiu Z, Yu B, et al. Peptide vaccine against ADAMTS-7 ameliorates atherosclerosis and postinjury neointima hyperplasia. Circulation. 2022; https://doi.org/10.1161/CIRCULATIONAHA.122.061516. This publication showed that therapeutic intervention at the ADAMTS7 level can reduce atherosclerosis in mice.
Kessler T, Zhang L, Liu Z, Yin X, Huang Y, Wang Y, et al. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation. 2015;131(13):1191–201. https://doi.org/10.1161/CIRCULATIONAHA.114.014072.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. https://doi.org/10.1038/s41572-019-0106-z.
• Colige A, Monseur C, Crawley JTB, Santamaria S, de Groot R. Proteomic discovery of substrates of the cardiovascular protease ADAMTS7. J Biol Chem. 2019;294(20):8037–45. https://doi.org/10.1074/jbc.RA119.007492. This publication was the first to employ TAILS in ADAMTS7 and revealed the ECM as the most likely site of ADAMTS7 action.
• MacDonald BT, Keshishian H, Mundorff CC, Arduini A, Lai D, Bendinelli K, et al. TAILS identifies candidate substrates and biomarkers of ADAMTS7, a therapeutic protease target in coronary artery disease. Mol Cell Proteomics. 2022;21(4):100223. https://doi.org/10.1016/j.mcpro.2022.100223. This publication performed TAILS in SMCs and endothelial cells and expanded on the list of candidate substrates of ADAMTS7.
Kleifeld O, Doucet A, Prudova A, Auf dem Keller U, Gioia M, Kizhakkedathu JN, et al. Identifying and quantifying proteolytic events and the natural N terminome by terminal amine isotopic labeling of substrates. Nat Protoc. 2011;6(10):1578–611. https://doi.org/10.1038/nprot.2011.382.
Su CT, Urban Z. LTBP4 in health and disease. Genes (Basel). 2021;12(6). https://doi.org/10.3390/genes12060795.
Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347(1):155–75. https://doi.org/10.1007/s00441-011-1189-3.
Djokic J, Fagotto-Kaufmann C, Bartels R, Nelea V, Reinhardt DP. Fibulin-3, -4, and -5 are highly susceptible to proteolysis, interact with cells and heparin, and form multimers. J Biol Chem. 2013;288(31):22821–35. https://doi.org/10.1074/jbc.M112.439158.
Bekhouche M, Leduc C, Dupont L, Janssen L, Delolme F, Vadon-Le Goff S, et al. Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-beta signaling as primary targets. FASEB J. 2016;30(5):1741–56. https://doi.org/10.1096/fj.15-279869.
Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55(1):1–11. https://doi.org/10.1111/j.1432-0436.1993.tb00027.x.
Funding
This work was supported by an American Heart Association predoctoral fellowship to A.C. (909206), grants from the National Institutes of Health to M.P.R. (R01HL150359, R01HL166916, and UL1TR001873), a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to R.C.B (R01HL141745), and institutional funds from Columbia University to R.C.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to disclose.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chung, A., Reilly, M.P. & Bauer, R.C. ADAMTS7: a Novel Therapeutic Target in Atherosclerosis. Curr Atheroscler Rep 25, 447–455 (2023). https://doi.org/10.1007/s11883-023-01115-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-023-01115-0