Abstract
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome and a risk factor for both cardiovascular and hepatic related morbidity and mortality. The increasing prevalence of this disease requires novel therapeutic approaches to prevent disease progression. Farnesoid X receptors are bile acid receptors with roles in lipid, glucose, and energy homeostasis. Synthetic farnesoid X receptor (FXR) agonists have been developed to specifically target these receptors for therapeutic use in NAFLD patients. Here, we present a review of bile acid physiology and how agonism of FXR receptors has been examined in pre-clinical and clinical NAFLD. Early evidence suggests a potential role for synthetic FXR agonists in the management of NAFLD; however, additional studies are needed to clarify their effects on lipid and glucose parameters in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a metabolic disease, the prevalence of which parallels that of the obesity epidemic in developed and developing nations [1, 2]. Classically, patients with NAFLD have hepatic steatosis and features of the metabolic syndrome, namely, central obesity, dyslipidemia, insulin resistance, and hypertension [3]. NAFLD predicts development of both cardiovascular disease [4•] and diabetes [5], and mortality in NAFLD patients is primarily due to cardiometabolic disease [6]. Non-hepatic malignancy and liver disease account for the second and third causes of death in these patients, respectively [6], and by 2020, end-stage liver disease due to NAFLD is expected to be the leading indication for liver transplantation in the USA [7] making this disease a significant public health problem.
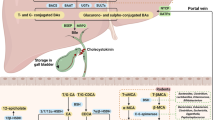
The histologic spectrum of NAFLD includes simple hepatic steatosis, so-called non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), i.e., hepatic steatosis and inflammation with or without fibrosis, and cirrhosis [8••]. The NAFLD activity (NAS) score is the most widely accepted scoring system for NASH diagnosis. The NAS score ranges from 0 to 8 and a score of ≥5 correlates with NASH diagnosis [9]. Only 15 % of NAFLD patients have NASH but NASH patients have an 18-year liver-related mortality of 17.5 % compared with 2.7 % for NAFL patients [6]. Because of the disproportionate mortality associated with NASH compared with NAFL, most NAFLD therapeutic studies have focused on treatments to ameliorate NASH and prevent advanced fibrosis (Fig. 1).
The mainstay of treatment in NASH patients is weight loss by calorie reduction and exercise [8••]. A randomized controlled trial examining the effect of diet, exercise, and behavior modification (i.e., self-monitoring, stimulus-control, relapse prevention, and problem-solving techniques) on NASH progression showed that an average weight loss of 9.3 % from baseline correlates with histologic improvement in NASH severity [10]. Seventy-two percent of patients in the experimental group had at least a 3-point improvement of the NAS score compared with 30 % of patients in the control group (p = 0.03). Long-term maintenance of weight loss in these patients was not examined, but an earlier study of patients with the metabolic syndrome suggests that after 2 years, most patients are unable to sustain weight loss [11]. As NASH is often considered the hepatic manifestation of the metabolic syndrome [12], NASH patients are likely to have difficulty maintaining weight loss as well.
The development of NASH therapeutics is a rather recent area of study, beginning with the use of weight loss medications. Orlistat is the only weight loss medication to date that has been studied in NASH patients. Overweight patients with NASH were randomized to receive either orlistat with vitamin E and dietary modification or vitamin E alone with dietary modification. Although this randomized controlled trial demonstrated improvements in both hepatic inflammation and insulin sensitivity in those patients who achieved weight loss 9 % or greater, it failed to show any significant benefit of orlistat over vitamin E [13].
The challenges with weight loss maintenance and the lack of efficacy with weight loss medications have led, in part, to increasing interest in the development of NASH therapies that target the underlying pathogenesis. It is presumed that NAFL progresses to NASH; however, there is little evidence of a linear progression. Rather, NASH likely results from a complex interaction of genetic, nutritional, hormonal, and immunologic factors that incite “multiple parallel hits” [14] ultimately causing impaired insulin signaling, dysregulated lipid metabolism, oxidative stress, and hepatic inflammation. Not surprisingly, medications already approved for management of insulin resistance and diabetes were among the first category of medications studied for treatment of NASH. Biguanides and thiazolidinediones (TZDs) are insulin sensitizers that have been studied for this indication. The biguanide metformin improves hepatic insulin sensitivity through activation of AMP-activated kinase and subsequent inhibition of gluconeogenesis [15]. Although several small studies and open-label trials showed promise, results of a meta-analysis of these studies showed that metformin improves neither serologic nor histologic markers of hepatic inflammation in NASH patients [16] thwarting its approval for treatment of NASH.
In contrast, TZDs have shown promise as treatment for NASH. Pioglitazone is a TZD currently approved and recommended for the management of biopsy-proven NASH in both diabetic and non-diabetic patients [8••]. In diabetic patients with NASH, a randomized controlled trial demonstrated that pioglitazone significantly improves steatohepatitis compared with placebo [17] and a subsequent RCT in non-diabetic patients with NASH showed that pioglitazone improves both steatohepatitis and fibrosis compared with lifestyle interventions in subjects with NASH [18]. The largest RCT of NASH therapeutics to date is the Pioglitazone versus Vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis (PIVENS) trial of non-diabetic patients randomized to pioglitazone, vitamin E, or placebo. Results from this trial show greater histologic improvement of NASH in patients who received pioglitazone compared with those who received placebo [19]. Despite the encouraging results of these pioglitazone studies, the adverse effect of TZD-induced weight gain and concerns about the long-term safety profile of TZDs has limited the use of pioglitazone in patients with NASH.
Therapeutics that target oxidative stress and inflammation have also been studied in NASH patients. The antioxidants vitamin E and vitamin C were examined in a small prospective randomized controlled trial. The combination of 1000 IU vitamin E, 1000 mg vitamin C, a low-fat diet, and weight loss counseling improved fibrosis scores in NASH patients but did not improve inflammation scores [20]. This study was confounded, however, by the higher body mass index (BMI) and larger percentage of diabetics in the treatment group. Although vitamin C is not FDA-approved for the treatment of NASH, vitamin E is approved based on results of the PIVENS trial that demonstrated improvement of steatohepatitis in NASH patients who took 400 IU of vitamin E daily [19]. The uncertain interaction of vitamin E with cardiac [21] and oncologic disease risk [22] and its lack of efficacy in improving insulin sensitivity [19], however, make this medication of limited utility in the general NASH population.
Given the encouraging results of TZD insulin sensitizing medications, it is not surprising that a class of medication that both improves insulin sensitivity and reduces hepatic inflammation without the PPARy-associated side effect of weight gain has become the holy grail of NASH therapeutics. Hydrophilic bile acids (BAs) are the latest candidates for NASH therapeutics as they both regulate glucose and lipid homeostasis [23] and have anti-inflammatory properties [24]. BAs signal through two receptors, farnesoid X receptor (FXR) [25–27] and TGR5 [28]. Although agonists for both receptors have been developed and are currently being investigated for use in metabolic diseases, the FXR agonist obetacholic acid (OCA) is the first in-class synthetic BA under investigation for the treatment of NASH [29]. This review will make evident both the rationale for the use of FXR agonism in NASH and the potential challenges by outlining (1) the physiology of BA metabolism, (2) FXR signaling effects on glucose and lipid homeostasis, and (3) pre-clinical and clinical data of FXR agonism in hepatic steatosis with a particular focus on OCA.
Bile Acid Physiology
BAs are amphipathic steroid molecules derived from cholesterol precursors. The sources of cholesterol include dietary cholesterol and return of cholesterol to the liver from tissues via reverse cholesterol transport. The liver is the site of synthesis of the primary BAs, cholic acid, and chenodeoxycholic acid, while intestinal bacteria are responsible for the conversion of these primary BAs into the secondary BAs, deoxycholic, and lithocholic acids. The details of secondary BA synthesis are extensively reviewed in Ridlon et al. [30] and are beyond the scope of this current review. In the liver, the classic BA synthetic pathway begins with hydrolysis of cholesterol to 7α-hydroxycholesterol by the microsomal enzyme CYP7A1, the rate-limiting step of classic BA synthesis. An alternative pathway also exists in which side-chain hydroxylated cholesterols (oxysterols) are converted into 7α-hydroxycholesterol by CYP7A1, CYP39A1, or CYP7BI microsomal enzymes. 7α-Hydroxycholesterol is subsequently isomerized and oxidized by the microsomal enzyme, 3β-hydroxy-∆5-C27-steroid oxireductase. A series of downstream events involving multiple enzymes results in the synthesis of two cholanic acids: cholic acid and chenodeoxycholic acid which differ by addition of one hydroxyl group by CYP8B1. Cholic acid and chenodeoxycholic become the primary constituents of bile after conjugation primarily with taurine or glycine by the peroxisomal enzyme bile acid coenzyme A:amino acid N-acyltransferase (BAAT). Such conjugation confers a more hydrophilic property to the BAs for secretion into bile canaliculi by bile salt export pump (BSEP).
BAs are secreted with phospholipids and cholesterol creating primary bile and stored in the gallbladder before release into the small intestine. Cholecystokinin-mediated gallbladder contraction pumps primary bile into the small intestine in response to intestinal fat. Bile then aids in fat emulsification and digestion of lipid-soluble vitamins. BAs subsequently enter the enterohepatic circulation after re-absorption in the intestine terminal ileum. At the level of the terminal ileum, FGF-19 (FGF-15 in mice) is secreted into the portal circulation to inhibit hepatocyte BA synthesis. Notably, insulin-resistant NAFLD patients have normal baseline FGF-19 levels but an impaired post-prandial hepatic response to FGF-19 resulting in a failure to reduce bile salt synthesis [31]. Only a small percentage of primary BAs passes into the colon where colonic bacteria convert primary BAs into the secondary BAs, deoxycholic and lithocholic acids. These BAs can be excreted in feces or passively reabsorbed into the portal circulation. This elaborate BA circulation is reviewed in detail in Lefebvre et al. [23].
BAs have both an enterohepatic and systemic circulation through which they help regulate key lipid and glucose homeostatic mechanisms, processes of great relevance for NASH, and other metabolic diseases. For example, mice fed sodium cholic acid are protected from hepatic steatosis, muscle insulin resistance, hyperglycemia, hyperinsulinemia, and high-fat diet-induced obesity [32]. Further, rats fed a salmon-protein rich diet have higher plasma BA content, are protected from obesity, and have reduced hepatic triglyceride accumulation [33]. These rats also have lower serum triglyceride level compared with casein protein-fed mice. In addition, they have improved hepatic insulin sensitivity as suggested by the higher glycogen levels and lower expression of PEPCK, the liver’s major gluconeogenic enzyme. Energy expenditure is also increased thought to be the result of upregulation of uncoupling and energy expenditure genes in white and brown adipose tissue and muscle. These improvements in whole body metabolism were largely prevented by pharmacologic binding of BAs by cholestyramine.
In humans, circulating BAs have been implicated in the regulation of glucose and lipid homeostasis, as well. Patients with prior gastric bypass anti-obesity surgery have higher circulating BA levels than BMI-matched control patients [34]. These BA levels are inversely correlated with post-prandial glucose and fasting triglyceride levels and positively correlated with serum adiponectin, an insulin sensitizing hormone. The relationship between BA levels and metabolic disease risk, however, remains unclear as type 2 diabetic patients have been reported to have both higher conjugated BA levels than control patients [35–37] and unchanged BA levels [36]. The discrepancy in findings may in part be explained by relative differences in concentrations of primary and secondary BAs, as type 2 diabetic men have higher total BA pool due to higher cholic acid synthesis rate and deoxycholic acid (DCA) input rate. Notably, these diabetic men have lower serum levels of chenodeoxycholic acid (CDCA) [37], the BA ligand with the greatest FXR affinity [25].
Upon return to the liver from the circulation, BAs cross the basolateral membrane of hepatocytes via both sodium-dependent and sodium-independent transporters [38]. Using Forster resonance energy transfer sensor (FRET) analysis, van der Velden et al. observed that BAs are able to directly enter the nucleus after entry into the cell [39]. There, BAs can bind FXR and it is this binding and resultant downstream signaling events that establish the basis for the therapeutic use of FXR agonism in metabolic diseases.
Farnesoid X Receptor Signaling
At the cellular level, BAs affect changes in lipid and glucose metabolism (reviewed below) primarily through binding FXR. FXR is a nuclear receptor transcription factor encoded by nuclear receptor subfamily 1, group H, member 4 (NR1H4) and was originally named for its suspected ability to bind farnesoid. BAs were ultimately found to be the natural ligands for FXR [26] through which BAs feedback negatively on their own synthesis. The net effect, therefore, of FXR agonism on bile acid homeostasis is the inhibition of additional BA synthesis, reduction of BA hepatic uptake, and promotion of BA hepatocellular export [40]. FXR exists in four isoforms (FXRα 1–4) [41] that appear to have tissue specificity. To date, these isoforms have been found in multiple tissues and cell types in both rodents and humans. In humans, FXRα1/2 is predominantly found in liver and adrenal glands, while colon, duodenum, and kidney have the highest expressions of FXRα3-4 [42]. Data in mice suggest that these isoforms have differential binding to promoters that regulate BAs. For example, the mouse BA intestinal transporter, bile acid binding protein, is more sensitive to binding by FXRα4 (also called FXRβ2) than all other isoforms consistent with the high expression of FXRα4 in mouse intestine. On the other hand, there is no difference in induction of BSEP and short hairpin protein (SHP, detailed below) by the individual isoforms [41].
FXRs bind FXR DNA response elements as monomers or heterodimers with the nuclear receptor RXR to regulate transcription of genes involved in lipid and glucose homeostasis and inflammation [23] (Fig. 2). Evidence of FXR’s critical role in lipid and glucose homeostasis include the dyslipidemic and hepatic steatotic phenotype of FXR null mice [43, 44] and upregulation of FXR in response to glucose and downregulation in response to insulin [45]. In addition, although there are no known human FXR gene polymorphisms associated with NAFLD risk, two human NR1H4 gene polymorphisms have been associated with impaired lipid and glucose homeostasis. Namely, rs4764980 and rs11110390 are associated with impaired fasting and post-glucose load free fatty acid levels, and rs4764980 is associated with impaired fasting glucose [46]. A comprehensive review of all the target genes of FXR signaling is beyond the scope of this current review; however, a few representative examples of FXR targets relevant to NASH pathophysiology include SREBP1c, PPARα, PEPCK, and NF-κB and will be discussed below.
Schematic of FXR blinding and regulation of key genes involved in NASH. BA bile acid, FXR farnesoid X receptor, FXRe farnesoid X response element, RXR retinoid X receptor, SREBP1 sterol regulatory binding protien 1, PPAR α peroxisomal proliferator activator receptor α, MTTP microsomal triglyceride transfer protein, PEPCK phosphoenolpyruvate carboxykinase, G6Pase glucose 6-phosphatase, GSK glycogen synthase kinase
FXR and SREBP1
Sterol regulatory element-binding transcription factor 1 (SREBP1) is a nuclear transcription factor important for de novo lipogenesis, a process upregulated in NASH patients and NAFLD experimental models that results in the synthesis of lipids [47]. FXR activation by both natural and synthetic FXR agonists downregulates SREBP1 thereby reducing expression of several genes involved in de novo lipogenesis [48]. Watanabe et al. demonstrated this phenomenon by treating chow and high-fat diet fed male KK-Ay mice with the synthetic FXR agonist GW4064 and measuring hepatic triglycerides. KK-Ay mice are diabetic KK mice [49] with a mutation in the agouti gene that results in severe obesity, florid diabetes and other features of the metabolic syndrome. In the absence of GW4064, these mice have severe steatosis from hepatic accumulation of triglycerides. In this pivotal study, treatment of chow-fed wild-type and KK-Ay mice with cholic acid reduced hepatic SREBP1c expression and expression of several genes involved in fatty acid and triglyceride synthesis (e.g., AceCS, ME, SCD-1). This downregulation of SREBP1 was associated with upregulation of short hairpin protein (SHP), a gene that inhibits nuclear receptors (e.g., LRH-1 and LXR) required for transcription of CYP7A1, the rate-limiting enzyme of BA synthesis. The authors also performed in vitro studies in mouse primary hepatocytes to demonstrate that CDCA reduces expression of SREBP-1c and its target lipogenic genes through an SHP-dependent pathway. Importantly, these authors also demonstrated an increase in serum LDL cholesterol in their in vivo model likely as a result of downregulation of the hepatic LDL receptor due to downregulation of SREBP1. This result is consistent with prior reports and may impact the clinical utility of FXR agonists.
FXR and PPARα
FXR agonists appear to have opposite effects on peroxisome proliferator-activated receptor alpha (PPARα) compared with SREBP1. PPARα is a nuclear transcription factor whose activation results in expression of genes important for fatty acid uptake, binding, and oxidation, i.e., biochemical processes required for the cellular utilization of fatty acids as fuel. Reduced PPARα expression contributes to NASH pathogenesis [50]. PPARα is upregulated in response to both CDCA and the FXR agonist GW4064 in human hepatoma HepG2 cells, human primary hepatocytes [51], and rat hepatoma cells [52]. Pineda Torra et al. also demonstrated the existence of an FXR response element on the human PPARα promoter and upregulation of carnitine palmitoyltransferase, a PPARα target oxidative gene [51]. The combined effects of SREBP1 inhibition and PPARα activation likely contribute to the improved hepatic lipid profile upon FXR activation in experimental models.
FXR and PEPCK
Phosphoenolpyruvate carboxykinase (PEPCK) is a cataplerotic enzyme responsible for the conversion of oxalacetate to phosphoenolpyruvate and a key enzyme for gluconeogenesis and glyceroneogenesis [53]. Conflicting evidence exists regarding the role of FXR in regulation of PEPCK. Duran-Sandoval et al. reported that FXR gene expression is reduced in a streptozotocin-induced diabetic rat model and in diabetic Zucker rats. They observed increased FXR expression in primary rat hepatocytes in response to glucose and reduced expression with exposure to insulin [45]. Following publication of the previous study, Stayrook et al. sought to examine the effect of FXR agonism on PEPCK in rat hepatoma cells and mice. Unexpectedly, they observed that incubation of cells with either CDCA or the FXR agonists GW4064 or fexaramine caused dose-related increases in PEPCK mRNA without increasing other key gluconeogenic enyzmes. Conversely, incubation of cells with the natural FXR antagonist, guggulsterone, reduced PEPCK mRNA expression.
In primary rat hepatocytes, both PEPCK expression and glucose production were increased in response to FXR agonism and CDCA. These findings were replicated in C57BL6 mice [52] and are in contrast to findings by DeFabiani et al., who observed that CDCA reduces PEPCK expression through an FXR-independent mechanism [54] and those of Yamagata et al., who demonstrated a reduction of hepatic PEPCK gene expression in cholic acid fed mice compared with chow-fed mice and in HepG2 cells incubated with CDCA [55]. They did not specifically examine whether reduced PEPCK was due to FXR agonism or independent of FXR. However, they observed upregulation of SHP, the induction of which has been linked previously with FXR activation [56]. Based on data demonstrating relevance of the fed or fasted state to PEPCK expression [54], the variability of these study results is likely due to differences in the models used and/or nutrient availability. Clarifying these discrepancies is critical to the development of NASH therapeutics given the prevalence of hyperglycemia and insulin resistance in this population and underscore the need to examine these questions directly in humans.
FXR and NF-κB
Increased intestinal permeability, translocation of gut microbiota, and resultant increased LPS production in the portal circulation incite hepatic inflammatory pathways in NASH patients [57]. NF-κB is a nuclear factor whose activation in response to LPS and other stimuli has been linked to inflammatory cell recruitment and carcinogenesis [58], both features of advanced stages of NASH. FXR activation is implicated in the NF-κB-mediated hepatic inflammatory response. In vitro pharmacologic activation of the NF-κB pathway in primary hepatocytes from FXR null mice increases inflammatory cytokines iNOS and COX-2 when compared to untreated cells while incubation with the FXR agonists GW4064 and CDCA reduces cytokine expression, inhibits expression of NF-κB target genes, and reduces NF-κB activity and signaling. In vivo, FXR null mice have increased levels of inflammatory cytokines both at baseline and in response to LPS compared with wild-type mice and adenoviral hepatic expression of FXR ameliorates these effects [59].
Together, these studies demonstrate critical roles for FXR in hepatocellular energy homeostasis and inflammation and lay the framework for further study of FXR pharmacologic agonists in experimental in vivo models of hepatic injury.
FXR Agonism in Pre-clinical In Vivo Models of Liver Injury
The FXR null mouse is one of the few models whose liver disease encompasses the full spectrum of NAFLD pathology, including development of hepatocellular carcinoma [60, 61]. Given the poor availability of NASH experimental models, most researchers have used animal models that focus on individual stages of NASH pathogenesis to study FXR in liver disease. Results of studies using both types of models link FXR expression and/or agonism with reduced hepatic steatosis [62–64], inflammation [65], fibrosis [65, 66], and carcinogenesis [60, 61], and improved regenerative capacity [67–70]. The following sections summarize the use of pharmacologic FXR agonists in pre-clinical in vivo NAFLD studies.
FXR Agonism in Hepatic Steatosis and Steatohepatitis
Hepatic steatosis is considered the earliest, most benign stage of NAFLD. Both genetic and nutritional models can be used to study this phenotype. A commonly used genetic model for promotion of hepatic steatosis is a model of leptin deficiency. Leptin is an adipokine that regulates feeding and energy balance [71]. The Zucker (fa/fa) rat has a loss of function mutation of the leptin receptor and develops hyperleptinemia with resultant hyperphagia, obesity, insulin resistance, and hepatic steatosis [72]. Oral administration of obeticholic acid (OCA) to these rats results in improved glucose and insulin tolerance and weight loss and reduced steatohepatitis [73]. Improvement in the NASH phenotype is associated with reduced SREBP1 and TNFα and increased PPARα. Additionally, hepatic insulin signaling improves as assessed by downregulation of glucose synthetic enzymes and upregulation of key insulin signaling molecules. Cipriani et al. also demonstrate a reduction in HDL with OCA administration [73], a consistent yet unexplained finding in the human trials (discussed below).
Xiong et al. observed an association between hepatic steatosis and reduced hepatic FXR in old wild-type mice. Further, administration of the synthetic FXR agonist GW4064 improves steatosis and serum triglyceride levels in old mice compared with younger cohorts of mice possibly due to improvements in endoplasmic reticulum stress [63].
In another model of hepatic steatosis, Liu et al. compared mice drinking a 30 % fructose water solution with mice fed tap water for 8 weeks. Fructose-fed mice developed hepatic steatosis that was ameliorated by systemic administration of the synthetic FXR agonist WAY-362450 possibly through improvements in the intestinal barrier function [64]. These authors further demonstrated that WAY-362450 improves hepatic steatosis and LPS-mediated hepatic cytokine surge. Notably, these improvements corresponded with reduced perilipin 2 expression, a lipid droplet protein highly expressed in human and experimental steatotic diseases and linked to insulin resistance [74–76].
Aged FXR null mice develop steatohepatitis [77] but modeling steatohepatitis in younger rodents is challenging as mice are rather resistant to hepatic inflammation when fed standard high-fat diets. Several diets, including the methionine and choline deficient (MCD) diet, have been developed to model NASH liver pathology. The MCD diet promotes hepatic steatosis, inflammation, and fibrosis when fed to mice, but does not cause weight gain, hypertriglyceridemia, or insulin resistance seen in human NASH patients [78]. Nevertheless, this model is the most well-established rodent NASH model. Mice fed an MCD diet for 8 weeks have increased serum bile acids compared with control-diet fed mice possibly due to cytokine induced bile acid dysregulation [79]. Although such evidence of bile acid dysregulation in experimental NASH is intriguing, few studies have followed up these findings with FXR agonism in NASH experimental models. In fact, much of the data regarding FXR agonism in the pathologic stage of NASH come from work done in models investigating non-hepatic organs.
One such study examined pharmacologic FXR agonism in presumed steatohepatitis in the context of an erectile dysfunction study. This study examined penile pathology in high-fat diet fed rabbits and streptozotocin treated rats, a model of type I diabetes [80]. Although not the primary aim of the research, Vignozzi et al. demonstrated impaired glucose tolerance and an increase in fasting blood glucose, cholesterol, triglyceride, liver transaminases, blood pressure, and visceral adiposity in high-fat diet-fed rabbits. Administration of the FXR agonist 6α-ethyl chenodeoxycholic acid (OCA) normalized visceral adiposity, fasting glucose, and glucose tolerance, but had only modest effects on the other metabolic parameters and liver transaminases. OCA did not improve glycemia in stretozotocin-treated rats suggesting important pathologic differences between FXR agonism in type 1 and type 2 diabetic experimental models.
Examination of FXR agonism in apolipoprotein E-deficient mice (a model of premature atherosclerosis) revealed that 10 mg/kg OCA administered by oral gavage reduces liver triglycerides but has no effect on serum transaminases, and it also reduces total cholesterol, HDL, and VLDL [81]. Further investigation of hepatic metabolic pathways demonstrated downregulation of several lipid synthetic genes (including SREBP1) in the 10 mg/kg OCA-treated group. There was significant improvement in plaque size but also a reduction in the LDL receptor gene expression which may relate to the increase in LDL subsequently observed in the clinical trials (discussed below).
FXR Agonism in Hepatic Fibrosis and Liver Regeneration
Recapitulation of NASH fibrosis in experimental models is wrought with challenges as with steatohepatitis. Three established models to incite hepatic fibrosis in rodents are the carbon tetrachloride (CCL4) [82], bile duct ligation (BDL) [83], and porcine serum (PS) [84] models. FXR null mice have worse liver injury and cholestasis in response to CCL4 compared with wild-type mice [70]. FXR is expressed in hepatic stellate cells but, perhaps counterintuitively, fibrosis in PS rats is not associated with reduced FXR gene expression or FXR-regulated genes [66]. Despite this observation, pharmacologic FXR agonism with OCA to BDL and PS rats protects against histologic fibrosis and increase of profibrogenic markers. This anti-fibrogenic action is mediated by the nuclear receptor SHP whose induction inhibits BA synthesis.
The liver’s regenerative response is critical in preventing the harmful sequelae of hepatic fibrosis. Here, too, FXR agonism has been shown to have a beneficial role. Huang et al. demonstrated that wild-type mice fed cholic acid have 30 % increased liver mass as a result of increased hepatocyte DNA replication [67]. Liver repair is hindered in the setting of FXR deficiency [70], is particularly stunted in early stages of regeneration in FXR null mice [67], and contributes to significantly higher mortality in FXR null mice after partial hepatectomy [67]. Huang et al. also investigated the effect of FXR agonism on specific cell cycle regulators. Through a series of elegant experiments, including adenoviral overexpression of FXR in mice livers, they observed that FXR regulates Foxm1b, a transcriptional factor required for normal liver regeneration. They also noted that induction of Foxm1b is impaired in aged mice. Administration of a synthetic FXR agonist Px20350 reversed this defect in aged mice [68]. Together, these studies outline a significant role of FXR in protection against hepatic fibrosis and promotion of liver regeneration.
FXR Agonism in Hepatocellular Carcinoma
There is an increased risk of hepatocellular carcinoma in patients with NASH [85] and FXR may play a role in tumorigenesis. Near simultaneous publications from two different groups established the spontaneous development of liver tumors in aged FXR null mice [60, 61]. In both studies, the majority of the tumors were hepatocellular adenomas or carcinomas. Reduction of the bile acid pool with a bile acid resin reduces malignant tumor formation [61]. The mechanism of bile acid induced carcinogenesis may relate to tumor suppressor gene expression. The tumor suppressor N-myc downstream-regulated gene 2 is downregulated in the livers of FXR null mice and in human hepatocellular carcinoma [86]. Using a nude mouse hepatocellular carcinoma xenograft model, Deuschle et al. demonstrated reduction of tumor growth and metastases in mice treated with the FXR agonist Px20606 [86]. These findings are quite provocative as there are currently no pharmacologic treatments for hepatocellular carcinoma which offer potential for cure.
FXR Agonism as Treatment for Human NAFLD
Ursodeoxycholic Acid
The history of BAs for treatment of NAFLD began with trials of the naturally occurring bile acid ursodeoxycholic acid (ursodiol, UDCA). UDCA is a partial FXR agonist [87] approved in the USA for the treatment of primary biliary cirrhosis and primary sclerosing cholangitis, rare cholestatic liver diseases that can progress to cirrhosis. Because of its antioxidant and anti-apoptotic effects [88], it was the first bile acid investigated for the treatment of NASH. Initial studies included a heterogenous array of study designs and sample sizes with varied results. A US-based randomized, controlled trial of low dose UDCA (13–15 mg/kg) in biopsy-proven NASH patients [89] failed to demonstrate significant histologic improvement after 2 years of treatment. It is possible, however, that a lack of efficacy with UDCA may have been due to underdosing, as a subsequent European trial showed improvement of serologic markers of hepatic inflammation and fibrosis and insulin sensitivity with high-dose UDCA (28–35 mg) in biopsy-proven NASH patients [90]. The European findings have yet to be replicated in the USA.
OCA and Clinical NAFLD
The synthetic bile acid analogue OCA is the first-in class synthetic bile acid under investigation for treatment of patients with NAFLD [29]. OCA is a 6-α ethyl derivative of CDCA and dual FXR/TGR5 agonist with 100-fold greater FXR agonism than CDCA [91]. A double-blind, randomized, placebo controlled phase IIb study was performed to examine the safety and efficacy of OCA in patients with type 2 diabetes and presumed NAFLD [92••]. NAFLD was defined by elevated transaminases, hepatomegaly by imaging, or standard liver histologic criteria in type 2 diabetic patients without other causes of liver disease. Of note, >80 % of patients were diagnosed non-invasively by imaging reflecting real-world clinical practice. Two patients were diagnosed by liver biopsy. Patients were randomized to receive 25 mg OCA, 50 mg OCA, or placebo once daily by mouth for 6 weeks. A total of 64 patients (25 mg n = 20; 50 mg n = 21; placebo n = 23) from four health care centers were enrolled and 56 patients completed the study. A fasting euglycemic-hyperinsulinemic clamp was performed using both low-dose and high-dose insulin infusions prior to the start of the study drug and after the last dose. Primary outcomes were efficacy as measured by improvements in insulin sensitivity, measured as glucose infusion rate (GIR), and safety.
Patients receiving the 25-mg dose of OCA demonstrated a statistically significant increase in GIR of 28 and 18 %, respectively, with both the low- (60 mU/m2/min) and high-dose (120 mU/m2/min) insulin infusions (p = 0.019 and p = 0.036, respectively). Although there was a trend toward improvement in insulin sensitivity in the 50-mg group, these results did not reach statistical significance. This improvement in GIR may represent modest clinical improvement as a prior study of NAFLD and diabetic patients revealed a 52 and 44 % improvement in GIR in control patients undergoing euglycemic-hyperinsulinemic clamp with 40 mU/m2/min compared with NAFLD and diabetic patients, respectively (p < 0.001) [93].
The authors’ safety data revealed a greater percentage of treatment-related adverse events in the 50-mg group (38 %) compared with the 25-mg group (5 %) or placebo group (26 %). The majority of these were gastrointestinal side effects and dermatologic disorders. There were two patients (one each in the placebo and 50-mg groups) who withdrew consent because of an adverse event but there were no deaths due to treatment [92••].
Secondary outcome measures were changes in body weight, liver enzymes, serum lipids, FGF19, 7a-hydroxy-4-cholesten-3-one (C4, a cholesterol metabolite formed by the rate-limiting enzyme CYP7A1), endogenous BAs, and serologic measures of liver fibrosis and apoptosis. Patients in the 50-mg OCA treatment group experienced a statistically significant average weight loss of 2 % (p = 0.008) compared with placebo patients [92••]. Consistent with its known mechanism of action, FXR agonism resulted in dose-dependent increases in FGF19 and reductions in endogenous BA production and C4.
The enhanced liver fibrosis (ELF) score is a panel of serum markers used to estimate fibrosis. Patients who received the 25-mg dose of OCA had small, but statistically significant improvements in this score compared with placebo patients (p = 0.004), while patients who received the 50 mg dose had no change in their overall score. Cytokeratin-18 is a circulating marker of hepatic apoptosis and tracks with NASH severity [94]. This value was only available for 5–7 patients per group and was unchanged in all groups.
There were mixed results for serum liver tests. Patients who received OCA had reductions in ALT and gamma glutamyltransferase (GGT) but increases in alkaline phosphatase. The discordance of these results requires further analysis including alkaline-phosphatase fractionation and may elucidate the etiology of pruritus experienced in the sister FLINT trial (see below). Serum lipid analysis revealed an average final total cholesterol level in both OCA arms that was comparable with control levels (174, 181, 183 mg/dL in control, 25-mg, and 50-mg groups, respectively). Mean total LDL cholesterol was significantly higher in the 25-mg OCA groups (120 mg/dL, p = 0.01) and 50-mg group (129 mg/dL, p = 0.008) compared with control patients (107 mg/dL). By study end, the 50-mg OCA group had a lower HDL cholesterol compared with controls (37 versus 40 mg/dL, respectively, p = 0.01). Serum triglycerides improved in the 50-mg OCA patients compared with control (121 versus 178 mg/dL, respectively, p = 0.02), and there was a trend toward improvement in the 25-mg group. The reasons for the difference in lipid profile in OCA-treated patients compared with control patients are unknown and whether these changes translate into increased cardiovascular events needs further evaluation in long-term studies.
OCA and Clinical NASH
Based on the safety results of the above trial, researchers explored OCA in a randomized controlled trial of non-diabetic NASH patients [95••]. The Farnesoid X Receptor (FXR) Ligand Obeticholic Acid in Nonalcoholic Steatohepatitis (NASH) Treatment Trial (FLINT trial) was a double-blinded, placebo-controlled trial of non-cirrhotic patients with biopsy-proven NASH. Liver enzymes, histology, and serologic markers were examined in patients receiving either 25 mg of OCA or placebo daily for 72 weeks. An end-of-treatment liver biopsy was planned and patients were subsequently followed for an additional 24 weeks of therapy. The primary endpoint was a 2-point improvement in the NAS score [9], and no worsening of fibrosis. Secondary endpoints included NASH resolution, and any improvement of histologic inflammation, fibrosis, serum liver enzymes, insulin resistance, anthropometric measures, and quality of life. An interim analysis demonstrated significantly improved steatohepatitis in the treatment group compared with placebo. This resulted in early cessation of the treatment phase of the trial after approximately 50 % of patients completed 72 weeks of treatment. All patients were subsequently followed for an additional 24 weeks without performance of additional liver biopsies.
A total of 110 treatment and 109 placebo patients were included in the primary analysis. The treatment and placebo groups were well matched in terms of demographics, comorbidities, and baseline liver histology. Although the authors did not report a statistical difference, baseline insulin levels and homeostasis model assessment of insulin resistance (HOMA-IR) were higher in the treatment group compared with the placebo group. In addition, despite incorporation of liver biopsies for diagnosis of NASH, 20 % of OCA-treated patients and 12 % of placebo patients did not have hepatocellular ballooning on baseline histology, a criterion required for definitive diagnosis of NASH [8••]. This difference did not reach statistical significance, however.
Forty-five percent of the treatment group and 21 % of the placebo group met the primary endpoint of a 2-point improvement in the NAS score (p = 0.0002) independent of patient baseline demographics or clinical parameters. In addition, patients who received OCA had statistically significant improvements in individual features of inflammation and fibrosis. Notably, in the NAS scoring system, improvement in fibrosis is the only feature that has been associated with reduced risk of death, liver transplantation or liver-related outcomes [96]. The histologic improvements in the FLINT study translated into a trend toward NASH resolution, but this endpoint did not reach statistical significance. Similar to the OCA NAFLD study in diabetics, the authors again observed lower ALT levels in the OCA group but an increase in serum alkaline phosphatase despite lower GGT levels. These levels returned to normal after study completion indicative of the drug’s reversible action.
Anthropometric assessments revealed approximately 2-kg weight loss by week 72 in patients who took OCA compared with placebo. These changes, however, were not reflected in the HOMA-IR, perhaps because of a lack of improvement in central adiposity, a metric that more closely estimates insulin resistance and health risk [97]. Fasting glucose was similar between the two groups at the end of the study, but patients taking OCA had higher insulin levels resulting in a statistically significant higher HOMA-IR. It is noteworthy that there was a wide distribution of both baseline and end-of-study insulin values which may have factored into this unexpected result. If these findings are replicated in future studies, it will be important to determine the tissue-specific contribution to insulin resistance through performance of a euglycemic-hyperinsulinemic clamp with tracer kinetics.
Lipid analyses of the enrolled patients demonstrated a similar dyslipidemic profile of OCA-treated patients as in the NAFLD diabetes study. There was no difference in serum triglycerides between the two groups at 72 weeks. Patients who received OCA had an average increase of 0.16 mmol/L (6 mg/dL) in cholesterol compared with an average decrease of 0.19 mmol/L (7 mg/dL) in the placebo group (p = 0.0009). Additionally, there was a −0.02 mmol/L (0.8 mg/dL) reduction in HDL cholesterol (p = 0.01) and 0.22 mmol/L (8.5 mg/dL) increase in LDL cholesterol in the OCA group (p < 0.0001). The peak effect on total, LDL, and HDL cholesterol was at 12 weeks with reversion of these changes toward baseline for the remainder of the study (Fig. 3). The interim analysis of these data resulted in escalation of medical management for dyslipidemia during the trial. Although enhanced lipid management likely contributed to the improvement in lipid profile by the end of the study in OCA-treated patients, the details of lipid management were not included in the published report. Follow-up trials should include data regarding both the intensity of lipid management and additional safety data on the interaction between lipid-lowering therapies and OCA. It is also of interest to explore the role of individual FXR gene polymorphisms on the lipid management strategies in OCA-treated patients, as the FXR single nucleotide polymorphism -1G > T has been found to impact the efficacy of rosuvastatin [98], a statin medication commonly used in patients with hyperlipidemia.
Serum lipid levels in FLINT trial. Republished with permission of The Lancet by Lancet Publishing Group from [95••]. Permission conveyed through Copyright Clearance Center, Inc.
The lipid abnormalities with OCA treatment are being further evaluated in a separate study according to study authors and have not been associated with increased cardiovascular events. Three OCA patients and one placebo patient suffered cardiovascular death or non-fatal myocardial infarction or non-fatal stroke. Two patients in the OCA group died during the study: one of sepsis and congestive heart failure and the other of cardiac ischemia or infarction. Neither event was considered related to the study drug, and the lipid profiles and duration of study enrollment were not reported.
The most common adverse effect of OCA treatment was the development of pruritus in 23 % of OCA patients compared with 6 % of placebo patients. The majority of patients judged this symptom as intense or widespread requiring medications or periods of withholding treatment. One patient discontinued OCA as a result of this symptom. The incidence of pruritus in the treatment group is not unexpected given that OCA is also an agonist for TGR5 whose activation promotes the release of itch and analgesia neurotransmitters from sensory nerves in experimental models of cholestasis [99]. This adverse event may limit the tolerability of OCA in some patients and require symptomatic management. The IND and comprehensive FLINT trial datasets were transferred from NIDDK in December 2014 and the effect of OCA on NASH, lipid parameters and side effects will continue to be explored in a phase 3 trial whose program initiation is planned for 2015 [100].
Ongoing NAFLD Clinical Trials of FXR Agonists
OCA is the first FXR agonist to be studied in NAFLD patients; however, several additional FXR agonists are under active investigation. Study designs include enrollment of healthy adults, adults with metabolic syndrome, and adults with NAFLD. A list of current studies most relevant to patients with NAFLD is provided in Table 1.
FXR Agonists in Alcoholic Steatohepatitis
The development of insulin resistance is an early feature of alcoholic liver disease in experimental disease models [101] making FXR agonism an attractive target. To date there are two pre-clinical studies examining FXR agonism in experimental alcoholic liver disease. Wu et al. fed mice a standard Lieber-DeCarli alcohol diet or control diet and observed that alcohol reduces FXR activity while administration of the FXR agonist WAY-362450 protects mice from development of alcoholic steatosis and oxidative stress [102]. A subsequent study with OCA similarly demonstrated improvement in alcoholic steatosis and oxidative stress in mice fed a 10 % ethanol and a low protein diet [103]. There are as yet no completed clinical trials examining FXR agonism in patients with alcoholic liver disease, but The Trial of Obeticholic Acid in Patients With Moderately Severe Alcoholic Hepatitis is a phase 2 trial currently recruiting patients [29]. Patients will be assigned to either placebo or 10 mg OCA for 6 weeks with a primary outcome of change in MELD score (a well-established liver severity score [104]) and adverse events. Unlike NASH patients, patients with alcoholic hepatitis suffer from cholestasis in addition to steatohepatitis. It is, therefore, conceivable that effects of OCA may be intermediate between those seen in PBC patients [105] and NASH patients [95••].
Conclusions
Innovative targeted approaches to management are needed to curb the NAFLD epidemic. BAs and their synthetic analogues are attractive therapeutic agents as their biology is well understood, they are orally available, and they affect key energy homeostatic mechanisms. It is this last characteristic, however, that requires fine-tuning. The development of FXR agonists is the first attempt to reap the benefits of BAs without exposure to the adverse effects.
The majority of pre-clinical data investigating the effects of FXR agonism in glucose homeostasis, liver lipid metabolism, and NASH pathology demonstrate important roles for FXR in these processes. However, the use of different models and methods of FXR agonism have resulted in some incongruent findings. Results of the two NAFLD OCA clinical trials require additional follow-up, as there were clear improvements of both non-invasive and invasive measures of steatohepatitis and fibrosis, but there are discrepant results on insulin sensitivity between the two studies. Short-term (i.e., 6 weeks) OCA administration appeared to improve insulin sensitivity and weight in diabetic patients with NAFLD [92••], but long-term administration (i.e. 72 weeks) failed to show a similar benefit in non-diabetic, NASH patients [95••]. The difference may pertain to an augmented role of FXR agonism in the diabetic population compared with non-diabetics where FXR-independent BA signaling may play a greater role in glucose homeostasis. Or, more simply, the differences may be due to differences in methodology and sampling. These data need to be clarified in larger clinical trials.
The mechanism of the dyslipidemia that developed in patients treated with OCA requires further investigation as both low HDL cholesterol and high LDL cholesterol are associated with increased cardiovascular risk. Rodent and primate models demonstrate different FXR-dependent lipoprotein profiles from those seen in humans taking OCA. Nevertheless, these models reveal several mechanisms that may affect serum lipoprotein concentration including FXR-dependent effects on hepatic synthesis and secretion of apolipoproteins, expression of reverse cholesterol synthesis pathway genes, expression of FXR responsive hepatic genes, and intestinal absorption [106, 107]. Additionally, lipoprotein levels may be both dependent and independent of the hepatic receptors [108] and involve hepatic and non-hepatic pathways [107]. Formal studies using radiolabeled tracers in human subjects are therefore needed to fully assess the fate of lipids in patients taking OCA. These studies should include patients taking both low and high cholesterol diets.
In summary, NAFLD is a complex disease that results in part from dysregulated lipid and glucose metabolism. Naturally occurring BAs as well as synthetic BA analogues may represent novel therapeutic interventions for this disease. It is conceivable that FXR agonism has a role in the management of NAFLD and experimental and clinical studies of FXR agonism have yielded some promising results. However, the unresolved effects on glucose and lipid homeostasis merit further investigation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bellentani S et al. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):155–61.
Browning JD et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95.
Marchesini G et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23.
Stepanova M. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2012;10(6):646–50. This study is an analysis of NHANES III data examining cardiovascular disease risk in NAFLD patients by comparing ultrasound and mortality data.
Adams LA et al. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104(4):861–7.
Rafiq N et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2009;7(2):234–8.
Charlton MR et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–53.
Chalasani N et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. This guideline establishes the standard of care for management of NAFLD patients.
Kleiner DE et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Promrat K et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–9.
Svetkey LP et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–48.
Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(1):27–38.
Harrison SA et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49(1):80–6.
Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–46.
Zhou G et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–74.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85.
Belfort R et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307.
Aithal GP et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–84.
Sanyal AJ et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85.
Harrison SA et al. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98(11):2485–90.
Saremi A, Arora R. Vitamin E and cardiovascular disease. Am J Ther. 2010;17(3):e56–65.
Lippman SM et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51.
Lefebvre P et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–91.
Hollman DA et al. Anti-inflammatory and metabolic actions of FXR: insights into molecular mechanisms. Biochim Biophys Acta. 2012;1821(11):1443–52.
Makishima M et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–5.
Parks DJ et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–8.
Wang H et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53.
Kawamata Y et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–40.
Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G852–8.
Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–59.
Schreuder TC et al. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G440–5.
Ikemoto S et al. Cholate inhibits high-fat diet-induced hyperglycemia and obesity with acyl-CoA synthetase mRNA decrease. Am J Physiol. 1997;273(1 Pt 1):E37–45.
Liaset B et al. Nutritional regulation of bile acid metabolism is associated with improved pathological characteristics of the metabolic syndrome. J Biol Chem. 2011;286(32):28382–95.
Patti ME et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17(9):1671–7.
Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med. 1977;296(24):1365–71.
Abrams JJ, Ginsberg H, Grundy SM. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes. 1982;31(10):903–10.
Brufau G et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52(4):1455–64.
St-Pierre MV et al. Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001;204(Pt 10):1673–86.
van der Velden LM et al. Monitoring bile acid transport in single living cells using a genetically encoded Forster resonance energy transfer sensor. Hepatology. 2013;57(2):740–52.
Fuchs C, Claudel T, Trauner M. Bile acid-mediated control of liver triglycerides. Semin Liver Dis. 2013;33(4):330–42.
Zhang Y, Kast-Woelbern HR, Edwards PA. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem. 2003;278(1):104–10.
Huber RM et al. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 2002;290(1–2):35–43.
Sinal CJ et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–44.
Ma K et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–9.
Duran-Sandoval D et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53(4):890–8.
Heni M et al. Genetic variation in NR1H4 encoding the bile acid receptor FXR determines fasting glucose and free fatty acid levels in humans. J Clin Endocrinol Metab. 2013;98(7):E1224–9.
Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118(3):829–38.
Watanabe M et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–18.
Ikeda H. KK mouse. Diabetes Res Clin Pract. 1994;24(Suppl):S313–6.
Stienstra R et al. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148(6):2753–63.
Pineda Torra I et al. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17(2):259–72.
Stayrook KR et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146(3):984–91.
Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284(40):27025–9.
De Fabiani E et al. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem. 2003;278(40):39124–32.
Yamagata K et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279(22):23158–65.
Goodwin B et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–26.
Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7(12):691–701.
Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46(2):590–7.
Wang YD et al. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48(5):1632–43.
Kim I et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28(5):940–6.
Yang F et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67(3):863–7.
Lu Y et al. Yin Yang 1 promotes hepatic steatosis through repression of farnesoid X receptor in obese mice. Gut. 2014;63(1):170–8.
Xiong X et al. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J Hepatol. 2014;60(4):847–54.
Liu X et al. Activation of farnesoid X receptor (FXR) protects against fructose-induced liver steatosis via inflammatory inhibition and ADRP reduction. Biochem Biophys Res Commun. 2014;450(1):117–23.
Zhang S et al. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51(2):380–8.
Fiorucci S et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127(5):1497–512.
Huang W et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):233–6.
Chen WD et al. Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription. Hepatology. 2010;51(3):953–62.
Zhang L et al. Significance and mechanism of CYP7a1 gene regulation during the acute phase of liver regeneration. Mol Endocrinol. 2009;23(2):137–45.
Meng Z et al. FXR regulates liver repair after CCl4-induced toxic injury. Mol Endocrinol. 2010;24(5):886–97.
Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37.
Durham HA, Truett GE. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R652–8.
Cipriani S et al. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51(4):771–84.
Carr RM et al. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One. 2014;9(5):e97118.
Chang BH et al. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26(3):1063–76.
Varela GM et al. Inhibition of ADRP prevents diet-induced insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G621–8.
Bjursell M et al. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS One. 2013;8(5):e64721.
Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87(1):1–16.
Tanaka N et al. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56(1):118–29.
Vignozzi L et al. Farnesoid X receptor activation improves erectile function in animal models of metabolic syndrome and diabetes. J Sex Med. 2011;8(1):57–77.
Mencarelli A et al. Antiatherosclerotic effect of farnesoid X receptor. Am J Physiol Heart Circ Physiol. 2009;296(2):H272–81.
Perez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3(1):112–20.
Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65(3):305–11.
Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology. 1992;16(6):1452–73.
Ascha MS et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–8.
Deuschle U et al. FXR controls the tumor suppressor NDRG2 and FXR agonists reduce liver tumor growth and metastasis in an orthotopic mouse xenograft model. PLoS One. 2012;7(10):e43044.
Campana G et al. Regulation of ileal bile acid-binding protein expression in Caco-2 cells by ursodeoxycholic acid: role of the farnesoid X receptor. Biochem Pharmacol. 2005;69(12):1755–63.
Amaral JD et al. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50(9):1721–34.
Lindor KD et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39(3):770–8.
Ratziu V et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54(5):1011–9.
Pellicciari R et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47(18):4559–69.
Mudaliar S et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–82 e1. This is the first clinical trial of the first-in-class FXR synthetic agonist OCA in diabetic NAFLD patients that establishes the safety of OCA in NAFLD patients.
Marchesini G et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–50.
Feldstein AE et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–8.
Neuschwander-Tetri BA, et al., Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2014. This is the first clinical trial of OCA in NASH patients.
Angulo P, The prognostic relevance of liver histology features in nonalcoholic fatty liver disease: the PRELHIN study. AASLD 2014. Abstract. 2014.
Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–84.
Hu M et al. The farnesoid X receptor -1G > T polymorphism influences the lipid response to rosuvastatin. J Lipid Res. 2012;53(7):1384–9.
Alemi F et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123(4):1513–30.
Intercept Provides 2014 Year-End Update and Planned 2015 Milestones. Jan. 15, 2015 Accessed January 26, 2015]; Available from: http://ir.interceptpharma.com/releasedetail.cfm?releaseid=890713.
Carr RM et al. Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol Clin Exp Res. 2013;37(7):1091–9.
Wu W et al. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem Biophys Res Commun. 2014;443(1):68–73.
Livero FA et al. The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chem Biol Interact. 2014;217:19–27.
Dunn W et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41(2):353–8.
Kowdley KV, Jones D, Luketic V, C.R.B.A., et al., An International Study Evaluating the Farnesoid X Receptor Agonist Obeticholic Acid as Monotherapy in PBC. J Hepatol. 2011(Suppl 1): p. S13.
Lambert G et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278(4):2563–70.
Hambruch E et al. Synthetic farnesoid X receptor agonists induce high-density lipoprotein-mediated transhepatic cholesterol efflux in mice and monkeys and prevent atherosclerosis in cholesteryl ester transfer protein transgenic low-density lipoprotein receptor (−/−) mice. J Pharmacol Exp Ther. 2012;343(3):556–67.
Rohrl C et al. Bile acids reduce endocytosis of high-density lipoprotein (HDL) in HepG2 cells. PLoS One. 2014;9(7):e102026.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) grant funding from the following sources: K08-AA021424, P30-DK-50306 pilot, and feasibility grant program and Robert Wood Johnson Foundation, Harold Amos Medical Faculty Development Award, 71586 (RMC)
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Rotonya M. Carr and Andrea E. Reid declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Nonstatin Drugs
Rights and permissions
About this article
Cite this article
Carr, R.M., Reid, A.E. FXR Agonists as Therapeutic Agents for Non-alcoholic Fatty Liver Disease. Curr Atheroscler Rep 17, 16 (2015). https://doi.org/10.1007/s11883-015-0500-2
Published:
DOI: https://doi.org/10.1007/s11883-015-0500-2