Abstract
Purpose of Review
This review aims to summarize the most recently published literature highlighting the potential of pharmacological inhibition of ANGPTL3 in treating patients suffering from dyslipidemias. The rational for this strategy will be discussed considering evidence describing the role of ANGPTL3 in lipid metabolism and the consequences of its deficiency in humans.
Recent Findings
Recent trials have demonstrated the efficacy and safety of ANGPTL3 inhibition in treating homozygous familial hypercholesterolemia even in those patients carrying biallelic null/null variants, thus supporting the notion that the LDL-lowering effect of ANGPLT3 inhibition is LDLR-independent. The use of ANGPTL3 inhibition strategies has expanded its indications in hypertrygliceridemic patients with functional lipoprotein lipase activity. Contemporarily, the pharmacological research is exploring novel approaches to ANGPTL3 inhibition such as the use of a small interfering RNA targeting the ANGPTL3 transcript in the liver, a protein-based vaccine against ANGPTL3, and a CRISP-Cas-9 method for a liver-selective knock-out of ANGPTL3 gene.
Summary
First, we will describe the molecular function of ANGPTL3 in lipoprotein metabolism. Then, we will revise the clinical characteristics of individuals carrying loss-of-function mutations of ANGPTL3, a rare condition known as familial hypobetalipoproteinemia type 2 (FHBL2) that represents a unique human model of ANGPTL3 deficiency. Finally, we will examine the lipid-lowering potential of pharmacological inhibition of ANGPTL3 based on the results of clinical trials employing Evinacumab, the first approved fully humanized monoclonal antibody against ANGPTL3. The future perspectives for ANGPTL3 inhibition will also be revised.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-density lipoprotein cholesterol (LDL-C) is a well-established causal factor of atherosclerotic cardiovascular disease (ASCVD) [1]. This strong causal relationship is the rationale for the intensive LDL-C lowering in patients at high risk for ASCVD events [1, 2], which requires in many cases therapies beyond statins and ezetimibe [1, 2]. The inhibitors of the pro-protein convertase subtilisin-kexin type 9 protein (PCSK9i) has been proven to be very effective in LDL-C lowering and in reducing the risk of ASCVD recurrence in patients at high and extremely high cardiovascular risk [3]. These drugs have also shown to be effective in individuals affected by familial hypercholesterolemia (FH), where the functionality of LDL receptor (LDLR) is impaired [4]. However, this effect requires the presence of a residual LDLR activity, hence their efficacy in homozygous FH carrying the LDLRnull/null mutation is poor [4, 5].
It must be noted that although LDL-C is the most relevant factor involved in ASCVD development, it does not reflect the global plasma atherogenicity accounting for all circulating apolipoprotein B (ApoB)-containing lipoproteins: very low-density lipoprotein (VLDL), intermediate density lipoprotein (IDL), and their remnants [6,7,8]. VLDLs are directly secreted by the liver and transport triglycerides (TGs) together with cholesterol [9, 10]. The hydrolysis of their triglycerides (TGs) content by the lipoprotein lipase (LPL) progressively transforms VLDLs into remnants (or IDL) and then into LDLs. VLDLs and their remnants are gaining growing interest in cardiovascular prevention since these lipoproteins have the ability to penetrate the endothelium where they can be oxidized, thus leading to endothelial dysfunction inflammation [9,10,11]. The role of VLDLs and their remnants is particularly important as these lipoproteins are mainly increased in the postprandial state lasting in plasma for 4–6 h after a fat-rich meal [12, 13]. In fact, considering the western type diet, epidemiological projections showed that populations at higher ASCVD risk consume 3 to 4 meal a day containing 20–40 g of fat, resulting in a 14–20 h of continuous postprandial lipemia [12, 13]. Despite that VLDL particles and their remnants are nowadays considered potential targets for preventing atherosclerosis, very few effective treatments are actually available to manage increased levels of these lipoproteins [12, 13].
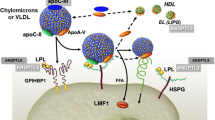
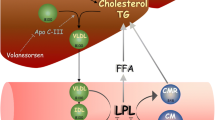
The angiopoietin-like 3 (ANGPTL3) is a circulating inhibitor of LPL and endothelial lipase (EL). It acts in a coordinated manner with two other angiopoietins (ANGPTL4 and ANGPTL8) [14] to control the breakdown of TGs and the partitioning of derived free fatty acids (FFA) between oxidative and storage tissues [15]. Therefore, ANGPTL3 may represent an interesting pharmacological target to reduce atherosclerotic risk by modulating TG-rich lipoproteins. The potential use of its pharmacological inhibition has been prompted by both animal and human genetic models of ANGPTL3 deficiency [16, 17]. Particularly interesting have been the results of these investigations considering individuals carrying the loss-of-function mutations in the ANGPTL3 gene that show a comprehensive reduction of ApoB- and Apolipoprotein AI (ApoAI)-containing lipoproteins and reduced ASCVD risk [18].
In this review, we will briefly summarize the molecular aspects related to the role of ANGPTL3 in lipid metabolism and the characteristics of individuals with genetically determined ANGPTL3 deficit. Then, we will describe the currently available ANGPTL3 inhibiting drugs, the future perspective in ANGPTL3 inhibition and their applications in the treatment of patients affected by dyslipidemias.
The ANGPTL3 Gene and Protein
The gene coding for the ANGPTL3 protein was discovered in 1999 by Cancklin et al. [19] as being located on chromosome 1p3 and comprises 7 exons [20]. The ANGPTL3 transcription is mainly regulated by the liver X receptor (LXR) and the hepatocyte nuclear factor 1α (HNF-1α). The first one upregulates ANGPTL3 transcription in response to insulin or leptin [21] in the feeding state, whereas the second one seems to inhibit ANGPTL3 transcription indirectly through the recruitment of the thyroid hormone receptor [22]. Plasmatic levels of ANGPTL3 is stable in plasma irrespective to feeding or fasting state [23].
ANGPTL3 is a 70 kDa glycoprotein, secreted almost exclusively by the liver [19], and catabolized in the kidney [19, 24]. The main known function of ANGPTL3 is the inhibition of both LPL and EL [25, 26], the extracellular lipases involved in the metabolism of TG-rich lipoproteins and phospholipids contained in circulating lipoproteins [25, 26]. ANGPTL3 comprises two distinct domains: the N-terminus coiled coil domain (CCD, residues 17–224) and the C-terminus fibrinogen-like domain (residues 237–455) linked with the first one by a hinge region. The latter one is where ANGPTL3 is recognized and cleaved by both the intracellular protease furin (PCSK3) and the extracellular protease PACE4 (PCSK6) [25, 26]. The derived N-terminal domain of ANGPTL3 binds in a tetramer fashion with another liver-secreted glycoprotein, the angiopoietin-like 8 (ANGPTL8). Studies have shown that the ANGPTL3-ANGPTL8 tetramers are 100-times more powerful in inhibiting LPL than ANGPTL3 alone [15, 26, 27]. While the function of the N-terminal domain has been extensively studied, the function of the fibrinogen-like domain has been only partially elucidated. Preliminary studies have demonstrated that it might be involved in angiogenesis as it interacts with the integrin receptor α3βν [28]. However, some recent evidence from our group showed that it is able to enhance β-adrenergic mediated lipolysis [29], suggesting its potential role in regulating lipid trafficking in response to specific signals.
The ANGPTL3-4–8 Model
The angiopoietin-like protein system is emerging as a regulator of whole-body energy metabolism and TG partitioning, capable of regulating key adipose tissue functions. To this aim, the ANGPTL3 works in an integrated network with ANGPTL4 and 8 as regulators of the LPL function in both the feeding and fasting conditions [14]. In the feeding state ANGPTL3 forms tetramers with ANGPTL8 and this complex inhibits the LPL, predominantly in the skeletal muscle, whereas in the adipose tissue, ANGPTL8 binds to ANGPTL4, thus blocking its LPL-inhibitory effect. The result is that the flux of energy substrates, namely free fatty acids, is redirected to storage tissues. On the contrary, during the fasting state, the secretion of ANGPTL8 is abolished and the circulating ANGPTL3 is much less active. In this condition, the LPL expressed in oxidative tissues is now active, while that in storage tissues is inhibited by free ANGPTL4, thus allowing a FFA flux to be directed from fat deposits to oxidative tissues and converted into energy [15].
ANGPTL3 Deficiency and Plasma Lipids
Due the above reported actions, ANGPTL3 has a profound effect on plasma lipid and lipoprotein transport. This has been well illustrated by conditions associated with ANGPTL3 deficiency.
ANGPTL3 deficiency was first observed in an obese animal model, the KK-San mouse, not expressing ANGPTL3 and showing reduced levels of circulating TGs [30]. Additional animal models, where the expression of ANGPTL3 was knocked-down, did not show fat accumulation either in the white adipose tissue (WAT), or in the brown adipose tissue (BAT), or in the heart as well as in the liver even when fed with the high-fat-high-cholesterol diet [31, 32].
After the first studies in mice, genome-wide associations studies (GWAS) in humans identified the association of several ANGPTL3 polymorphisms with lower TGs levels [33]. Then, Musunuru et al. identified loss-of-function (LOF) mutations in the ANGPTL3 locus in a family characterized by a comprehensive reduction of ApoB and ApoA1-containing lipoproteins [34]. This unique phenotype, subsequently identified in several other families and denominated as familial hypobetalipoproteinemia type 2 (FHBL2), allowed a deeper characterization of the consequences of ANGPTL3 inhibition on lipid and lipoprotein metabolism [34].
A pooled analysis, including 115 FHBL2 individuals carrying 13 different LOF mutations in the ANGPTL3 gene (14 homozygotes, 8 compound heterozygotes, and 93 heterozygotes) and 402 controls, highlighted that homozygous FHBL2 presented almost undetectable plasma levels of ANGPTL3, and this was associated with very low levels of circulating ApoB- and ApoA1-containing lipoproteins (TG 71%, LDLC 67%, HDL-C 39%, ApoB 48%) [35].
Additional experiments demonstrated that the complete absence of ANGPTL3 resulted in an increased LPL activity and mass with a marked reduction in the fasting levels of circulating free fatty acid (FFA) [35]. Then, the impact of ANGPTL3 deficit on postprandial metabolism of TG-rich lipoproteins was investigated. To this aim, 7 FHBL2, 31 heterozygous LOF carriers, and 35 wild-type controls underwent an oral fat load [23]. Results of this study highlighted that FHBL2 is characterized by a marked attenuation in the post-prandial lipemia probably explainable with faster catabolism of intestinally derived lipoproteins and faster uptake by the liver of their remnant. In addition, a larger expansion of the postprandial FFA pool was observed probably reflecting a more rapid TG-chylomicron lipolysis due to the increased LPL activity.
The Safety of ANGPTL3 Deficiency
Together with explorative and dynamic studies on lipoprotein metabolism, studies concerning safety have also been carried out in individuals with FHBL2.
The first study carried out on the Campodimele population [36] showed that the overall mortality within this community did not differ from the general population both when analyzing the tumor-related and the cardiovascular related mortality [36].
One of the most important questions were if the extremely low LDL-C levels per se could provoke adverse effects in humans [3]. Sterol metabolism has been investigated in both homozygous and heterozygous carriers of ANGPTL3 LOF. Results showed that circulating levels of markers of cholesterol synthesis, absorption and catabolism, as well as of bile acid metabolism in FHBL2 were comparable with those of individuals from the general population [18, 37].
It is well known that another condition causing a marked reduction of ApoB-containing lipoproteins is familial hypobetalipoproteinaemia (FHBL1; OMIM #615,558), due to heterozygous mutations in the APOB gene FHBL1 associated with accumulation of TGs in hepatocytes leading to hepatic steatosis. It is thought that this consequence is linked to the impairment of synthesis and export of VLDLs by the liver and the increased fractional catabolic rate of ApoB-containing lipoproteins [38]. As it was reported that also homozygous carriers of FHBL2 showed a reduced synthesis of VLDL, it is interesting to establish whether the FHBL2 phenotype carries an increased risk of liver steatosis [18]. Nevertheless, Di Costanzo et al. [38], performed a study comparing the clinical and liver characteristics of carriers of APOB with carriers of ANGPTL3 mutations. They found that FHBL2, differently from FHBL1, was not associated with higher prevalence or severity of liver steatosis [38]. Furthermore, a preliminary evaluation of the impact of FHBL2 phenotype on the liver content of fat measured by magnetic resonance has been presented by D’Erasmo et al. [39] during the 88th edition of the EAS congress. The results confirmed that unlike other genetic causes of hypolipidemia, ANGPTL3 deficiency does not appear to cause hepatic fat accumulation.
In the meanwhile, in a study from Stitziel et al. [40] it was tested whether ANGPTL3 deficiency translates into a reduced risk of coronary artery disease (CAD). In a very large cohort, the cardiovascular risk in carriers of ANGPTL3 LOF was compared with that of non-carriers. In heterozygous carriers of ANGPTL3 LOF, it was possible to detect a 34% reduction of cardiovascular ischemic events [40]. These results were further confirmed by Dewey et al. [41] showing that, among the participant of the DiscovEHR study and participants of four follow-up studies, LOF variants in ANGPTL3 were found in 0.33% of case patients with coronary artery disease and in 0.45% of controls (adjusted odds ratio, 0.59; 95% CI, 0.41 to 0.85; P = 0.004) [41].
Altogether, the evidence produced in the recent years pushed toward the development of specific drugs inhibiting ANGPTL3 in order to mimic the FHBL2 phenotype in patients suffering from dyslipidemia and atherosclerotic cardiovascular disease (ASCVD) [37].
Targeting ANGPTL3 in the Clinical Setting
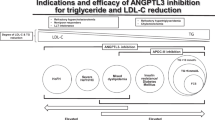
The ANGPTL3 protein is produced only by the liver at low-constant rates, and thus, it could be easily targeted with both monoclonal antibodies (Table 1) and novel RNA-based drugs (Table 2). Nevertheless, novel pharmacological technologies inhibiting ANGPTL3 are currently in development (Fig. 1).
The figure represents all drugs targeting ANGPTL3, either in use or under investigation for future clinical development. Drugs under clinical or pre-clinical research are marked with a gray label. The drug in clinical use (Evinacumab) is marked with a black label. A blood vessel carrying the compounds is shown in the middle. On the left, hepatocytes are targeted by most of the ANGPTL3-inhibitig drugs. On the right, B-memory cells produce a ANGPTL3-inhibiting antibody
Evinacumab
Evinacumab (commercial name Evkeeza, previously called REGN1500) is a fully humanized monoclonal antibody (mAb) targeting circulating ANGPTL3 (Fig. 1).
The pharmacological inhibition of ANGPTL3 trough the monoclonal antibody (REGN1500) in mice was found to reduce plasma cholesterol levels. The clearance of rabbit 125I- βVLDL or mouse 125I-LDL was lowered in case of treatment with mAb against ANGPTL3. Despite a 61% reduction in VLDL-TG production, VLDL-ApoB-100 production was unchanged in REGN1500-treated animals. Hepatic TG content, fatty acid synthesis, and fatty acid oxidation were similar in REGN1500 and control antibody-treated animals [42]. Interestingly, these effects were not affected by knocking out key proteins involved in the clearance of ApoB-containing lipoproteins (APOE, LDL-R, LRP-1, and SD-1) [31, 32].
Preclinical results pushed the research on the use of Evinacumab in humans and results of the phase I, II, and III trials are summarized in Table 1.
Due to the mechanism of action of ANGPTL3, one would expect that its inhibition is particularly efficacious in patients suffering from hypertriglyceridemia [43••]. A recent randomized clinical trial studied the effect of Evinacumab in severe hypertriglyceridemia. It enrolled three different cohorts of hypertrigliceridemic patients (NCT03452228). Those suffering from familial chylomicronemia syndrome (FCS) (cohort 1), where the activity of LPL is almost abolished, and the ones with multifactorial familial hypertriglyceridemia (MCS) with (cohort 2) and without (cohort 3) mutation in the LPL gene but residual or normal LPL activity. After 12 weeks of treatment with 15 mg/kg of Evinacumab iv, there was a significant reduction in the median TG levels vs. placebo in the two MCS cohorts (− 64.8% in cohort 2 and − 81.7% in cohort 3. Therefore, the presence of a residual LPL activity is essential for the TG-lowering effect of Evinacumab. These results would suggest that Evinacumab could be very effective in patients with hypertriglyceridemia associated with diabetes mellitus, obesity, or metabolic syndrome as well as in those with combined hyperlipidemia, but not in those carrying complete LPL deficiency (LPLD).
Due to the LDL-lowering phenotype associated with ANGPTL3 deficiency, the potential of Evinacumab was further explored in severe forms of hypercholesterolemia.
The most surprising results were those reported in the phase 3 randomized, controlled ELIPSE trial where patients with HoFH were enrolled. It was shown that treatment with Evinacumab at the dose of 15 mg/kg intravenously reduced LDL-C by approximately 50% with 47% of treated patients achieving an LDL-C below 100 mg/dl [44••]. Surprisingly, the degree of LDL-C lowering was higher in the Evinacumab group than in the placebo group among patients with null–null variants (–43.4% and + 16.2%, respectively) and non-null variants (–49.1% and –3.8%, respectively) suggesting that the mechanism by which Evinacumab reduces LDL-C is independent from the LDL receptor [44••]. A clue about the mechanisms by which Evinacumab may produce its LDL-lowering effect independently from the functionality of the LDLR pathway, has been proposed by a recent kinetic study from Reeskamp et al. [45•] These authors investigated four HoFH patients carrying mutations with different effects on LDLR activity and revealed that Evinacumab determines predominately an increased removal of IDL, which are the precursors of LDL particles. In the ELIPSE HoFH trial, the use of Evinacumab was associated with the occurrence of serious adverse events (urosepsis and suicide attempt, respectively) in 2 patients (5%) while 5 out of 44 patients (11%) in the Evinacumab and none in the placed group reported influenza-like symptoms. No signs of impaired hepatic safety were observed in patients exposed to Evinacumab. Indeed, increases in liver function test (LFT) were detected in 5% of patients randomized to Evinacumab and in 10% of those randomized to placebo with a more severe elevation in the latter group. It must be note that in both cases, the elevation in LFT was transient with values returning to a normal range during treatment.
The reassuring safety profile observed in the ELIPSE HoFH trial induced the US Food and Drug Administration (FDA) and the European Medicine Agency (EMA) to approve Evinacumab (Evkeeza) as second line treatment in HoFH patients aged above 12 [46]. The recommended dose is 15 mg/kg intravenously, administered in 60 min once every 4 weeks [46].
Evinacumab has also been tested in patients with refractory hypercholesterolemia, e.g., who have high LDL-C levels despite treatment with lipid-lowering therapies at maximum tolerated doses [47]. In a double-blind, placebo-controlled, phase 2 trial, 272 patients with or without heterozygous FH who had refractory hypercholesterolemia defined as LDL-C > 70 mg/dl or higher with atherosclerosis or > 100 mg/dl or higher without atherosclerosis (NCT03452228). Patients were randomly assigned to the following groups: subcutaneous Evinacumab at a dose of 450 mg weekly (40 patients), 300 mg weekly (43 patients), or 300 mg every 2 weeks (39 patients) or placebo (41 patients); or intravenous Evinacumab at a dose of 15 mg per kilogram of body weight every 4 weeks (39 patients) or 5 mg per kilogram every 4 weeks (36 patients) or placebo (34 patients). At week 16, patients receiving the maximum dose of Evinacumab (15 mg/kg every 4 weeks intravenously) experienced an LDL-C decrease of approximately 50%. Serious adverse events were observed in 3 to 16% of patients across treatment groups but none experienced elevation of LFT. The most common side effects were urinary tract infection, injection site erythema, arthralgia, myalgia in the subcutaneous group and abdominal pain, back pain, dizziness, fatigue, arm or leg pain, nausea, and nasopharyngitis in the intravenous group.
Vupanorsen
Vupanorsen (also called IONIS-ANGPTL3-LRx or AKCEA-ANGPTL3-LRx) is a Gal-NAc-conjugated antisense oligonucleotide targeting ANGPTL3 mRNA. GalNac domain selectively targets the asialoglycoprotein receptor (ASGPR) that is highly expressed in hepatocytes [48] thus specifically directing ASO to the liver where ANGPTl3 is almost exclusively produced (Fig. 1). GalNAc also has the function to stabilize the ASOs in hepatocytes, plasma, as well as the lymphatic system potentially reducing the immunogenicity [48]. These effects may potentially avoid the side effects commonly observed with ASO treatment such as thrombocytopenia and injection site reaction [49].
Results of the phase I and II trials on the use of Vupanorsen are summarized in Table 2. It must be acknowledged that the clinical development of Vupanorsen has foreseen its experimentation mainly in subjects with mild to moderate or severe hypertriglyceridemia. The only clinical trial on HoFH (NCT03455777) was withdrawn due to lack of available patients meeting entry criteria.
Vupanorsen was tested in an open-label phase II study of four patients with familial partial lipodystrophy [50] resulting in a reduction in fasting levels of triglycerides by 59.9%, ANGPTL3 by 54.7%, and in several other lipoproteins/lipids, including very low-density lipoprotein cholesterol by 53.5%, non-high-density lipoprotein cholesterol by 20.9%, and free fatty acids (FFA) by 41.7%.
Then, its use was evaluated in double-blind, placebo-controlled, dose-ranging, phase 2 study on 105 patients with diabetes, hepatic steatosis, and hypertriglyceridaemia [51]. The addition of vupanorsen 80 mg Q4W reduced Apolipoprotein C-III (58%), remnant cholesterol (38%), total cholesterol (19%), non-high-density lipoprotein cholesterol (HDL-C; 18%), HDL-C (24%), and apolipoprotein B (9%). These results were apparently positive highlighting a favorable lipid/lipoprotein profile with potential as cardiovascular risk reduction strategy [51].
Despite these promising results, the clinical development of Vupanorsen was recently stopped [52]. This was decided following a deep analysis of the phase IIb RCT named TaRgeting ANGPTL3 with an aNtiSense oLigonucleotide in AdulTs with dyslipidEmia (TRANSLATE-TIMI 70) [52]. This was a double blinded, placebo controlled, randomized, multicentric trial in dyslipidemic subjects under statin treatment. The study aimed at evaluating the non-HDL-C (total cholesterol – HDL cholesterol), TGs, and ANGPTL3 reduction in patients in the Vupanorsen arm, and results showed a significant reduction in non-HDL-C and additional lipid parameters. Despite this, treatment was associated with a dose dependent increase in liver steatosis and high dosage was associated with significant increase in AST and ALT liver enzymes raising some concern about the safety of pharmacological inhibition of ANGPTL3 synthesis [52]. Cellular studies reported the accumulation of ApoB within hepatocytes knocked out for ANGPTL3 [53], thus suggesting that the lack of this protein may have an adverse impact on the intracellular trafficking of neutral lipids. However, this conclusion appears in contrast with the observation that FHBL2 subjects do not develop liver steatosis [38, 39]. It is possible that in case of congenital loss of activity of ANGPTL3, compensatory mechanisms are activated that prevent the accumulation of lipids in the liver. Further studies are needed to confirm whether the development of liver steatosis during Vupanorsen is drug or target specific as well as to understand the metabolic pathways underlying this effect.
The Future for ANGPTL3 Targeting
siRNA ARO-ANG3
The use of small interfering RNAs (siRNAs) as a therapy in dyslipidemias has been proved to be safe and effective [54, 55]. SiRNAs are longer molecules than antisense oligonucleotides (ASOs) and have longer circulating half-life. Similar to ASOs, they could be tagged with three molecules of N-acetyl-Galactosamine, in order to be recognized by the asialoglycoprotein receptor (ASPGR) specifically expressed by the liver [48]. One construct using siRNA technology is now under clinical investigation for ANGPTL3 inhibition (ARO-ANG3).
The phase I randomized, placebo-controlled single and multiple dose study (NCT03747224) has recently closed. It involved 93 adult healthy volunteers as well as dyslipidemic patients. The aim was to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of ARO-ANG3. Results of this study will hopefully be available shortly.
A phase 2 trial on ARO-ANG3 (NCT04832971) was developed to evaluate the efficacy and safety of ARO-ANG3 in participants with mixed dyslipidemia. Participants initially received two subcutaneous injections of ARO-ANG3 or placebo. Participants who completed the double-blind treatment period may opt to continue in an open-label extension receiving up to 8 doses of ARO-ANG3. The study is currently running, and the estimated completion date is November 2024.
In June 2022, a phase 2 trial (NCT05217667) began with the aim to evaluate the safety and efficacy of ARO-ANG3 in subjects affected by homozygous FH. Sixteen participants who have provided informed consent will receive two open-label doses of ARO-ANG3 and be evaluated for safety and efficacy parameters through 36 weeks. The study is currently recruiting, and the estimated completion data is October 2023.
Vaccine Targeting ANGPTL3
The technology of a protein-based vaccine against ANGPTL3 is also under development [56]. Until now, the use of monoclonal antibodies directed toward ANGPTL3 has been the only effective approach in the treatment of severe dyslipidemias. The possibility to produce endogenous antibodies against native ANGPTL3 might produce the same effect of Evinacumab after a single or a seldom repeated subcutaneous shot of ANGPTL3 protein-based vaccine [56]. Fukami et al. recently produced three potential immunogenic peptides for a protein-based ANGPTL3 vaccine (named E1-E2-E3). E2 and E3 proved to be more immunogenic in the leptin deficient ob/ob mouse. After 6-weeks from immunization the treated mice showed a small but significant reduction in both serum TGs, LDL-C, and HDL-C levels. Moreover, they showed a significant reduction in the liver steatosis and immune cell infiltration that are typical of this murine model. The ANGPTL3 vaccine has also been trialed on mouse models showing fast progressing atherosclerosis, such as Apoeshl mice. The treatment with E3-vaccine inhibited atherosclerosis in this model with absence of evident atherosclerotic lesion up to the 22nd week after treatment [56].
CRISPR-Cas9 Technology
The “CRISPR-Cas9” was first discovered in 2012. It is based on an evolutionary conserved mechanism in procaryotic organisms used as a defense against non-self-DNA infections [57]. This mechanism uses RNA fragments called CRISPR that “guide” and regulate the Cas9 activity in order to induce double-strand breaks in non-self-DNA that could have infected the bacterial cell [57]. Differently from other gene-editing approaches based on viral particles, CRISPR-Cas9 technology allows high specificity targeting in generating double-strand breaks [58]. Moreover, the technology is extremely flexible, it is sufficient to produce different RNA guides to target potentially the entire genome. This makes CRISPR-Cas9 useful to silence the expression of small proteins encoded by small genomic regions, a situation that is well fitted with ANGPTL3 gene expression.
The silencing system for ANGPTL3 used for the first time a second generation Cas9 called “BE3”. This mutagenesis system is based on a specific DNA base-editing, it does not make double-strand breaks, hence reducing both on-target and off-target mutations due to the loose specificity of the RNA guide [59]. The produced BE3 system against ANGPTL3 targeted the Gln-135 codon of the gene. Murine models treated with this strategy showed more than 35% of ANGPTL3 alleles edited and no evidence of off-target mutations. This effect was associated with a reduction of − 50% in circulating ANGPTL3, − 35% in fasting TGs and − 20% in plasma cholesterol [59].
Despite being interesting, this technology is vastly unknown in terms of efficacy and safety in the short and long term. Although the base-editing could be delivered to specific organs (e.g., the liver), it is still unknown whether the selective KO could be dangerous or whether a low, yet undetectable frequency of potential off-targets could be a serious issue in the long term.
Conclusion
In conclusion, ANGPTL3 is clearly an interesting pharmacological target for the treatment of dyslipidemias and to prevent atherosclerosis. Despite this, some aspects of the role of ANGPTL3 need to be clarified (the mechanism of LDL reduction in case of ANGPTL3 inhibition, FFA flux, lipoprotein kinetics, intracellular role of ANGPTL3) as well as the effective cardiovascular protection in case of ANGPTL3 inhibition.
Although carriers of ANGPTL3 LOF apparently do not have any related organ damage or dysfunction, the safety profile of ANGPTL3 inhibiting drugs remains to be determined. Since the liver will be the principal target for the forthcoming ANGPTL3-inihibiting drugs, it will be crucial to verify the liver safety of ANGPTL3 inhibition, particularly considering results of the last trial on Vupanorsen (TRANSLATE TIMI 70). Data collected from clinical trials suggest that extracellular inhibition of ANGPTL3 (Evinacumab) does not produce the same effect of intracellular ANGPTL3 inhibition (Vupanorsen), thus suggesting a possible intracellular function of ANGPTL3 and compensatory mechanisms in lipid production when ANGPTL3 mRNA is inhibited.
This novel therapeutic approach for dyslipidemia will be extremely useful alone or in association with other drugs (e.g., PCSK9i) in the most severe forms of familial hypercholesterolemia (FH), like HoFH or severe, resistant hypercholesterolemia. It is worth highlighting that the treatment with Evinacumab determined a marked reduction in LDL-C even in patients with LDLRnull /LDLRnull mutations. This suggests that this drug acts by a mechanism that is independent from the LDLR machinery, making it highly suitable for HoFH patients with almost abolished LDLR activity or in severe hypercholesterolemic patients where information about the LDLR status is not available. Until now, available treatments of HoFH consisted of lipoprotein apheresis or Lomitapide, a small molecule, inhibitor of the microsomal triglyceride transfer protein (MTTP). The lipoprotein apheresis although effective is not available worldwide and is associated with a great burden both for the public health and for patients. The use of Lomitapide is limited, in some cases, by gastrointestinal side effects and by concerns on its long-term liver safety [60, 61]. On the contrary, so far, Evinacumab has not shown any concern on liver safety, thus increasing the interest on its use in the real-world setting. Again, the use of Evinacumab in association with a very low dose Lomitapide might be an attractive possibility to improve the tolerance of this latter medication.
Severe hypertriglyceridemia is also an unmet clinical need and results of trials have suggested that Evinacumab can be effectively used to reduce TGs in MCS patients. MCS is caused by the concomitant presence of an oligo/polygenic susceptibility and metabolic comorbidities such as obesity and type 2 diabetes. MCS patients could suffer from recurrent pancreatitis and differently from FCS and are more prone to the development of early onset of atherosclerosis being associated with great morbidity and mortality [62, 63]. MCS is a very heterogeneous condition, while some patients could be easily managed with conventional therapy for hypertriglyceridemia, others are much more challenging. These hypertriglyceridemic patients carrying residual LPL activity might achieve a reduction in TG levels up to 88% (NCT02107872) when exposed to drugs against ANGPTL3. The use of Evinacumab in this type of patients might be extremely beneficial and, in our opinion, its clinical indications should be enlarged.
The introduction of novel lipid lowering therapies, based on cutting-edge drug technologies, need an accurate patients’ selection based on both genetic and non-genetic factors in order to personalize the treatment of single patients and avoid unnecessary healthcare costs with the aim to maximize the cost-effectiveness balance.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–97. https://doi.org/10.1001/JAMA.2016.13985.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290(1):140–205. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2019.08.014.
Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet (London, England). 2017;390(10106):1962–71. https://doi.org/10.1016/S0140-6736(17)32290-0.
Thedrez A, Blom DJ, Ramin-Mangata S, et al. Homozygous familial hypercholesterolemia patients with identical mutations variably express the LDLR (low-density lipoprotein receptor): implications for the efficacy of Evolocumab. Arterioscler Thromb Vasc Biol. 2018;38(3):592–8. https://doi.org/10.1161/ATVBAHA.117.310217.
Bansal S, Ruzza A, Sawhney JPS, et al. Evolocumab in patients with homozygous familial hypercholesterolemia in India. J Clin Lipidol. 2021;15(6):814–21. https://doi.org/10.1016/J.JACL.2021.10.003.
Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019;4(12):1287–95. https://doi.org/10.1001/JAMACARDIO.2019.3780.
Pinzon Grimaldos A, Bini S, Pacella I, et al. The role of lipid metabolism in shaping the expansion and the function of regulatory T cells. Clin Exp Immunol. 2022;208(2):181–92. https://doi.org/10.1093/cei/uxab033.
Feingold KR. Introduction to Lipids and Lipoproteins. MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK305896/. Accessed August 29, 2021.
Björnson E, Adiels M, Taskinen MR, Borén J. Kinetics of plasma triglycerides in abdominal obesity. Curr Opin Lipidol. 2017;28(1):11–8. https://doi.org/10.1097/MOL.0000000000000375.
Borén J, Taskinen MR, Björnson E, Packard CJ. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat Rev Cardiol. 2022. https://doi.org/10.1038/S41569-022-00676-Y.
Klop B, Proctor SD, Mamo JC, Botham KM, Castro Cabezas M. Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int J Vasc Med. 2012;2012. https://doi.org/10.1155/2012/947417.
Lopez-Miranda J, Williams C, Larion D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98(3):458–73. https://doi.org/10.1017/S000711450774268X.
Berry SE, Valdes AM, Drew DA, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26(6):964–73. https://doi.org/10.1038/S41591-020-0934-0.
Zhang R, Zhang K. An updated ANGPTL3–4–8 model as a mechanism of triglyceride partitioning between fat and oxidative tissues. Prog Lipid Res. 2021;85. https://doi.org/10.1016/J.PLIPRES.2021.101140.
Bini S, D’Erasmo L, Di Costanzo A, Minicocci I, Pecce V, Arca M. The interplay between angiopoietin-like proteins and adipose tissue: another piece of the relationship between adiposopathy and cardiometabolic diseases? Int J Mol Sci. 2021;22(2):1–16. https://doi.org/10.3390/ijms22020742.
Tikkanen E, Minicocci I, Hällfors J, et al. Metabolomic signature of angiopoietin-like protein 3 deficiency in fasting and postprandial state. Arterioscler Thromb Vasc Biol. 2019;39(4):665–74. https://doi.org/10.1161/ATVBAHA.118.312021.
Banfi S, Gusarova V, Gromada J, et al. Increased thermogenesis by a noncanonical pathway in ANGPTL3/8-deficient mice. https://doi.org/10.1073/pnas.1717420115.
Minicocci I, Montali A, Robciuc MR, et al. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J Clin Endocrinol Metab. 2012;97(7):E1266–75. https://doi.org/10.1210/jc.2012-1298.
Conklin D, Gilbertson D, Taft DW, et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62(3):477–82. https://doi.org/10.1006/GENO.1999.6041.
Quagliarini F, Wang Y, Kozlitina J, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci USA. 2012;109(48):19751–6. https://doi.org/10.1073/PNAS.1217552109.
Inaba T, Matsuda M, Shimamura M, et al. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J Biol Chem. 2003;278(24):21344–51. https://doi.org/10.1074/jbc.M213202200.
Li H, Liu J. The novel function of HINFP as a co-activator in sterol-regulated transcription of PCSK9 in HepG2 cells. Biochem J. 2012;443(3):757–68. https://doi.org/10.1042/BJ20111645.
Minicocci I, Tikka A, Poggiogalle E, et al. Effects of angiopoietin-like protein 3 deficiency on postprandial lipid and lipoprotein metabolism. J Lipid Res. 2016;57(6):1097–107. https://doi.org/10.1194/jlr.P066183.
Li Y, Sun L, Xu H, et al. Angiopoietin-like protein 3 modulates barrier properties of human glomerular endothelial cells through a possible signaling pathway involving phosphatidylinositol-3 kinase/protein kinase B and integrin alphaVbeta3. Acta Biochim Biophys Sin (Shanghai). 2008;40(6):459–65. https://doi.org/10.1111/J.1745-7270.2008.00421.X.
Shimamura M, Matsuda M, Kobayashi S, et al. Angiopoietin-like protein 3, a hepatic secretory factor, activates lipolysis in adipocytes. Biochem Biophys Res Commun. 2003;301(2):604–9. https://doi.org/10.1016/S0006-291X(02)03058-9.
Ono M, Shimizugawa T, Shimamura M, et al. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J Biol Chem. 2003;278(43):41804–9. https://doi.org/10.1074/jbc.M302861200.
Chen YQ, Pottanat TG, Siegel RW, et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res. 2020;61(317):jlr.RA120000781. https://doi.org/10.1194/jlr.ra120000781.
Camenisch G, Pisabarro MT, Sherman D, et al. ANGPTL3 Stimulates endothelial cell adhesion and migration via integrin αvβ3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277(19):17281–90. https://doi.org/10.1074/JBC.M109768200.
Bini S, Pecce V, Di Costanzo A, et al. The fibrinogen-like domain of ANGPTL3 facilitates lipolysis in 3T3-L1 cells by activating the intracellular erk pathway. Biomolecules. 2022;12(4):585. https://doi.org/10.3390/biom12040585.
Fujimoto K, Koishi R, Shimizugawa T, Ando Y. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp Anim. 2006;55(1):27–34. https://doi.org/10.1538/EXPANIM.55.27.
Wang Y, Gusarova V, Banfi S, Gromada J, Cohen JC, Hobbs HH. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J Lipid Res. 2015;56(7):1296–307. https://doi.org/10.1194/JLR.M054882.
Wang Y, McNutt MC, Banfi S, et al. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc Natl Acad Sci U S A. 2015;112(37):11630–5. https://doi.org/10.1073/pnas.1515374112.
Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–97. https://doi.org/10.1038/NG.75.
Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–7. https://doi.org/10.1056/NEJMoa1002926.
Minicocci I, Santini S, Cantisani V, et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J Lipid Res. 2013;54(12):3481–90. https://doi.org/10.1194/jlr.P039875.
Fazio S, Sidoli A, Vivenzio A, et al. A form of familial hypobetalipoproteinaemia not due to a mutation in the apolipoprotein B gene. J Intern Med. 1991;229(1):41–7. https://doi.org/10.1111/J.1365-2796.1991.TB00304.X.
Arca M, D’Erasmo L, Minicocci I. Familial combined hypolipidemia : ANGPTL3 deficiency. Curr Opin Lipidol. 2020;31(2):41–8. https://doi.org/10.1097/MOL.0000000000000668.
Di Costanzo A, Di Leo E, Noto D, et al. Clinical and biochemical characteristics of individuals with low cholesterol syndromes: a comparison between familial hypobetalipoproteinemia and familial combined hypolipidemia. J Clin Lipidol. 2017;11(5):1234–42. https://doi.org/10.1016/j.jacl.2017.06.013.
D’Erasmo L, Neufeld T, Di Martino M, et al. The impact of ANGPTL3 deficiency on hepatic steatosis: Observations from carriers of loss-of-function mutations. Atherosclerosis. 2020;315: e17. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2020.10.064.
Stitziel NO, Khera AV, Wang X, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol. 2017;69(16):2054–63. https://doi.org/10.1016/j.jacc.2017.02.030.
Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211–21. https://doi.org/10.1056/nejmoa1612790.
Gusarova V, Alexa CA, Wang Y, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res. 2015;56(7):1308–17. https://doi.org/10.1194/JLR.M054890.
•• Ahmad Z, Banerjee P, Hamon S, et al. Inhibition of angiopoietin-like protein 3 with a monoclonal antibody reduces triglycerides in hypertriglyceridemia. Circulation. 2019;140(6):470–86. https://doi.org/10.1161/CIRCULATIONAHA.118.039107. This paper shows the results of 2 Phase 1 studies proving the efficacy and safety of in hypertriglyceridemic subjects showing comparable results that that observed in carrying loss of function mutations in ANGPTL3.
•• Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711–20. https://doi.org/10.1056/nejmoa2004215. This Phase III trials showed that homozygous familial hypercholesterolemia receiving maximum doses of lipid-lowering therapy plus Evinacumab had a 49% reduction from baseline in LDL-C at 24 weeks as compared the small increase in the placebo group.
• Reeskamp LF, Millar JS, Wu L, et al. ANGPTL3 inhibition with evinacumab results in faster clearance of IDL and LDL apoB in patients with homozygous familial hypercholesterolemia-brief report. Arterioscler Thromb Vasc Biol. 2021;41(5):1753–9. https://doi.org/10.1161/ATVBAHA.120.315204. In this small kinetic study, ANGPTL3 inhibition with evinacumab associates with an increase in the fractional catabolic rate of IDL apoB and LDL apoB.
Evkeeza: Pending EC decision | European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/evkeeza. Accessed June 7, 2021.
Rosenson RS, Burgess LJ, Ebenbichler CF, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383(24):2307–19. https://doi.org/10.1056/nejmoa2031049.
Prakash TP, Graham MJ, Yu J, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42(13):8796–807. https://doi.org/10.1093/NAR/GKU531.
Wang Y, Yu RZ, Henry S, Geary RS. Pharmacokinetics and clinical pharmacology considerations of GalNAc 3-conjugated antisense oligonucleotides. Expert Opin Drug Metab Toxicol. 2019;15(6):475–85. https://doi.org/10.1080/17425255.2019.1621838.
Foss-Freitas MC, Akinci B, Neidert A, et al. Selective targeting of angiopoietin-like 3 (ANGPTL3) with vupanorsen for the treatment of patients with familial partial lipodystrophy (FPLD): results of a proof-of-concept study. Lipids Health Dis. 2021;20(1). https://doi.org/10.1186/S12944-021-01589-4.
Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, Hurh E, Kingsbury J, Bartlett VJ, Figueroa AL, Piscitelli P, Singleton W, Witztum JL, Geary RS, Tsimikas S. Louis St. L O'Dea, the Vupanorsen Study Investigators, Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41(40):3936–3945. https://doi.org/10.1093/eurheartj/ehaa689.
Bergmark BA, Marston NA, Bramson CR, et al. Effect of vupanorsen on non-high-density lipoprotein cholesterol levels in statin-treated patients with elevated cholesterol: TRANSLATE-TIMI 70. Circulation. 2022;145(18):1377–86. https://doi.org/10.1161/CIRCULATIONAHA.122.059266.
Xu YX, Redon V, Yu H, et al. Role of angiopoietin-like 3 (ANGPTL3) in regulating plasma level of low-density lipoprotein cholesterol. Atherosclerosis. 2018;268:196–206. https://doi.org/10.1016/j.atherosclerosis.2017.08.031.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–30. https://doi.org/10.1056/nejmoa1913805.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa1912387.
Fukami H, Morinaga J, Nakagami H, et al. Vaccine targeting ANGPTL3 ameliorates dyslipidemia and associated diseases in mouse models of obese dyslipidemia and familial hypercholesterolemia. Cell Rep Med. 2021;2(11). https://doi.org/10.1016/J.XCRM.2021.100446.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. https://doi.org/10.1126/SCIENCE.1225829.
Zalatan JG, Lee ME, Almeida R, et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160(1–2):339–50. https://doi.org/10.1016/J.CELL.2014.11.052.
Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation. 2018;137(9):975–7. https://doi.org/10.1161/CIRCULATIONAHA.117.031335.
D’Erasmo L, Bini S, Arca M. Rare treatments for rare dyslipidemias: new perspectives in the treatment of homozygous familial hypercholesterolemia (HoFH) and familial chylomicronemia syndrome (FCS). Curr Atheroscler Rep. 2021;23(11):65. https://doi.org/10.1007/s11883-021-00967-8.
D’Erasmo L, Minicocci I, Nicolucci A, et al. Autosomal recessive hypercholesterolemia: long-term cardiovascular outcomes. J Am Coll Cardiol. 2018;71(3):279–88. https://doi.org/10.1016/j.jacc.2017.11.028.
Moulin P, Dufour R, Averna M, et al. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): Expert panel recommendations and proposal of an “FCS score.” Atherosclerosis. 2018;275:265–72. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2018.06.814.
D’Erasmo L, Di Costanzo A, Cassandra F, et al. Spectrum of mutations and long-term clinical outcomes in genetic chylomicronemia syndromes. Arterioscler Thromb Vasc Biol. 2019;39(12):2531–41. https://doi.org/10.1161/ATVBAHA.119.313401.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Laura D’Erasmo has received personal fees for public speaking, consultancy or grant support from Amryt Pharmaceuticals, Akcea Therapeutics, Pfizer, Amgen, SOBI and Sanofi.
Marcello Arca has received research grant support from Amryt Pharmaceutical, Amgen, IONIS, Akcea Therapeutics, Pfizer and Sanofi; has served as a consultant for Amgen, Aegerion, Akcea Therapeutics, Regeneron, Sanofi and Alfasigma and received lecturing fees from Amgen, Amryt Pharmaceutical, Pfizer, Sanofi and AlfaSigma.
Simone Bini has received personal fees for public speaking from Akcea Therapeutics and SOBI.
The other authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bini, S., Tramontano, D., Minicocci, I. et al. How ANGPTL3 Inhibition Will Help Our Clinical Practice?. Curr Atheroscler Rep 25, 19–29 (2023). https://doi.org/10.1007/s11883-022-01076-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-022-01076-w