Abstract
Purpose of Review
The objective of this article is to provide a recent update of the association between allergic inflammation and chronic rhinosinusitis. The systematic approach of this review article critically evaluates the literature published over the past few years and summarizes the specific pathophysiologic pathway of chronic sinonasal inflammation that has been postulated.
Recent Findings
From a systematic search of the Ovid Medline and Embase, 11 studies were included in a qualitative analysis of the association between systemic allergy and chronic rhinosinusitis (CRS). Of the 11 studies, four showed an association, three were inconclusive, and four did not show any association. From the systematic search, 50 studies suggested four possible pathophysiologic pathways that may explain the association of allergic inflammation and CRS, namely, (1) staphylococcal enterotoxin, (2) the innate immunity pathway, (3) mast cell–associated inflammation, and (4) dysbiosis of microbiota.
Summary
The association of systematic allergy and CRS remains inconclusive. The recent advances in the study of the pathophysiologic pathway of CRS may lead to the possibility of a targeted treatment option for CRS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is a disease with multiple etiologies. One of the possible etiologies is allergic inflammation. There are several studies about the association between systemic allergy and CRS [1, 2]. However, there is no clear consensus on the pathophysiologic role of allergy on CRS development. The association could be caused by mechanistic links between allergic inflammation and CRS. Alternatively, they could be merely multimorbidity, which is the coexistence of two or more chronic diseases.
Local allergic inflammation was proposed as a part of the pathophysiologic mechanism that may lead to the development of CRS and may increase the severity, especially in CRS with nasal polyps (CRSwNP) and allergic fungal rhinosinusitis (AFRS) [3, 4, 5••].
To identify the phenotypes of CRS, several markers have been used, such as eosinophilic count, immunohistochemical staining, enzyme-linked immunosorbent assay (ELISA) for measuring cytokines of eosinophils (e.g., interleukin (IL)-5 and IL-13), reverse transcription polymerase chain reaction (RT-PCR) for measuring transcription factors, serum (e.g., eosinophilia, periostin, lymphocyte) by flow cytometry, and nasal secretion for microparticle or heat shock protein [6,7,8,9].
In the last 5 years, there have been several studies about the association of systemic allergy and CRS [1, 10••] and studies to determine local allergic inflammation involvement in the chronic process of CRS [9, 11, 12]. Therefore, we aimed to evaluate the evidence of the association between systemic allergies and to summarize the findings of studies investigating the pathophysiologic pathway involving local allergic inflammation that could contribute to the chronic process of CRS.
Material and Methods
Search Strategy
A systematic search of the literature was performed by two reviewers (Tantilipikorn P and Ngaotepprutaram P) who independently conducted a search using the OVID Medline and Embase databases from 1990 to September 2019. The following keywords were used: “chronic rhinosinusitis,” “rhinosinusitis,” “sinusitis,” “allergy,” “skin test,” “prick test,” “immunoglobulin E,” and “sensitization”. Additional articles from the references of retrieved literature were also selected if they contained the same keywords to expand the scope of searching. The abstracts included in the first step were then screened independently by both reviewers. The abstracts that were written in English language and had full text availability were included for further study selection.
Study Selection
We set up the inclusion and exclusion criteria before the selection of relevant studies. The inclusion criteria were primary research, which included different study types (descriptive studies, observational studies, randomized trials, and basic science articles), recently published within the last 5 years (after January 2014). We included both adult and pediatric populations. The studies addressing the CRS and allergy with or without a control group were retained for the analysis of the association and were considered for meta-analysis if possible. We excluded secondary research studies (e.g., review articles or systematic review), animal studies, and non-English language articles. Articles were then read and selected independently by the two reviewers. Any disagreements between the two reviewers were subsequently resolved by their consensus.
To address the mechanistic links between allergic inflammation and CRS, the articles summarizing the possible specific pathophysiologic pathway of chronic sinonasal inflammation were narratively reviewed.
Results
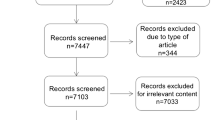
The details of the systematic search performed are shown in Fig. 1. The initial search from both databases yielded 112 articles, and one that was a duplicate was excluded. Then, 85 studies were excluded based on the selection criteria. Twenty-six full text articles were screened, and 13 were removed because they were not the original article. Among the remaining 13 publications, 2 were excluded because of irrelevant content. Finally, 11 articles which encompassed 8 articles studied in adult and remaining 3 articles in children about the association between allergy and CRS were included in this review (Table 1).
The Association Between Allergy and CRS
Bakhshaee et al. showed that the prevalence of allergic rhinitis (AR) in CRS patients was as high as 64%, and no difference in prevalence between CRSwNP and chronic rhinosinusitis without nasal polyp (CRSsNP) was seen [2]. The most common allergen in the whole CRS patients group was Salsala. However, the terms AR and allergy were used interchangeably in this study.
Green et al. described the pattern of allergic sensitization in CRS in Canadian patients, and the investigators compared CRS and non-CRS groups [13]. Ragweed was the most common outdoor allergen (40.4%), and both mite and cat were the most common indoor allergens (29.3%) in CRS patients. Alternaria alternata was also common in a large percentage of all allergens (36.4%). The study showed that the allergic sensitization in CRS group was much higher than in the control group (73% vs 32%, respectively). Mite and timothy grass were found to be more prevalent in CRS patients than their non-CRS counterparts. However, these differences were not significant. It is notable that the level of specific immunoglobulin E (sIgE) > 65 kU/L was considered to be a positive allergy test, which is higher than in other studies.
Sedaghat et al. retrospectively reviewed 4044 pediatric CRS patients over a 10-year period [14]. Their study showed that 3376 patients (83.5%) were CRS without any concomitant diagnosis such as cystic fibrosis, immunodeficiency, or primary ciliary dyskinesia. The prevalence of AR in CRS was found much lower (26.5%) than in other studies, and asthma was considered to be strongly positively associated with AR in CRS (OR 8.25 [95% CI 6.77–10.04]). The low prevalence may have resulted from the inclusion criteria, which used AR patients instead of allergic sensitization. In addition, data in these studies were collected using ICD-9 coding screening, and the method diagnosis was not clear. Criteria to specify disease may vary from person to person. The same investigators also reported the characterization of aeroallergen sensitization in a subset of patients with allergic testing data from their previous studies [14, 15]. Mites accounted for 51.4% of all sensitized allergens and were the most common in 140 pediatric CRS patients, followed by trees (42.9%).
Tomljenovic et al. compared 14 patients with CRSsNP and allergic sensitization with 18 patients with CRSsNP and no allergic sensitization [16]. There was no difference in visual analogue scale score for CRS symptoms and measures of perceived stress scale between the two groups except for postnasal drip, back pain, and startle response, which were higher in the allergy groups. Although the total Sinonasal Outcome Test-22 (SNOT-22) score was markedly increased in the atopy group, no significant difference was found. The method of allergy evaluation was not reported clearly in this study.
Brook et al. demonstrated that 61.9% of CRS patients had allergic sensitization, and atopic status was not associated with the Lund-Mackay score from computerized tomography (CT) scan [17]. However, the investigators included both rhinitis and chronic rhinosinusitis in their regression model. In addition, the diagnosis of both diseases was identified by the ICD-9 coding without clear definitions. Hence, we rate their findings as inconclusive.
Hamizan et al. studied the pattern of central compartment atopic disease (CCAD) from CT scan and inhalant allergen sensitization [18]. Investigators found that 63 of 112 CRS patients (56.2%) had allergen sensitization. Among the allergen sensitization, grass and mites were the dominant allergens (68.2% and 65.1%, respectively). Allergic sensitization was more prevalent in CCAD radiologic patterns (limited disease involving the floor/medial wall of sinus or normal sinus mucosa) than in the non-CCAD group (73.5% vs 53.2%, respectively; p = 0.030). Among the tested allergens, mites were the only one that reached significance (58.8% vs 32.6%; p < 0.010). Similar to Brook et al. [17], Hamizan et al. found no association between Lund-Mackay CT score and atopic status. The CCAD CT phenotype demonstrated diagnostic test performance results of sensitivity, specificity, positive predictive value, and negative predictive value of 19.8%, 90.8%, 73.5%, and 46.8%, respectively. Finally, investigators concluded that aeroallergen sensitization was associated with CCAD radiological phenotype in CRS.
Mady et al. estimated that the prevalences of positive allergy in overall study populations of CRS, CRSwNP, and CRSsNP were 64.0%, 62.1%, and 65.7%, respectively [19]. Among 80 patients who tested positive, 65.8% tested positive to more than 6 allergens, and the majority of patients who tested positive to 7–14 allergens were CRSwNP (66.7%). Overall, CRS patients tended to have sensitization to perennial allergen more than seasonal allergen (55% vs 45%, respectively). Of CRSwNP patients, there was a significant difference in proportions of those with asthma in CRSwNP patient with a positive allergen test compared with those with a negative one (66.7% vs 40.9%, respectively; p = 0.045). In contrast, no such difference was found in CRSsNP patients.
Anamika et al. estimated that the prevalence of the sensitization to at least one allergen was 52.7% in CRS patients [20]. The dominant allergen was insects (43.6%). Among insect allergens, male cockroach was the most common allergen (30.9%). Quality of life and Lund-Mackay endoscopy scores were significantly higher in atopic patients than non-atopic patients (p < 0.001 and p = 0.007, respectively).
Chen et al. collected data from CRS patients who underwent endoscopic sinus surgery. They found no difference in allergy positive rate between CRSwNP and CRSsNP patients (21.7% vs 22.3%) [21]. However, the CRSwNP group had a higher proportion of elevated peripheral blood eosinophil count than the non-polyp group (33.1% vs 13.5%; p < 0.001). In univariable logistic regression analysis, elevated peripheral blood eosinophil count was significantly associated with the incidence of the nasal polyp (crude OR 3.17 [95% CI 1.80–5.59]; p < 0.001). After adjusting for confounding variables in the model, elevated peripheral blood eosinophil count was an independent predictor of incidence of nasal polyp (adjusted OR 2.02 [95% CI 1.08–3.76]; p = 0.027). From a stratified analysis of the relationship between peripheral blood eosinophil and nasal polyps, elevated serum eosinophil was an independent predictor of increased risk of nasal polyp formation in non-atopic CRS patients (adjusted OR 2.95 [95% CI 1.39–6.33]; p = 0.005). However, all patients in this study were recruited because of failed medical therapy that required surgical intervention and thus represented severe disease.
Ho et al. studied the impact of allergy on quality of life in CRS patients. The prevalence of atopy in these groups was 59.9%, which was comparable to other studies [22]. They found that positive allergen sensitization patients more frequently were asthma, were CRSwNP, and had elevated peripheral blood eosinophil count. Overall, SNOT-22 was not associated with atopic status in CRS patients, but the positive allergic sensitized patients had higher nasal symptom score subdomain compared with their non-allergic sensitized counterparts. However, there was no association between atopy and SNOT-22 score in the CRSwNP subgroup both in total and subdomain scores.
Conclusion
Direct comparison among studies cannot be done, and the meta-analysis is not feasible. The main reason was notable differences in study design, methods of CRS diagnosis, and allergy testing among all included studies.
The Role of Allergy in CRS with and Without Nasal Polyps
The present study found mixed results regarding the association between allergy and CRS, allowing no definite conclusion to be drawn. This is because of limitations in study methodologies and data quality in the included studies. Traditionally, the phenotype of CRS was assigned according to the presence of nasal polyps on endoscopy and radiologic imaging. However, CRS is being increasingly recognized as a disease spectrum including a range of inflammatory states in the sinonasal cavity, with non-type 2 and type 2 (T2) inflammation. An overlapping inflammation of both T2 and non-T2 inflammation in differing proportions make interpretation of older studies complicated [23]. A better definition of population subgroup, standardized outcome measurement, and seeking more insight into the local IgE inflammation process in future studies may allow for a firm conclusion on this issue.
Despite the limitations of the included observational studies, there are data supporting the biological plausibility of the pathophysiological relationship between allergy and CRS. A proposed hypothesis by which allergy (AR and atopy) may lead to the development of sinusitis is an allergic inflammation of the sinonasal mucosa, followed by ostial obstruction, promoting bacterial overgrowth and continuation of inflammation (Fig. 2) [24]. AR and eosinophilic inflammation could impair mucociliary clearance, which is often observed in patients with CRS. Although persistent inflammation found in CRS could cause ciliary abnormalities over time, concurrent AR could possibly exacerbate CRS and have an impact on the disease process [25].
Pathophysiologic pathways of CRS and allergic inflammation include staphylococcal enterotoxin, an innate immunity pathway, mast cell–associated inflammation, and dysbiosis of the microbiota and involve a systemic interaction between peripheral lymphoid organs and the nasal-associated lymphoid tissue leading to local IgE production. Allergic inflammation recruits tissue eosinophils in a complex process regulated by various inflammatory cells and cytokines. Epithelial barrier dysfunction and disturbance in balance of nasal microbiome allow penetration of aeroallergens that induce an innate immune response shift to T-helper 2 hypersensitivity. Subsequently, chronic sinus mucosal inflammation and ostial obstruction develop. B, B lymphocyte; DC, dendritic cell; ECP, eosinophil cationic protein; EDN, eosinophil derived neurotoxin; EPO, eosinophil peroxidase; IL, interleukin; ILC2, innate lymphoid cells type 2; sIgE, specific immunoglobulin E; SE, staphylococcal enterotoxin; TSLP, thymic stromal lymphopoietin; ICAM1, intercellular adhesion molecule 1; VCAM1, vascular cell adhesion protein 1
Furthermore, parallel inflammation within both the nasal passage and maxillary sinuses was demonstrated after performing nasal allergen provocation in skin prick test (SPT)–positive patients [26]. The investigators found a significant increase in inflammatory cytokines from both nasal passage and maxillary sinuses lavage. Although the cytokines obtained from the maxillary sinuses had a smaller amount of inflammatory cytokines than the nasal passage, this was the first study to demonstrate parallel allergic inflammation, which might play roles in the development of rhinosinusitis in allergic subjects.
Since there are limitations to the evidence on the association and the existence of a mechanistic link between allergy and CRS, a firm conclusion on the causal inference cannot be drawn yet.
High Allergen Sensitization in CRSwNP Patients
The prevalence of CRSwNP was reported to be 25–30% among all patients with CRS [27]. Several studies have demonstrated an increased prevalence of perennial allergies in patients with CRSwNP compared with controls. Two studies revealed a strong association between perennial allergies and CRSwNP [28]. In contrast, many studies did not demonstrate an increased prevalence of seasonal allergies in CRSwNP patients [28, 29]. Therefore, the evidence for an association between seasonal allergies and CRSwNP is still inconclusive, but perennial allergies may contribute to CRSwNP.
The high allergen sensitization rate reported in CRSwNP patients suggests a possible link with the disease process. Munoz del Castillo et al. demonstrated 63.2% sensitization rate for at least one aeroallergen in 190 patients with nasal polyp compared with 31.1% in 190 controlled group (p < 0.001) [30]. Another evidence that supported this claim is a prospective study on CRS patients who had surgery after failing maximal medical therapy for allergen sensitization (defined by the skin tests) compared with rhinitis patients without CRS and the general population. This study revealed that there was a trend of increasing allergen sensitization from rhinitis patients (72%) to CRSsNP patients (79.4%) and thence to CRSwNP patients (85.5%). However, the overall sensitization rates were not significantly different between each group. CRS patients had higher rates of allergen sensitization compared with the general population but not compared with rhinitis patients. Interestingly, a bidirectional process may have occurred. CRSwNP patients were more likely to have multiple positive skin tests compared with the CRSsNP group (p = 0.330) and with the rhinitis group (p < 0.010). Epithelial break in CRS itself increased the probability of allergen exposure and further development of sensitization, which might lead to the perpetuation of inflammation [29]. Conversely, other studies comparing CRSwNP with CRSsNP subjects revealed no significant difference in the prevalence of allergies [31]. From the biggest epidemiological study on nasal polyps in Spain demonstrated the same result, but quality of life in allergen-sensitized patients with nasal polyps were worse than non-allergic group [32]. There are many studies that showed a lower prevalence of allergy in CRSwNP patients [33, 34]. However, the prevalence of allergen sensitization in CRSwNP is still higher than that of the general population, but substantially lower than that found in the study of Tan et al. [29]
Despite the contradictory results from observational studies, allergen sensitization may play a role in the progression of CRSwNP. Repeated exposure to aeroallergen in sensitized patients might contribute to persistent inflammation in CRSwNP patients. To support the role of IgE-mediated inflammation in CRSwNP by analogy, analogous phenomena have been described in AFRS. AFRS is a non-invasive, eosinophilic rhinosinusitis subtype, occurring in immunocompetent hosts, which has evidence that strongly demonstrates the role of IgE-mediated hypersensitivity inflammation in its pathogenesis. Fungal allergen sensitization is observed in 90% of AFRS patients and was considered as one of the Bent and Kuhn diagnostic criteria [35]. Fungal-specific IgE is linked to the pathophysiologic basis, accounting for an inflammatory response within the nasal cavity and paranasal sinuses [36, 37]. AFRS patients have demonstrated elevated serum total IgE and fungal-specific IgE compared with patients with CRSwNP, CRSsNP, and normal controls [38]. The elevation of fungal-specific IgE in the sinonasal mucosa of AFRS patients correlates with sinonasal eosinophilia [39, 40]. However, not all patients with fungal allergy develop AFRS, and not all AFRS patients have a fungal allergy. Therefore, AFRS might result from a local rather than a systemic allergic process [41].
Interestingly, CCAD was recently reported as another CRS variant [42]. The clinical description of CCAD is the pathological involvement of central structures, including the posterior-superior nasal septum, middle turbinates, and superior turbinates. Radiologically, this is seen as a soft tissue thickening in the central portion of the sinonasal cavity [18]. The majority of patients with the entity were allergen sensitization and seemed to have a stronger relationship to allergy [42, 43]. However, further study is needed to better clarify the etiology and clinical course of this CRS subtype [44].
High Local IgE Presence in Nasal Polyps: A Mechanistic Link
CRSwNP has overlapping pathophysiology with allergy with the presence of eosinophils and type 2 cytokines, such as IL-4, IL-5, IL-9, and IL-13, derived from both Th2 and innate lymphoid cells type 2 in the peripheral blood and nasal mucosa [45]. The mentioned features were described as T2 inflammation, which is also a major feature of AR and local allergic rhinitis [5, 46••]. Histomorphological features of polyp tissue reveal epithelial damage, thickened basement membrane, and edematous to sometimes fibrotic stromal tissue, containing numerous inflammatory cells and supporting fibroblasts [47]. Activated eosinophils (EG2+) are the predominate cells, accounting for 80% of inflammatory cells in polyp tissue [48]. The process of polyp growth has been studied. Numerous subepithelial EG2+ eosinophils with scarce mast cell population were observed in the luminal compartment of the early stage polyps. In contrast, the mature polyps contained degranulated mast cells and eosinophils, both of which were diffusely distributed in the polyp tissue.
Tantilipikorn et al. investigated the type of sinonasal inflammation among patients diagnosed with CRSsNP, CRSwNP, and chronic rhinitis by using transcription factor analysis. The results revealed hyperfunction of Th2 in patients with CRSwNP, which might result in hypereosinophilic infiltration in the polyps. The regulatory T cell transcription factor was significantly lower in the CRSwNP group than in the CRSsNP and rhinitis groups. The authors also concluded that decreased activity of Treg might explain these findings [49].
The evidence that we have described suggest that eosinophilic inflammation is involved in the pathogenesis of polyps formation and growth [50••]. Patients with CRSwNP also demonstrated both local mucosal and systemic circulating IgE production [51]. A previous study demonstrated that mucosal IgE in nasal polyp tissue was functional and was able to activate mast cells. These could be found independently of their presence in serum [52].
The bacterium Staphylococcal aureus has the ability to produce enterotoxin, which has superantigenic features [53]. It could bind directly to the T cell receptor outside the conventional antigen-binding site and bypass the class II major histocompatibility complex of the antigen-presenting cells. This could result in an excessive and uncoordinated T cell response with concurrent B cell proliferation, causing local polyclonal IgE production, and further eosinophil activation [5••, 54, 55]. S. aureus superantigens (SAgs) also play a role in CRS through the enhanced IgE response within the nose and the formation of polyps [5••, 55, 56]. Specific IgE produced in response to exotoxins could promote allergic inflammation and further cause the polyp to form within the nose [55, 57]. Therefore, Staphylococcal SAgs potentially increase the risk of developing CRSwNP and could be a marker for more severe disease.
The rate of S. aureus colonization is 27.3% in CRSsNP and is as high as 63.6% in CRSwNP [58]. CRSwNP patients had a significantly higher S. aureus colonization rate than in the control group (OR 4.85 [95% CI 1.80–13.05]). The detection of S. aureus SAgs and their specific IgE in CRSwNP patients were significantly higher than that of those in the control group (OR 12.07 [95% CI 4.57–31.90] and OR 17.03 [95% CI 5.43–53.39], respectively) [59].
Taken together, high allergen sensitization observed in CRSwNP patients, overlapping endotypes, and analogous mechanistic links may reflect the link between allergen sensitization, allergic inflammation, and CRSwNP. Although the causal association cannot be concluded yet, some authors suggest that allergy is a disease modifier of CRSwNP as part of its pathophysiology rather than it being a root cause of the disease [60]. We believed that future high-quality studies will lead to a firm conclusion.
The Role of Allergen Immunotherapy in CRS
Some CRS patients are refractory to medical and surgical treatment. Therefore, attempts to develop novel therapies are needed. There is a similarity of pathophysiology between CRS and allergic diseases, such as AR and atopic asthma. Allergen immunotherapy (AIT) is considered a hope for treating the allergic component in CRS pathomechanisms [46••, 49, 61]. There is a paucity of available data on the efficacy of AIT for the treatment of CRS. Weak evidence supports the use of AIT as an adjunctive treatment in CRS patients, particularly in the post-operative period. A recent systematic review from DeYoung et al. identified seven studies that assessed the efficacy of AIT on the clinical outcome of CRS [62]. Their study concluded that there was weak evidence supporting the role of AIT as an adjunctive treatment in CRS patients with atopy by improving symptom scores, endoscopic outcomes, radiologic outcomes, and decreased frequency in revision surgery [63, 64].
However, the limitations of this evidence includes the lack of randomized controlled trials, heterogeneous participants (CRSsNP, CRSwNP, and AFRS), as well as overlapping outcomes between AR and CRS. Therefore, the available evidence supports the use of AIT in CRS patients with weak evidence. The symptom improvement observed in the outcome might be due to an improvement of AR symptoms [65,66,67]. AR shares similar symptoms with CRS. No data of AIT on the disease-modulating effect for CRS is currently available.
Conclusion
A definite conclusion could not be drawn from the present study. This is due to several limitations of study methodologies and data quality among the studies included. Since an asymptomatic patient can be sensitized to one or more allergens (atopy), while allergic rhinitis is diagnosed if these atopic individuals have clinically relevant sensitization. This important limitation is that our systemic allergy includes atopy and allergic rhinitis. However, the evidence of the mechanistic link between CRS and allergy exists. We believed that more high-quality studies will lead to a firm conclusion.
Abbreviations
- AFRS:
-
Allergic fungal rhinosinusitis
- AIT:
-
Allergen immunotherapy
- AR:
-
Allergic rhinitis
- CCAD:
-
Central compartment atopic disease
- CRS:
-
Chronic rhinosinusitis
- CRSsNP:
-
Chronic rhinosinusitis without nasal polyp
- CRSwNP:
-
Chronic rhinosinusitis with nasal polyp
- ECP:
-
Eosinophil cationic protein
- EDN:
-
Eosinophil derived neurotoxin
- ELISA:
-
Enzyme-linked immunosorbent assay
- EPO:
-
Eosinophil peroxidase
- ICAM1:
-
Intercellular adhesion molecule 1
- ILC2:
-
Innate lymphoid cells type 2
- LAR:
-
Local allergic rhinitis
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- sIgE:
-
Specific IgE
- SE:
-
Staphylococcal enterotoxin
- SPT:
-
Skin prick test
- TSLP:
-
Thymic stromal lymphopoietin
- VCAM1:
-
Vascular cell adhesion protein 1
References
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Ahn JC, Kim JW, Lee CH, Rhee CS. Prevalence and risk factors of chronic rhinosinusitus, allergic rhinitis, and nasal septal deviation: results of the Korean National Health and Nutrition Survey 2008-2012. JAMA Otolaryngol Head Neck Surg. 2016;142(2):162–7.
Bakhshaee M, Jabari F, Ghassemi MM, Hourzad S, Deutscher R, Nahid K. The prevalence of allergic rhinitis in patients with chronic rhinosinusitis. Iran J Otorhinolaryngol. 2014;26(77):245–9.
Tantilipikorn P, Siriboonkoom P, Sookrung N, Thianboonsong A, Suwanwech T, Pinkaew B, et al. Prevalence of local allergic rhinitis to Dermatophagoides pteronyssinus in chronic rhinitis with negative skin prick test. Asian Pac J Allergy Immunol. 2019. https://doi.org/10.12932/AP-170918-0408.
Buntarickpornpan P, Veskitkul J, Pacharn P, Visitsunthorn N, Vichyanond P, Tantilipikorn P, et al. The proportion of local allergic rhinitis to Dermatophagoides pteronyssinus in children. Pediatr Allergy Immunol. 2016;27(6):574–9.
•• Rondon C, Campo P, Togias A, Fokkens WJ, Durham SR, Powe DG, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol. 2012;129(6):1460–7 Excellent comprehensive review of local allergic rhinitis.
Yao Y, Xie S, Yang C, Zhang J, Wu X, Sun H. Biomarkers in the evaluation and management of chronic rhinosinusitis with nasal polyposis. Eur Arch Otorhinolaryngol. 2017;274(10):3559–66.
Takahashi T, Kato A, Berdnikovs S, Stevens WW, Suh LA, Norton JE, et al. Microparticles in nasal lavage fluids in chronic rhinosinusitis: potential biomarkers for diagnosis of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017;140(3):720–9.
Jonstam K, Westman M, Holtappels G, Holweg CTJ, Bachert C. Serum periostin, IgE, and SE-IgE can be used as biomarkers to identify moderate to severe chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;140(6):1705–8 e1703.
Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138(5):1344–53.
•• Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2014;4(2):93–103 Outstanding review of association between allergy and chronic rhinosinusitis.
Ba L, Du J, Liu F, Yang F, Han M, Liu S, et al. Distinct inflammatory profiles in atopic and nonatopic patients with chronic rhinosinustis accompanied by nasal polyps in western China. Allergy Asthma Immunol Res. 2015;7(4):346–58.
Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014;44(5):690–700.
Green BJ, Beezhold DH, Gallinger Z, Barron CS, Melvin R, Bledsoe TA, et al. Allergic sensitization in Canadian chronic rhinosinusitis patients. Allergy Asthma Clin Immunol. 2014;10(1):15.
Sedaghat AR, Phipatanakul W, Cunningham MJ. Prevalence of and associations with allergic rhinitis in children with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol. 2014;78(2):343–7.
Sedaghat AR, Phipatanakul W, Cunningham MJ. Characterization of aeroallergen sensitivities in children with allergic rhinitis and chronic rhinosinusitis. Allergy Rhinol (Providence). 2014;5(3):143–5.
Tomljenovic D, Pinter D, Kalogjera L. Perceived stress and severity of chronic rhinosinusitis in allergic and nonallergic patients. Allergy Asthma Proc. 2014;35(5):398–403.
Brook CD, Kuperstock JE, Rubin SJ, Ryan MW, Platt MP. The association of allergic sensitization with radiographic sinus opacification. Am J Rhinol Allergy. 2017;31(1):12–5.
Hamizan AW, Loftus PA, Alvarado R, Ho J, Kalish L, Sacks R, et al. Allergic phenotype of chronic rhinosinusitis based on radiologic pattern of disease. Laryngoscope. 2018;128(9):2015–21.
Mady LJ, Schwarzbach HL, Moore JA, Boudreau RM, Kaffenberger TM, Willson TJ, et al. The association of air pollutants and allergic and nonallergic rhinitis in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(3):369–76.
Anamika A, Chakravarti A, Kumar R. Atopy and quality of life in pediatric chronic rhinosinusitis. Am J Rhinol Allergy. 2019;33(5):586–90.
Chen F, Wen L, Qiao L, Shi Z, Xue T, Chen X, et al. Impact of allergy and eosinophils on the morbidity of chronic rhinosinusitis with nasal polyps in Northwest China. Int Arch Allergy Immunol. 2019;179(3):209–14.
Ho J, Alvarado R, Rimmer J, Sewell WA, Harvey RJ. Atopy in chronic rhinosinusitis: impact on quality of life outcomes. Int Forum Allergy Rhinol. 2019;9(5):501–7.
Xu X, Ong YK, Wang Y. Novel findings in immunopathophysiology of chronic rhinosinusitis and their role in a model of precision medicine. Allergy. 2019. https://doi.org/10.1111/all.14044.
Halderman AA, Tully LJ. The role of allergy in chronic rhinosinusitis. Otolaryngol Clin N Am. 2017;50(6):1077–90.
Schuhl JF. Nasal mucociliary clearance in perennial rhinitis. J Investig Allergol Clin Immunol. 1995;5(6):333–6.
Baroody FM, Mucha SM, Detineo M, Naclerio RM. Nasal challenge with allergen leads to maxillary sinus inflammation. J Allergy Clin Immunol. 2008;121(5):1126–32 e1127.
Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4(4):565–72.
Houser SM, Keen KJ. The role of allergy and smoking in chronic rhinosinusitis and polyposis. Laryngoscope. 2008;118(9):1521–7.
Tan BK, Zirkle W, Chandra RK, Lin D, Conley DB, Peters AT, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1(2):88–94.
Munoz del Castillo F, Jurado-Ramos A, Fernandez-Conde BL, Soler R, Barasona MJ, Cantillo E, et al. Allergenic profile of nasal polyposis. J Investig Allergol Clin Immunol. 2009;19(2):110–6.
Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23(2):145–8.
Davila I, Rondon C, Navarro A, Anton E, Colas C, Dordal MT, et al. Aeroallergen sensitization influences quality of life and comorbidities in patients with nasal polyposis. Am J Rhinol Allergy. 2012;26(5):e126–31.
Gorgulu O, Ozdemir S, Canbolat EP, Sayar C, Olgun MK, Akbas Y. Analysis of the roles of smoking and allergy in nasal polyposis. Ann Otol Rhinol Laryngol. 2012;121(9):615–9.
Bonfils P, Malinvaud D. Influence of allergy in patients with nasal polyposis after endoscopic sinus surgery. Acta Otolaryngol. 2008;128(2):186–92.
Saravanan K, Panda NK, Chakrabarti A, Das A, Bapuraj RJ. Allergic fungal rhinosinusitis: an attempt to resolve the diagnostic dilemma. Arch Otolaryngol Head Neck Surg. 2006;132(2):173–8.
Callejas CA, Douglas RG. Fungal rhinosinusitis: what every allergist should know. Clin Exp Allergy. 2013;43(8):835–49.
Silva MP, Baroody FM. Allergic fungal rhinosinusitis. Ann Allergy Asthma Immunol. 2013;110(4):217–22.
Hutcheson PS, Schubert MS, Slavin RG. Distinctions between allergic fungal rhinosinusitis and chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(6):405–8.
Chang YT, Fang SY. Tissue-specific immunoglobulin E in maxillary sinus mucosa of allergic fungal sinusitis. Rhinology. 2008;46(3):226–30.
Wise SK, Ahn CN, Lathers DMR, Mulligan RM, Schlosser RJ. Antigen-specific IgE in sinus mucosa of allergic fungal rhinosinusitis patients. Am J Rhinol. 2008;22(5):451–6.
Pant H, Schembri MA, Wormald PJ, Macardle PJ. IgE-mediated fungal allergy in allergic fungal sinusitis. Laryngoscope. 119(6):1046–52.
White LJ, Rotella MR, DelGaudio JM. Polypoid changes of the middle turbinate as an indicator of atopic disease. Int Forum Allergy Rhinol. 2014;4(5):376–80.
Brunner JP, Jawad BA, McCoul ED. Polypoid change of the middle turbinate and paranasal sinus polyposis are distinct entities. Otolaryngol Head Neck Surg. 2017;157(3):519–23.
Marcus S, DelGaudio JM, Roland LT, Wise SK. Chronic rhinosinusitis: does allergy play a role? Med Sci (Basel). 2019 Feb 18;7(2). https://doi.org/10.3390/medsci7020030.
Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43(1):29–40.
•• Bachert C, Zhang N. Endotype-driven care pathways in chronic rhinosinusitis (CRS). Allergologie. 2018;41(12):571–86 Explanation of the role of type 2 inflammation/biomarkers and describing the use of biologics in chronic rhinosinusitis.
Kakoi H, Hiraide F. A histological study of formation and growth of nasal polyps. Acta Otolaryngol. 1987;103(1–2):137–44.
Stoop AE, van der Heijden HA, Biewenga J, van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol. 1993;91(2):616–22.
Tantilipikorn P, Sookrung N, Muangsomboon S, Lumyongsatien J, Bedavanija A, Suwanwech T. Endotyping of chronic rhinosinusitis with and without polyp using transcription factor analysis. Front Cell Infect Microbiol. 2018;27(8):82. https://doi.org/10.3389/fcimb.2018.00082.
•• Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464 Updated evidence-based recommendations in all aspect of rhinosinusitis.
Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107(4):607–14.
Zhang N, Holtappels G, Gevaert P, Patou J, Dhaliwal B, Gould H, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66(1):141–8.
Muluk NB, Altin F, Cingi C. Role of superantigens in allergic inflammation: their relationship to allergic rhinitis, chronic rhinosinusitis, asthma, and atopic dermatitis. Am J Rhinol Allergy. 2018;32(6):502–17.
Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer PH, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175(1):91–8.
Eguiluz-Gracia I, Layhadi JA, Rondon C, Shamji MH. Mucosal IgE immune responses in respiratory diseases. Curr Opin Pharmacol. 2019;46:100–7.
Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy. 2012;26(3):187–90.
Bernstein JM, Allen C, Rich G, Dryja D, Bina P, Reiser R, et al. Further observations on the role of Staphylococcus aureus exotoxins and IgE in the pathogenesis of nasal polyposis. Laryngoscope. 2011;121(3):647–55.
Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114(4):981–3.
Ou J, Wang J, Xu Y, Tao ZZ, Kong YG, Chen SM, et al. Staphylococcus aureus superantigens are associated with chronic rhinosinusitis with nasal polyps: a meta-analysis. Eur Arch Otorhinolaryngol. 2014;271(10):2729–36.
Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2014;4(2):93–103.
De Greve G, Hellings PW, Fokkens WJ, Pugin B, Steelant B, Seys SF. Endotype-driven treatment in chronic upper airway diseases. Clinical and Translational Allergy. 2017;12;7:22. https://doi.org/10.1186/s13601-017-0157-8.
DeYoung K, Wentzel JL, Schlosser RJ, Nguyen SA, Soler ZM. Systematic review of immunotherapy for chronic rhinosinusitis. Am J Rhinol Allergy. 2014;28(2):145–50.
Nathan RA, Santilli J, Rockwell W, Glassheim J. Effectiveness of immunotherapy for recurring sinusitis associated with allergic rhinitis as assessed by the sinusitis outcomes questionnaire. Ann Allergy Asthma Immunol. 2004;92(6):668–72.
Marple BF. Allergic rhinitis and inflammatory airway disease: interactions within the unified airspace. Am J Rhinol Allergy. 2010;24(4):249–54.
Purkey MT, Smith TL, Ferguson BJ, Luong A, Reisacher WR, Pillsbury HC 3rd, et al. Subcutaneous immunotherapy for allergic rhinitis: an evidence based review of the recent literature with recommendations. Int Forum Allergy Rhinol. 2013;3(7):519–31.
Erekosima N, Suarez-Cuervo C, Ramanathan M, Kim JM, Chelladurai Y, Segal JB, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope. 2014;124(3):616–27.
Nurmatov U, Dhami S, Arasi S, Roberts G, Pfaar O, Muraro A, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rhinosinusitis
Rights and permissions
About this article
Cite this article
Tantilipikorn, P., Sompornrattanaphan, M., Suwanwech, T. et al. Chronic Rhinosinusitis and Allergy: Increased Allergen Sensitization Versus Real Allergic Rhinitis Multimorbidity: a Systematic Review. Curr Allergy Asthma Rep 20, 19 (2020). https://doi.org/10.1007/s11882-020-00913-9
Published:
DOI: https://doi.org/10.1007/s11882-020-00913-9